Abstract
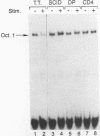
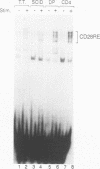
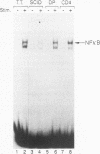
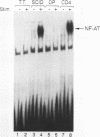
At least three stages in the intrathymic development of pre-T cells are demarcated by differences in the competence to express the interleukin-2 (IL-2) gene as an acute response to stimulation. IL-2 inducibility appears to be acquired relatively early, prior to T-cell receptor (TcR) gene rearrangement. It is then abrogated during the stage when cells are subject to positive and negative selection, i.e., the fate determination processes that select cells for maturation or death. IL-2 inducibility finally reappears in mature classes of thymocytes that have undergone positive selection. To provide a basis for a molecular explanation of these developmental transitions, we have examined the representation in different thymocyte subsets of a set of DNA-binding proteins implicated in IL-2 gene regulation. As the DNA-binding activities of many factors are elicited only by inductive stimuli, the cells were cultured in the presence or absence of the calcium ionophore A23187 and phorbol ester. Our results separate these factors into four regulatory classes: (i) constitutive factors, such as Oct-1 and probably Sp1, that are expressed in thymocytes at all stages; (ii) inducible factors, such as NF-kappa B and complexes binding to the region of a CD28 response element, that can be activated in all thymocytes, including those cells (CD4+ CD8+ TcRlow) that can undergo selection; (iii) inducible factors, such as NF-AT and AP-1, that can be activated in mature (CD4+ CD8- TcRhigh) and immature (CD4- CD8- TcR-) thymocytes alike but not in the transitional stages when the cells (CD4+ CD8+ TcRlow) are subject to selection; and (iv) a factor containing CREB, which can be activated in thymocytes of all developmental stages by culture but does not require specific induction. These results verify that inducible transcription factors are targets of intrathymic developmental change. They also identify NF-AT and AP-1 as factors that are particularly sensitive to the mechanism altering thymocyte responses during the stages when thymocytes may undergo positive and negative selection.
Full text
PDF



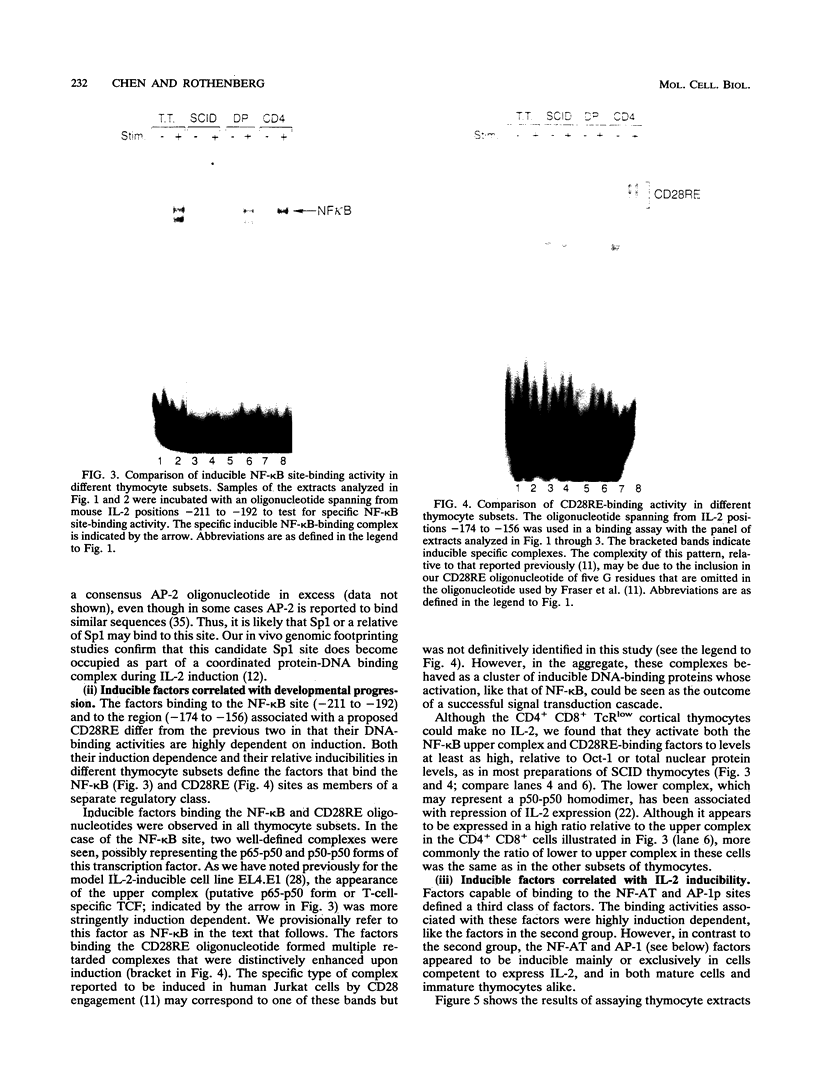

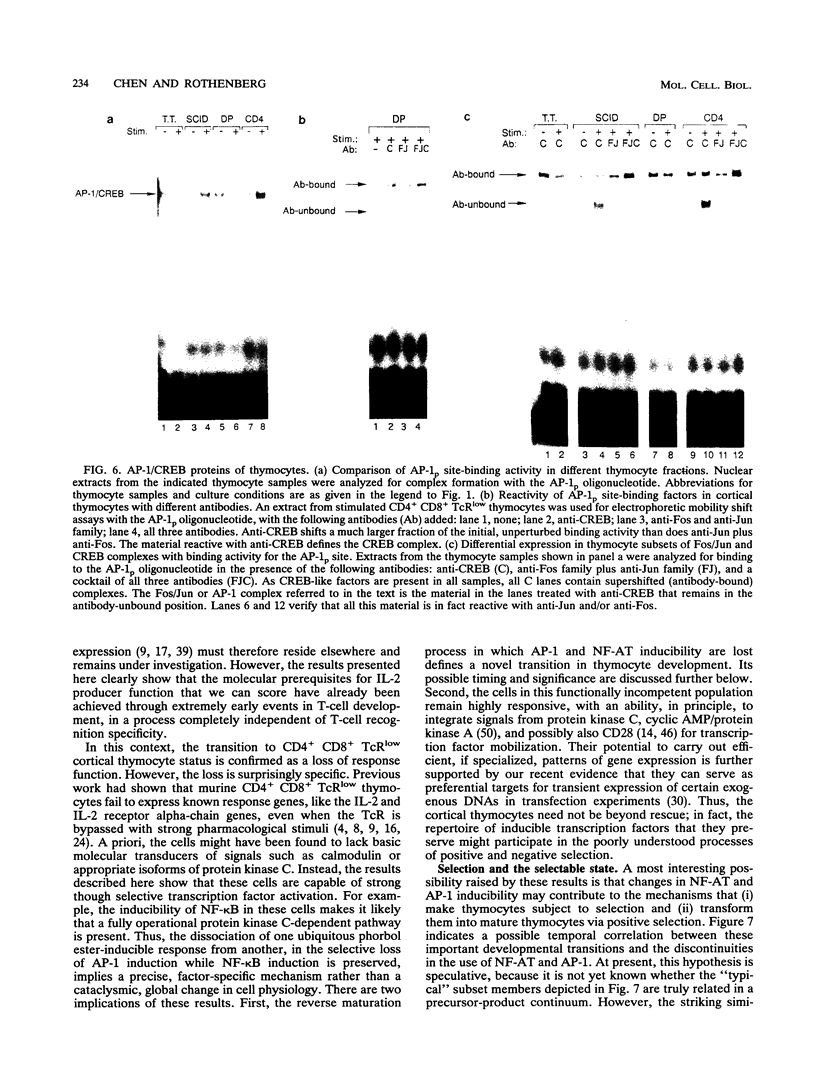
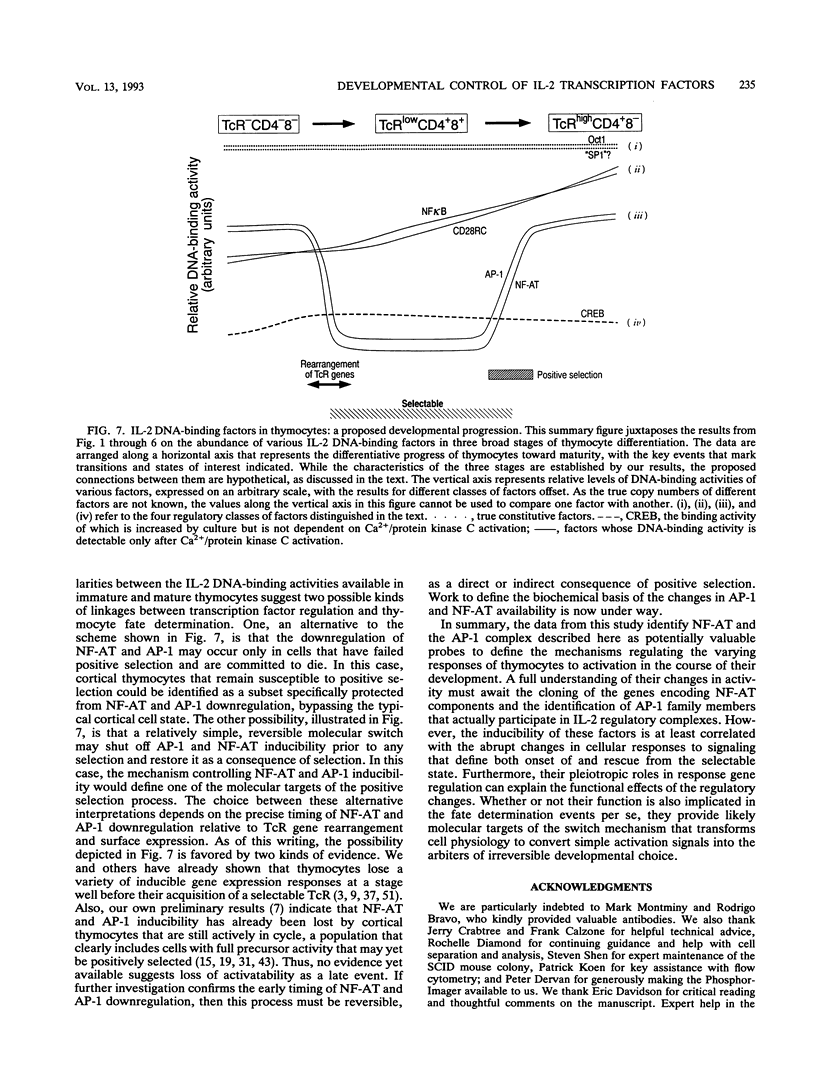


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham K. M., Levin S. D., Marth J. D., Forbush K. A., Perlmutter R. M. Delayed thymocyte development induced by augmented expression of p56lck. J Exp Med. 1991 Jun 1;173(6):1421–1432. doi: 10.1084/jem.173.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma M. J., Carroll A. M. The SCID mouse mutant: definition, characterization, and potential uses. Annu Rev Immunol. 1991;9:323–350. doi: 10.1146/annurev.iy.09.040191.001543. [DOI] [PubMed] [Google Scholar]
- Boyer P. D., Diamond R. A., Rothenberg E. V. Changes in inducibility of IL-2 receptor alpha-chain and T cell-receptor expression during thymocyte differentiation in the mouse. J Immunol. 1989 Jun 15;142(12):4121–4130. [PubMed] [Google Scholar]
- Boyer P. D., Rothenberg E. V. IL-2 receptor inducibility is blocked in cortical-type thymocytes. J Immunol. 1988 May 1;140(9):2886–2892. [PubMed] [Google Scholar]
- Brabletz T., Pietrowski I., Serfling E. The immunosuppressives FK 506 and cyclosporin A inhibit the generation of protein factors binding to the two purine boxes of the interleukin 2 enhancer. Nucleic Acids Res. 1991 Jan 11;19(1):61–67. doi: 10.1093/nar/19.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzone F. J., Thézé N., Thiebaud P., Hill R. L., Britten R. J., Davidson E. H. Developmental appearance of factors that bind specifically to cis-regulatory sequences of a gene expressed in the sea urchin embryo. Genes Dev. 1988 Sep;2(9):1074–1088. doi: 10.1101/gad.2.9.1074. [DOI] [PubMed] [Google Scholar]
- Cooke M. P., Abraham K. M., Forbush K. A., Perlmutter R. M. Regulation of T cell receptor signaling by a src family protein-tyrosine kinase (p59fyn). Cell. 1991 Apr 19;65(2):281–291. doi: 10.1016/0092-8674(91)90162-r. [DOI] [PubMed] [Google Scholar]
- Fischer M., MacNeil I., Suda T., Cupp J. E., Shortman K., Zlotnik A. Cytokine production by mature and immature thymocytes. J Immunol. 1991 May 15;146(10):3452–3456. [PubMed] [Google Scholar]
- Fowlkes B. J., Pardoll D. M. Molecular and cellular events of T cell development. Adv Immunol. 1989;44:207–264. doi: 10.1016/s0065-2776(08)60643-4. [DOI] [PubMed] [Google Scholar]
- Fraser J. D., Irving B. A., Crabtree G. R., Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991 Jan 18;251(4991):313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- Gonzalez G. A., Yamamoto K. K., Fischer W. H., Karr D., Menzel P., Biggs W., 3rd, Vale W. W., Montminy M. R. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature. 1989 Feb 23;337(6209):749–752. doi: 10.1038/337749a0. [DOI] [PubMed] [Google Scholar]
- Gross J. A., Callas E., Allison J. P. Identification and distribution of the costimulatory receptor CD28 in the mouse. J Immunol. 1992 Jul 15;149(2):380–388. [PubMed] [Google Scholar]
- Guidos C. J., Weissman I. L., Adkins B. Intrathymic maturation of murine T lymphocytes from CD8+ precursors. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7542–7546. doi: 10.1073/pnas.86.19.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havran W. L., Poenie M., Kimura J., Tsien R., Weiss A., Allison J. P. Expression and function of the CD3-antigen receptor on murine CD4+8+ thymocytes. Nature. 1987 Nov 12;330(6144):170–173. doi: 10.1038/330170a0. [DOI] [PubMed] [Google Scholar]
- Howe R. C., MacDonald H. R. Heterogeneity of immature (Lyt-2-/L3T4-) thymocytes. Identification of four major phenotypically distinct subsets differing in cell cycle status and in vitro activation requirements. J Immunol. 1988 Feb 15;140(4):1047–1055. [PubMed] [Google Scholar]
- Hoyos B., Ballard D. W., Böhnlein E., Siekevitz M., Greene W. C. Kappa B-specific DNA binding proteins: role in the regulation of human interleukin-2 gene expression. Science. 1989 Apr 28;244(4903):457–460. doi: 10.1126/science.2497518. [DOI] [PubMed] [Google Scholar]
- Huesmann M., Scott B., Kisielow P., von Boehmer H. Kinetics and efficacy of positive selection in the thymus of normal and T cell receptor transgenic mice. Cell. 1991 Aug 9;66(3):533–540. doi: 10.1016/0092-8674(81)90016-7. [DOI] [PubMed] [Google Scholar]
- Jain J., Valge-Archer V. E., Rao A. Analysis of the AP-1 sites in the IL-2 promoter. J Immunol. 1992 Feb 15;148(4):1240–1250. [PubMed] [Google Scholar]
- Kamps M. P., Corcoran L., LeBowitz J. H., Baltimore D. The promoter of the human interleukin-2 gene contains two octamer-binding sites and is partially activated by the expression of Oct-2. Mol Cell Biol. 1990 Oct;10(10):5464–5472. doi: 10.1128/mcb.10.10.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S. M., Tran A. C., Grilli M., Lenardo M. J. NF-kappa B subunit regulation in nontransformed CD4+ T lymphocytes. Science. 1992 Jun 5;256(5062):1452–1456. doi: 10.1126/science.1604322. [DOI] [PubMed] [Google Scholar]
- Kovary K., Bravo R. The jun and fos protein families are both required for cell cycle progression in fibroblasts. Mol Cell Biol. 1991 Sep;11(9):4466–4472. doi: 10.1128/mcb.11.9.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo J. P., Krishnan S. N., Sailor R. D., Rothenberg E. V. Early precursor thymocytes can produce interleukin 2 upon stimulation with calcium ionophore and phorbol ester. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1862–1866. doi: 10.1073/pnas.83.6.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire K. L., Yang J. A., Rothenberg E. V. Influence of activating stimulus on functional phenotype: interleukin 2 mRNA accumulation differentially induced by ionophore and receptor ligands in subsets of murine T cells. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6503–6507. doi: 10.1073/pnas.85.17.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinkoth J. L., Montminy M. R., Fink J. S., Feramisco J. R. Induction of a cyclic AMP-responsive gene in living cells requires the nuclear factor CREB. Mol Cell Biol. 1991 Mar;11(3):1759–1764. doi: 10.1128/mcb.11.3.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muegge K., Williams T. M., Kant J., Karin M., Chiu R., Schmidt A., Siebenlist U., Young H. A., Durum S. K. Interleukin-1 costimulatory activity on the interleukin-2 promoter via AP-1. Science. 1989 Oct 13;246(4927):249–251. doi: 10.1126/science.2799385. [DOI] [PubMed] [Google Scholar]
- Novak T. J., Chen D., Rothenberg E. V. Interleukin-1 synergy with phosphoinositide pathway agonists for induction of interleukin-2 gene expression: molecular basis of costimulation. Mol Cell Biol. 1990 Dec;10(12):6325–6334. doi: 10.1128/mcb.10.12.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak T. J., White P. M., Rothenberg E. V. Regulatory anatomy of the murine interleukin-2 gene. Nucleic Acids Res. 1990 Aug 11;18(15):4523–4533. doi: 10.1093/nar/18.15.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak T. J., Yoshimura F. K., Rothenberg E. V. In vitro transfection of fresh thymocytes and T cells shows subset-specific expression of viral promoters. Mol Cell Biol. 1992 Apr;12(4):1515–1527. doi: 10.1128/mcb.12.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penit C. Positive selection is an early event in thymocyte differentiation: high TCR expression by cycling immature thymocytes precedes final maturation by several days. Int Immunol. 1990;2(7):629–638. doi: 10.1093/intimm/2.7.629. [DOI] [PubMed] [Google Scholar]
- Radler-Pohl A., Pfeuffer I., Karin M., Serfling E. A novel T-cell trans-activator that recognizes a phorbol ester-inducible element of the interleukin-2 promoter. New Biol. 1990 Jun;2(6):566–573. [PubMed] [Google Scholar]
- Randak C., Brabletz T., Hergenröther M., Sobotta I., Serfling E. Cyclosporin A suppresses the expression of the interleukin 2 gene by inhibiting the binding of lymphocyte-specific factors to the IL-2 enhancer. EMBO J. 1990 Aug;9(8):2529–2536. doi: 10.1002/j.1460-2075.1990.tb07433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegel J. S., Richie E. R., Allison J. P. Nuclear events after activation of CD4+8+ thymocytes. J Immunol. 1990 May 1;144(9):3611–3618. [PubMed] [Google Scholar]
- Roesler W. J., Vandenbark G. R., Hanson R. W. Cyclic AMP and the induction of eukaryotic gene transcription. J Biol Chem. 1988 Jul 5;263(19):9063–9066. [PubMed] [Google Scholar]
- Rothenberg E. V., Diamond R. A., Pepper K. A., Yang J. A. IL-2 gene inducibility in T cells before T cell receptor expression. Changes in signaling pathways and gene expression requirements during intrathymic maturation. J Immunol. 1990 Mar 1;144(5):1614–1624. [PubMed] [Google Scholar]
- Rothenberg E. V., McGuire K. L., Boyer P. D. Molecular indices of functional competence in developing T cells. Immunol Rev. 1988 Aug;104:29–53. doi: 10.1111/j.1600-065x.1988.tb00758.x. [DOI] [PubMed] [Google Scholar]
- Rothenberg E. V. The development of functionally responsive T cells. Adv Immunol. 1992;51:85–214. doi: 10.1016/s0065-2776(08)60487-3. [DOI] [PubMed] [Google Scholar]
- Shaw J. P., Utz P. J., Durand D. B., Toole J. J., Emmel E. A., Crabtree G. R. Identification of a putative regulator of early T cell activation genes. Science. 1988 Jul 8;241(4862):202–205. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- Shibuya H., Yoneyama M., Taniguchi T. Involvement of a common transcription factor in the regulated expression of IL-2 and IL-2 receptor genes. Int Immunol. 1989;1(1):43–49. doi: 10.1093/intimm/1.1.43. [DOI] [PubMed] [Google Scholar]
- Shortman K., Vremec D., Egerton M. The kinetics of T cell antigen receptor expression by subgroups of CD4+8+ thymocytes: delineation of CD4+8+3(2+) thymocytes as post-selection intermediates leading to mature T cells. J Exp Med. 1991 Feb 1;173(2):323–332. doi: 10.1084/jem.173.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanopoulou E., Giguere V., Grosveld F. The functional domains of the murine Thy-1 gene promoter. Mol Cell Biol. 1991 Apr;11(4):2216–2228. doi: 10.1128/mcb.11.4.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B., Rahmsdorf H. J., Steffen A., Litfin M., Herrlich P. UV-induced DNA damage is an intermediate step in UV-induced expression of human immunodeficiency virus type 1, collagenase, c-fos, and metallothionein. Mol Cell Biol. 1989 Nov;9(11):5169–5181. doi: 10.1128/mcb.9.11.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turka L. A., Linsley P. S., Paine R., 3rd, Schieven G. L., Thompson G. B., Ledbetter J. A. Signal transduction via CD4, CD8, and CD28 in mature and immature thymocytes. Implications for thymic selection. J Immunol. 1991 Mar 1;146(5):1428–1436. [PubMed] [Google Scholar]
- Ullman K. S., Northrop J. P., Verweij C. L., Crabtree G. R. Transmission of signals from the T lymphocyte antigen receptor to the genes responsible for cell proliferation and immune function: the missing link. Annu Rev Immunol. 1990;8:421–452. doi: 10.1146/annurev.iy.08.040190.002225. [DOI] [PubMed] [Google Scholar]
- Verweij C. L., Geerts M., Aarden L. A. Activation of interleukin-2 gene transcription via the T-cell surface molecule CD28 is mediated through an NF-kB-like response element. J Biol Chem. 1991 Aug 5;266(22):14179–14182. [PubMed] [Google Scholar]
- Zick Y., Cesla R., Shaltiel S. Non-hormonal burst in the level of cAMP caused by a "temperature shock" to mouse thymocytes. FEBS Lett. 1978 Jun 15;90(2):239–242. doi: 10.1016/0014-5793(78)80376-7. [DOI] [PubMed] [Google Scholar]
- Zlotnik A., Godfrey D. I., Fischer M., Suda T. Cytokine production by mature and immature CD4-CD8- T cells. Alpha beta-T cell receptor+ CD4-CD8- T cells produce IL-4. J Immunol. 1992 Aug 15;149(4):1211–1215. [PubMed] [Google Scholar]
- von Boehmer H. Developmental biology of T cells in T cell-receptor transgenic mice. Annu Rev Immunol. 1990;8:531–556. doi: 10.1146/annurev.iy.08.040190.002531. [DOI] [PubMed] [Google Scholar]