Abstract
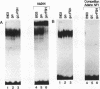
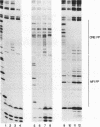
We have characterized tissue type plasminogen activator (tPA) promoter elements and nuclear factors required for follicle-stimulating hormone (FSH)-induced transcription of the rat tPA gene in granulosa cells and constitutive expression of the gene in the rat neuroblastoma cell line B103. Run-on transcription analysis of isolated nuclei revealed that B103 cells transcribe the tPA gene at a high and constitutive level, while FSH was found to induce tPA gene transcription in a rapid and transient manner in granulosa cells. The maximal FSH-induced transcription rate was obtained after 20 min and was similar in the absence or presence of the protein synthesis inhibitor cycloheximide. However, in the presence of cycloheximide, tPA transcription was not turned off but continued at a high rate for several hours. This phenomenon may at least partly explain the earlier finding that tPA mRNA is superinduced by FSH in the presence of cycloheximide. DNase I footprinting analysis of the first 621 bp of the tPA promoter revealed a total of six regions that interact with nuclear factors from B103 and granulosa cells. Deletion of the promoter region from positions -269 to -621, a region that includes the two most-upstream footprints, had no effect on constitutive or FSH-induced transcription in transient expression experiments. Nuclear extracts from both granulosa cells and B103 cells showed strong binding to a consensus cyclic AMP-responsive element (CRE) at positions -178 to -185 and a neighboring binding site for nuclear factor 1 (NF1) at positions -145 to -158. The factors binding to these two regions were identified as members of the CRE-binding protein and NF1 families of transcription factors, respectively. Footprints were also obtained over two GC boxes at positions -64 to -71 and -41 to -49. These footprints were more pronounced with nuclear extracts from B103 cells than with extracts from untreated or FSH-treated granulosa cells, but gel shift assays indicate that similar amounts of two distinct factors bind to the two GC boxes in both cell types. Transfection experiments using promoter constructs with inactivated promoter elements indicate that both the CRE and NF1 sites contribute to the FSH responsiveness of the rat tPA gene in granulosa cells, while only the NF1 site is important for constitutive expression in B103 cells. The two GC boxes were found to be necessary both for constitutive expression in B103 cells and for FSH-induced expression in granulosa cells, and inactivation of both GC boxes essentially eliminated the tPA promoter activity in both cell types.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altus M. S., Pearson D., Horiuchi A., Nagamine Y. Inhibition of protein synthesis in LLC-PK1 cells increases calcitonin-induced plasminogen-activator gene transcription and mRNA stability. Biochem J. 1987 Mar 1;242(2):387–392. doi: 10.1042/bj2420387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M. Transcriptional control by nuclear receptors. FASEB J. 1991 Apr;5(7):2044–2051. doi: 10.1096/fasebj.5.7.2010057. [DOI] [PubMed] [Google Scholar]
- Beers W. H. Follicular plasminogen and plasminogen activator and the effect of plasmin on ovarian follicle wall. Cell. 1975 Nov;6(3):379–386. doi: 10.1016/0092-8674(75)90187-7. [DOI] [PubMed] [Google Scholar]
- Beers W. H., Strickland S., Reich E. Ovarian plasminogen activator: relationship to ovulation and hormonal regulation. Cell. 1975 Nov;6(3):387–394. doi: 10.1016/0092-8674(75)90188-9. [DOI] [PubMed] [Google Scholar]
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Ushiro H., Stoscheck C., Chinkers M. A native 170,000 epidermal growth factor receptor-kinase complex from shed plasma membrane vesicles. J Biol Chem. 1982 Feb 10;257(3):1523–1531. [PubMed] [Google Scholar]
- Collen D. On the regulation and control of fibrinolysis. Edward Kowalski Memorial Lecture. Thromb Haemost. 1980 Jun 18;43(2):77–89. [PubMed] [Google Scholar]
- Comb M., Birnberg N. C., Seasholtz A., Herbert E., Goodman H. M. A cyclic AMP- and phorbol ester-inducible DNA element. 1986 Sep 25-Oct 1Nature. 323(6086):353–356. doi: 10.1038/323353a0. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R., Barr P. J., Cousens L. S., Fretto L. J., Williams L. T. Acidic and basic fibroblast growth factors stimulate tyrosine kinase activity in vivo. J Biol Chem. 1988 Jan 15;263(2):988–993. [PubMed] [Google Scholar]
- Damon D. H., D'Amore P. A., Wagner J. A. Nerve growth factor and fibroblast growth factor regulate neurite outgrowth and gene expression in PC12 cells via both protein kinase C- and cAMP-independent mechanisms. J Cell Biol. 1990 Apr;110(4):1333–1339. doi: 10.1083/jcb.110.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Darrow A. L., Rickles R. J., Pecorino L. T., Strickland S. Transcription factor Sp1 is important for retinoic acid-induced expression of the tissue plasminogen activator gene during F9 teratocarcinoma cell differentiation. Mol Cell Biol. 1990 Nov;10(11):5883–5893. doi: 10.1128/mcb.10.11.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. S., Farese R. V., Clark M. R. Gonadotropin-releasing hormone (GnRH) stimulates phosphatidylinositol metabolism in rat granulosa cells: mechanism of action of GnRH. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2049–2053. doi: 10.1073/pnas.80.7.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Sazer S., Tjian R., Schimke R. T. Transcription factor Sp1 recognizes a DNA sequence in the mouse dihydrofolate reductase promoter. Nature. 1986 Jan 16;319(6050):246–248. doi: 10.1038/319246a0. [DOI] [PubMed] [Google Scholar]
- Ephrussi A., Church G. M., Tonegawa S., Gilbert W. B lineage--specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science. 1985 Jan 11;227(4683):134–140. doi: 10.1126/science.3917574. [DOI] [PubMed] [Google Scholar]
- Evans M. I., Hager L. J., McKnight G. S. A somatomedin-like peptide hormone is required during the estrogen-mediated induction of ovalbumin gene transcription. Cell. 1981 Jul;25(1):187–193. doi: 10.1016/0092-8674(81)90243-9. [DOI] [PubMed] [Google Scholar]
- Feng P., Ohlsson M., Ny T. The structure of the TATA-less rat tissue-type plasminogen activator gene. Species-specific sequence divergences in the promoter predict differences in regulation of gene expression. J Biol Chem. 1990 Feb 5;265(4):2022–2027. [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galway A. B., Oikawa M., Ny T., Hsueh A. J. Epidermal growth factor stimulates tissue plasminogen activator activity and messenger ribonucleic acid levels in cultured rat granulosa cells: mediation by pathways independent of protein kinases-A and -C. Endocrinology. 1989 Jul;125(1):126–135. doi: 10.1210/endo-125-1-126. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton J. H., Nebes V. L., O'Dell L. G., Morris S. M., Jr, Gelehrter T. D. Glucocorticoid and cyclic nucleotide regulation of plasminogen activator and plasminogen activator-inhibitor gene expression in primary cultures of rat hepatocytes. Mol Endocrinol. 1989 Jan;3(1):185–192. doi: 10.1210/mend-3-1-185. [DOI] [PubMed] [Google Scholar]
- Hsueh A. J., Wang C., Erickson G. F. Direct inhibitory effect of gonadotropin-releasing hormone upon follicle-stimulating hormone induction of luteinizing hormone receptor and aromatase activity in rat granulosa cells. Endocrinology. 1980 Jun;106(6):1697–1705. doi: 10.1210/endo-106-6-1697. [DOI] [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Jia X. C., Ny T., Hsueh A. J. Synergistic effect of glucocorticoids and androgens on the hormonal induction of tissue plasminogen activator activity and messenger ribonucleic acid levels in granulosa cells. Mol Cell Endocrinol. 1990 Jan 22;68(2-3):143–151. doi: 10.1016/0303-7207(90)90187-d. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Kageyama R., Merlino G. T., Pastan I. A transcription factor active on the epidermal growth factor receptor gene. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5016–5020. doi: 10.1073/pnas.85.14.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama R., Merlino G. T., Pastan I. Nuclear factor ETF specifically stimulates transcription from promoters without a TATA box. J Biol Chem. 1989 Sep 15;264(26):15508–15514. [PubMed] [Google Scholar]
- Kolena J., Channing C. P. Stimulatory effects of LH, FSH and prostaglandins upon cyclic 3',5'-AMP levels in porcine granulosa cells. Endocrinology. 1972 Jun;90(6):1543–1550. doi: 10.1210/endo-90-6-1543. [DOI] [PubMed] [Google Scholar]
- LaPolt P. S., Yamoto M., Veljkovic M., Sincich C., Ny T., Tsafriri A., Hsueh A. J. Basic fibroblast growth factor induction of granulosa cell tissue-type plasminogen activator expression and oocyte maturation: potential role as a paracrine ovarian hormone. Endocrinology. 1990 Nov;127(5):2357–2363. doi: 10.1210/endo-127-5-2357. [DOI] [PubMed] [Google Scholar]
- Leegwater P. A., van Driel W., van der Vliet P. C. Recognition site of nuclear factor I, a sequence-specific DNA-binding protein from HeLa cells that stimulates adenovirus DNA replication. EMBO J. 1985 Jun;4(6):1515–1521. doi: 10.1002/j.1460-2075.1985.tb03811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin B. Commitment and activation at pol II promoters: a tail of protein-protein interactions. Cell. 1990 Jun 29;61(7):1161–1164. doi: 10.1016/0092-8674(90)90675-5. [DOI] [PubMed] [Google Scholar]
- Liu Y. X., Cajander S. B., Ny T., Kristensen P., Hsueh A. J. Gonadotropin regulation of tissue-type and urokinase-type plasminogen activators in rat granulosa and theca-interstitial cells during the periovulatory period. Mol Cell Endocrinol. 1987 Dec;54(2-3):221–229. doi: 10.1016/0303-7207(87)90160-2. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Wang C., Tjian R. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell. 1987 Sep 11;50(6):847–861. doi: 10.1016/0092-8674(87)90512-5. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss L. G., Moss J. B., Rutter W. J. Systematic binding analysis of the insulin gene transcription control region: insulin and immunoglobulin enhancers utilize similar transactivators. Mol Cell Biol. 1988 Jun;8(6):2620–2627. doi: 10.1128/mcb.8.6.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Hurwitz J. Specific binding of a cellular DNA replication protein to the origin of replication of adenovirus DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6177–6181. doi: 10.1073/pnas.80.20.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny T., Bjersing L., Hsueh A. J., Loskutoff D. J. Cultured granulosa cells produce two plasminogen activators and an antiactivator, each regulated differently by gonadotropins. Endocrinology. 1985 Apr;116(4):1666–1668. doi: 10.1210/endo-116-4-1666. [DOI] [PubMed] [Google Scholar]
- Ny T., Liu Y. X., Ohlsson M., Jones P. B., Hsueh A. J. Regulation of tissue-type plasminogen activator activity and messenger RNA levels by gonadotropin-releasing hormone in cultured rat granulosa cells and cumulus-oocyte complexes. J Biol Chem. 1987 Aug 25;262(24):11790–11793. [PubMed] [Google Scholar]
- O'Connell M. L., Canipari R., Strickland S. Hormonal regulation of tissue plasminogen activator secretion and mRNA levels in rat granulosa cells. J Biol Chem. 1987 Feb 15;262(5):2339–2344. [PubMed] [Google Scholar]
- Ohlsson H., Edlund T. Sequence-specific interactions of nuclear factors with the insulin gene enhancer. Cell. 1986 Apr 11;45(1):35–44. doi: 10.1016/0092-8674(86)90535-0. [DOI] [PubMed] [Google Scholar]
- Ohlsson M., Hsueh A. J., Ny T. Hormonal regulation of tissue-type plasminogen activator messenger ribonucleic acid levels in rat granulosa cells: mechanisms of induction by follicle-stimulating hormone and gonadotropin releasing hormone. Mol Endocrinol. 1988 Sep;2(9):854–861. doi: 10.1210/mend-2-9-854. [DOI] [PubMed] [Google Scholar]
- Opdenakker G., Ashino-Fuse H., Van Damme J., Billiau A., De Somer P. Effects of 12-O-tetradecanoylphorbol 13-acetate on the production of mRNAs for human tissue-type plasminogen activator. Eur J Biochem. 1983 Apr 5;131(3):481–487. doi: 10.1111/j.1432-1033.1983.tb07287.x. [DOI] [PubMed] [Google Scholar]
- Pascal E., Tjian R. Different activation domains of Sp1 govern formation of multimers and mediate transcriptional synergism. Genes Dev. 1991 Sep;5(9):1646–1656. doi: 10.1101/gad.5.9.1646. [DOI] [PubMed] [Google Scholar]
- Pecorino L. T., Darrow A. L., Strickland S. In vitro analysis of the tissue plasminogen activator promoter reveals a GC box-binding activity present in murine brain but undetectable in kidney and liver. Mol Cell Biol. 1991 Jun;11(6):3139–3147. doi: 10.1128/mcb.11.6.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh B. F., Tjian R. Mechanism of transcriptional activation by Sp1: evidence for coactivators. Cell. 1990 Jun 29;61(7):1187–1197. doi: 10.1016/0092-8674(90)90683-6. [DOI] [PubMed] [Google Scholar]
- Pugh B. F., Tjian R. Transcription from a TATA-less promoter requires a multisubunit TFIID complex. Genes Dev. 1991 Nov;5(11):1935–1945. doi: 10.1101/gad.5.11.1935. [DOI] [PubMed] [Google Scholar]
- Reich R., Miskin R., Tsafriri A. Follicular plasminogen activator: involvement in ovulation. Endocrinology. 1985 Feb;116(2):516–521. doi: 10.1210/endo-116-2-516. [DOI] [PubMed] [Google Scholar]
- Reich R., Miskin R., Tsafriri A. Intrafollicular distribution of plasminogen activators and their hormonal regulation in vitro. Endocrinology. 1986 Oct;119(4):1588–1593. doi: 10.1210/endo-119-4-1588. [DOI] [PubMed] [Google Scholar]
- Rickles R. J., Strickland S. Tissue plasminogen activator mRNA in murine tissues. FEBS Lett. 1988 Feb 29;229(1):100–106. doi: 10.1016/0014-5793(88)80806-8. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saksela O., Rifkin D. B. Cell-associated plasminogen activation: regulation and physiological functions. Annu Rev Cell Biol. 1988;4:93–126. doi: 10.1146/annurev.cb.04.110188.000521. [DOI] [PubMed] [Google Scholar]
- Schneider R., Gander I., Müller U., Mertz R., Winnacker E. L. A sensitive and rapid gel retention assay for nuclear factor I and other DNA-binding proteins in crude nuclear extracts. Nucleic Acids Res. 1986 Feb 11;14(3):1303–1317. doi: 10.1093/nar/14.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Heinemann S., Carlisle W., Tarikas H., Kimes B., Patrick J., Steinbach J. H., Culp W., Brandt B. L. Clonal cell lines from the rat central nervous system. Nature. 1974 May 17;249(454):224–227. doi: 10.1038/249224a0. [DOI] [PubMed] [Google Scholar]
- Short J. M., Wynshaw-Boris A., Short H. P., Hanson R. W. Characterization of the phosphoenolpyruvate carboxykinase (GTP) promoter-regulatory region. II. Identification of cAMP and glucocorticoid regulatory domains. J Biol Chem. 1986 Jul 25;261(21):9721–9726. [PubMed] [Google Scholar]
- Strickland S., Beers W. H. Studies on the role of plasminogen activator in ovulation. In vitro response of granulosa cells to gonadotropins, cyclic nucleotides, and prostaglandins. J Biol Chem. 1976 Sep 25;251(18):5694–5702. [PubMed] [Google Scholar]
- Tsafriri A., Bicsak T. A., Cajander S. B., Ny T., Hsueh A. J. Suppression of ovulation rate by antibodies to tissue-type plasminogen activator and alpha 2-antiplasmin. Endocrinology. 1989 Jan;124(1):415–421. doi: 10.1210/endo-124-1-415. [DOI] [PubMed] [Google Scholar]
- WILLIAMS J. R. B. The fibrinolytic activity of urine. Br J Exp Pathol. 1951 Dec;32(6):530–537. [PMC free article] [PubMed] [Google Scholar]
- Waller E. K., Schleuning W. D. Induction of fibrinolytic activity in HeLa cells by phorbol myristate acetate. Tissue-type plasminogen activator antigen and mRNA augmentation require intermediate protein biosynthesis. J Biol Chem. 1985 May 25;260(10):6354–6360. [PubMed] [Google Scholar]
- Wasylyk B., Derbyshire R., Guy A., Molko D., Roget A., Téoule R., Chambon P. Specific in vitro transcription of conalbumin gene is drastically decreased by single-point mutation in T-A-T-A box homology sequence. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7024–7028. doi: 10.1073/pnas.77.12.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Biggs W. H., 3rd, Montminy M. R. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988 Aug 11;334(6182):494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Menzel P., Rivier J., Montminy M. R. Characterization of a bipartite activator domain in transcription factor CREB. Cell. 1990 Feb 23;60(4):611–617. doi: 10.1016/0092-8674(90)90664-z. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zonneveld A. J., Curriden S. A., Loskutoff D. J. Type 1 plasminogen activator inhibitor gene: functional analysis and glucocorticoid regulation of its promoter. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5525–5529. doi: 10.1073/pnas.85.15.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]