Abstract
Progressive accumulation of α-synuclein (α-syn) in limbic and striatonigral systems is associated with the neurodegenerative processes in dementia with Lewy bodies (DLB) and Parkinson’s disease (PD). The murine Thy-1 (mThy1)-α-syn transgenic (tg) model recapitulates aspects of degenerative processes associated with α-syn accumulation in these disorders. Given that axonal and synaptic pathologies are important features of DLB and PD, we sought to investigate the extent and characteristics of these alterations in mThy1-α-syn tg mice and to determine the contribution of α-syn c-terminally cleaved at amino acid 122 (CT α-syn) to these abnormalities. We generated a novel polyclonal antibody (SYN105) against the c-terminally truncated sequence (amino acids 121 to 123) of α-syn (CT α-syn) and performed immunocytochemical and ultrastructural analyses in mThy1-α-syn tg mice. We found abundant clusters of dystrophic neurites in layers 2 to 3 of the neocortex, the stratum lacunosum, the dentate gyrus, and cornu ammonis 3 of the hippocampus, striatum, thalamus, midbrain, and pons. Dystrophic neurites displayed intense immunoreactivity detected with the SYN105 antibody. Double-labeling studies with antibodies to phosphorylated neurofilaments confirmed the axonal location of full-length and CT α-syn. α-Syn immunoreactive dystrophic neurites contained numerous electrodense laminated structures. These results show that neuritic dystrophy is a prominent pathologic feature of the mThy1-α-syn tg model and suggest that CT α-syn might play an important role in the process of axonal damage in these mice as well as in DLB and PD.
Dementia with Lewy bodies (DLB), Parkinson disease (PD) dementia, and idiopathic PD are common causes of movement impairment and cognitive dysfunction in the ageing population. Jointly, this heterogeneous group of disorders often is referred to as Lewy body disease (LBD). A common feature in LBD is the extensive accumulation of α-synuclein (α-syn) in cortical and subcortical regions. α-Syn is a 14-kDa natively unfolded protein, which in the central nervous system1 is found at the presynaptic terminal,2 where it is thought to play a role in synaptic plasticity.3
In LBD, α-syn accumulates in multiple cellular compartments including the synaptic terminals,4–6 axons,7 and neuronal cell bodies (Lewy bodies).8–11 Although Lewy bodies containing fibrillar α-syn are the pathologic hallmark of the disease, accumulation of other α-syn species in the synapses and axons has been suggested to be responsible for the impairment of the neural circuitries and neurodegeneration.12–14 Recent work suggests that α-syn oligomers rather than fibrils might be the neurotoxic species.15–17 C-terminally cleaved species of α-syn are thought to contribute to this process of increased oligomerization and toxicity.12,14
C-terminally truncated α-syn (CT α-syn) species consistently show a faster fibrillization rate than full-length α-syn.18 In transgenic (tg) mouse brain, C-terminal truncation (CT) leads to an enhanced pathology in various models of LBD.19–21 Truncated α-syn may originate from the activity of proteasomal or lysosomal enzymes,22,23 or may be cleaved by proteases such as matrix metalloproteinases24–26 or calpain-1.27,28 Tg α-syn murine models develop numerous functional deficits that likely relate to the widespread accumulation of insoluble α-syn in cortical and subcortical circuitries. For example, murine Thy-1 (mThy1)-α-syn tg mice display motor and nonmotor deficits reminiscent of those observed in DLB and the premanifest phase of PD.29 These mice also display neurodegenerative pathology and α-syn accumulation in the neocortex, hippocampus, basal ganglia, and brainstem, followed by a 40% loss of striatal dopamine and L-dopa–responsive motor deficits by 14 to 15 months of age, and deficits in Morris Water Maze performance.4,30
Given that axonal pathology is an important feature of LBD, the present study examined the extent and characteristics of axonal alterations in the mThy1-α-syn tg model and investigated the contribution of CT α-syn to these abnormalities. To this end, we used a novel antibody (SYN105) generated against α-syn c-terminally cleaved at amino acid (aa) 122 to perform immunohistochemical and electron microscopic analyses in mThy1-α-syn tg mice.
Materials and Methods
Transgenic α-Syn Mouse Model
This study was conducted in heterozygous tg mice overexpressing human wild-type α-syn under the mThy1 promoter (mThy1-α-syn of line 61).31 This well-characterized tg model was selected because of its noted behavioral deficits in tests of sensory-motor function. The robustness of these behavioral deficits is preserved across frequent rounds of repeated testing.32,33 These mice also display abnormal accumulation of detergent-insoluble α-syn and develop α-syn–immunoreactive inclusion-like structures in the brain. Although some nuclear staining has been observed in this model, other distinct cytoplasmic inclusion-like structures consistently have been identified by confocal and electron microscopy.31,34 The mThy1-α-syn tg mice were created and were maintained on a hybrid C57BL/6-DBA/2 background.31 Animals were kept on this background by mating N5 females hemizygous for the transgene with male wild-type mice on the hybrid background obtained from Charles River Laboratories, Inc. (Wilmington, MA).32,33 The mice used for this study were 6-month-old female mThy1-α-syn tg mice (n = 8), α-syn knockout mice (n = 8; ID: 003692; Jackson Laboratories, Bar Harbor, ME), and non-tg mice (n = 8). Additional control experiments were conducted with 6-month-old male (n = 5) and female (n = 5) mThy1-α-syn tg mice to evaluate gender differences in α-syn detection with the SYN105 antibody.
Human Specimens and Neuropathology
A total of 10 cases (n = 5 non-demented controls and n = 5 DLB) were included for the present study. Autopsy material was obtained from patients studied neurologically and psychometrically at the Alzheimer Disease Research Center/University of California, San Diego. The last neurobehavioral evaluation was performed within 12 months before death and included the Blessed score, Mini Mental State Examination, and dementia-rating scale.35,36 The demographics of the samples used are presented in Table 1.
Table 1.
Demographic Details of Human Samples Used
| Diagnosis | N = | Age, years | Disease duration, years | Sex, M/F | Blessed score range | Brain weight | Braak stage |
|---|---|---|---|---|---|---|---|
| Non-demented control | 5 | 84 ± 4 | NA | 3/2 | 0–1 | 1320 ± 98 | 0–I |
| Dementia with Lewy bodies | 5 | 79 ± 5 | 10 ± 3 | 3/2 | 20–33 | 1125 ± 174 | IV–V |
F, female; M, male; NA, not applicable.
Brains were processed and evaluated according to standard methods.37 At autopsy, brains were divided sagittally and the left hemibrain was fixed in formalin with 4% paraformaldehyde for neuropathologic analysis and the right hemibrain was frozen at −70°C for subsequent neurochemical analysis. Paraffin sections from 10% buffered formalin-fixed, neocortical, limbic system, and subcortical material stained with H&E, thioflavine-S, ubiquitin (Dako, Carpinteria, CA), and α-syn (Millipore, Temecula, CA) were used for routine neuropathologic analysis that included assessment of plaques, tangles, Lewy bodies, and Braak stage.37 The diagnosis of DLB was based on the initial clinical presentation with dementia followed by parkinsonism and the presence of α-syn and ubiquitin-positive Lewy bodies in cortical and subcortical regions.38,39
For human brains, sections from the putamen and hippocampus were used. Sections were incubated at 4°C overnight with the polyclonal antibodies against α-syn (Millipore) and CT α-syn (SYN105). Sections then were incubated in secondary antibody (1:75; Vector Laboratories, Inc., Burlingame, CA), followed by avidin D–horseradish peroxidase (ABC Elite; Vector Laboratories, Inc.) and reacted with diaminobenzidine (0.2 mg/mL) in 50 mmol/L Tris (pH 7.4) with 0.001% H2O2.
Generation and Characterization of the Rabbit SYN105 Antibody Against CT α-Syn
An immunogenic peptide with the sequence of CGGVDPDN, in which CGG are artificial aa added for conjugation and spacing and VDPDN are aa 118 to 122 of α-syn, was synthesized by Anaspec (San Jose, CA). This peptide was conjugated to Imject Maleimide Activated cationized bovine serum albumine (cBSA) from Pierce (Rockford, IL) following the manufacturer's protocol. The complete immunogen was supplied to Josman Labs (Napa, CA), who immunized rabbits following a modification of their standard protocol. Bleeds were collected monthly starting at week 7. Once a sufficient titer to the immunizing peptide was obtained, the antibody was purified on an affinity column prepared by coupling the immunizing peptide to SulfoLink Coupling Resin (Pierce) following the manufacturer's protocol.
The antibody was characterized by both selective binding to α-syn truncated at aa 122 versus full-length α-syn and by epitope mapping on overlapping 10-mer peptides. The comparative binding enzyme-linked immunosorbent assay (ELISA) was conducted by coating a plate with either purified full-length α-syn or truncated α-syn at 10 μg/mL. Plates were blocked, aspirated, and dilutions of the SYN105 antibody were incubated on the plates for 2 hours followed by washing. The plates then were incubated in goat anti-rabbit horseradish peroxidase for 1 hour and washed. Plates were developed using o-phenylenediamine dihydrochloride with an acid stop, and then read at 490 nm. The shift in 50% binding was determined to give a relative shift in affinity and we found that SYN105 had a 20-fold preference for α-syn truncated at aa 122.
For epitope screening, 10 aa peptides were synthesized at Biopeptide, Co., Inc. (San Diego, CA) with N-terminally added biotins encompassing aa 106 to 128 with a nine aa overlap. The peptides were bound to a Pierce neutravidin plate and a 10 μg/mL antibody solution was incubated with the peptides for 1 hour. Plates were washed and incubated for 1 hour with goat anti-rabbit IgG–horseradish peroxidase. Plates were washed and then developed with OPD, with an acid stop, and read at 490 nm.
Immunoblot Analysis
Briefly, as previously described, samples from the hippocampus and caudate putamen of mThy1-α-syn tg mice, non-tg mice, α-syn knockout controls, and DLB patients were homogenized and divided into Tris-buffered saline (TBS)-cytosolic, Triton X-100-membrane, and urea-insoluble fractions.19,40 For immunoblot analysis, 20 μg of TBS-soluble protein per lane was loaded into 4% to 12% Bis-Tris [Bis(2-hydroxyethyl) aminotris (hydroxymethyl) methane] SDS-PAGE (denaturing or on native/nondenaturating) gels and blotted onto nitrocellulose membranes. To characterize the SYN105 antibody, membranes were incubated with either SYN105 (1:1000, rabbit polyclonal antibody against α-syn c-terminally truncated at aa 122) or against full-length α-syn [1:1000, full-length (FL) α-syn rabbit polyclonal; Millipore]. Incubation with primary antibodies was followed by species-appropriate incubation with secondary antibodies tagged with horseradish peroxidase (1:5000; Santa Cruz Biotechnology, Santa Cruz, CA), visualization with enhanced chemiluminescence, and analysis with a Versadoc XL imaging apparatus (BioRad, Hercules, CA). Analysis of β-actin (1:1000; Sigma, St. Louis, MO) or tubulin (1:1000, β-tubulin; Chemicon, Temecula, CA) levels was used as a loading control.
Mass Spectrometry
Recombinant α-syn (± calpain-1 treatment) (30 μg) was electrophoresed on a 4% to 12% Bis-Tris gel, blotted for a short period (10 minutes, 30 volt) onto a PVDF membrane, and stained with the SYN105 antibody as described earlier. After transfer, the gel was rinsed with water and stained with the SilverQuest Staining Kit (Invitrogen Life Technologies, Carlsbad, CA), imaged, and sliced horizontally. Gel slices were de-stained, rinsed, and consecutively digested with sequencing-grade trypsin as previously described.41 Trypsin-digested peptides were analyzed by high-pressure liquid chromatography coupled with tandem mass spectroscopy using nanospray ionization. The nanospray ionization experiments were performed using a Triple-time-of-flight 5600 mass spectrometer (MS) (AB SCIEX, Framingham, MA), interfaced with nano-scale reversed-phase high-pressure liquid chromatography [Tempo (2,2,6,6-tetramethyl-1-piperidinyloxy)], using a 10-cm–100-micron internal diameter glass capillary packed with 5-μm C18 Zorbax beads (Agilent Technologies, Santa Clara, CA). Peptides were eluted from the C18 column into the mass spectrometer using a linear gradient (5% to 60%) of acetonitrile at a flow rate of 250 μL/min for 1 hour. The buffers used to create the acetonitrile gradient were as follows: buffer A [98% H2O, 2% acetonitrile, 0.2% formic acid, and 0.005% trifuoroacetic acid (TFA)] and buffer B (100% acetonitrile, 0.2% formic acid, and 0.005% (TFA). Tandem mass spectrometry data were acquired in a data-dependent manner in which the MS1 data were acquired for 250 ms at a mass to charge ratio of 400 to 1250 Da and the MS/MS tandem mass spectrometry data were acquired from a mass to charge ratio of 50 to 2000 Da. For independent data acquisition the following parameters were used: a 250 ms time of flight survey scan (MS1-TOF) was followed by a 50 product ion scan (MS2) of 25 ms each. For MS2 criteria, ions that had reached the treshold of 200 counts and the charge state of +2,+3 or +4 were selected and a four second exclusion criteria was chosen to limit the number of MS2 repetitive elements on the same ion. Finally, the collected data were analyzed using ProteinPilot 4.0 (ABSCIEX) for peptide identifications.
Immunocytochemical and Neuropathologic Analyses
Analysis of α-syn accumulation was performed in serially sectioned, free-floating, blind-coded 40-μm vibratome sections by incubating the sections overnight at 4°C with either the SYN105 (1:500, rabbit polyclonal antibody against α-syn c-terminally truncated at aa 122), a polyclonal antibody against full-length α-syn (1:500; Millipore),42 an antibody against pathologic α-syn found in Lewy bodies [1:500, mouse monoclonal (LB 509); Abcam, Cambridge, MA], or against α-syn phosphorylated at serine 129 [1:500, rabbit polyclonal; (pSer129) Millipore]. Incubation with primary antibodies was followed by incubation with species-appropriate secondary antibodies including biotinylated goat anti-rabbit IgG1 (1:100; Vector Laboratories, Inc.), avidin D–horseradish peroxidase (1:200, ABC Elite; Vector Laboratories, Inc.), and, finally, detection with diaminobenzidine. Sections were analyzed using an Olympus BX-41 upright pathology microscope (Olympus America Inc., Center Valley, PA).
Double Immunolabeling and Fluorescence Co-labeling
To determine the co-localization between α-syn with different antibodies and with other cellular markers, double-labeling experiments were performed, as previously described.43 For this purpose, vibratome sections were immunolabeled with the mouse monoclonal antibodies against human α-syn (SYN211, 1:500; Santa Cruz Biotechnology) and the rabbit polyclonal CT α-syn (SYN105). Sections also were double-labeled with SYN105 and mouse monoclonal antibodies against the synaptic vesicle marker, synaptophysin (1:500; Abcam), the axonal phosphorylated low-molecular-weight neurofilaments (SMI312, 1:500; Abcam), the dendritic microtubule-associated protein-2 (1:100; Millipore), or glial fibrillary acidic protein (1:500; Millipore). The CT α-syn immunoreactive structures were detected with the Tyramide Signal Amplification-Direct (Red) system (1:100; NEN Life Sciences, Boston, MA); whereas SYN211, SMI312, and microtubule-associated protein-2 were detected with fluorescein isothiocyanate–tagged antibodies (1:75; Vector Laboratories, Inc.). All sections were processed simultaneously under the same conditions and experiments were performed in triplicate to assess the reproducibility of results. Sections were imaged with a Zeiss (Oberkochen, Germany) high magnification (×63) objective (numerical aperture, 1.4) on an Axiovert 35 microscope (Zeiss) with an attached MRC1024 laser scanning confocal microscope system (BioRad).42 A series of 10 Z optical sections were acquired at an average depth of 2 μm and were used to estimate the proportion of SYN105-immunoreactive neurites that co-localized with synaptophysin.
Electron Microscopy and Immunogold Analysis
Briefly, vibratome sections were postfixed in 1% glutaraldehyde, treated with osmium tetraoxide, embedded in epon araldite, and sectioned with the ultramicrotome (Leica, Nussloch, Germany). Grids were analyzed with a Zeiss OM 10 electron microscope as previously described.44 For immunogold labeling, sections were mounted in nickel grids, etched, and incubated with the SYN105 antibody, followed by labeling with 10-nm Aurion ImmunoGold particles (1:50; Electron Microscopy Sciences, Fort Washington, PA) with silver enhancement. Electron micrographs were obtained at a magnification of ×25,000.
Results
The Polyclonal Antibody SYN105 Preferentially Recognizes C-Terminal–Truncated α-Syn
To investigate the biochemical properties and axonal distribution patterns of CT α-syn in mThy1-α-syn tg mice and human tissue, we generated a novel antibody, SYN105. The SYN105 antibody was raised to recognize a major calpain-1 cleavage site (amino acid 122) of human α-syn28 (Figure 1A). By ELISA, the antibody had a 20-fold preference for aa 122–truncated α-syn (Figure 1B). Epitope mapping showed that the SYN105 antibody bound preferentially to peptides ending at aa 121, aa 122, and aa 123 (Figure 1C). There was no reaction to a peptide ending at aa 125 even though it contained the entire immunogen sequence (Figure 1C).
Figure 1.
Characterization of the novel CT α-syn antibody SYN105. A: Schematic diagram of the α-syn peptide indicating epitopes relevant to the antibodies used in this study. B: ELISA analysis of the relative specificities of the SYN105 antibody for FL α-syn and α-syn truncated at amino acid (aa) residue 122. C: Epitope-mapping of purified SYN105 with 10-mer overlapping peptides. D: Immunoblot analysis on a denaturing SDS-PAGE gel with the SYN105 antibody and the FL α-syn antibody of TBS-soluble protein extracts from the caudate putamen (CPu) and hippocampal (HPc) regions of 6-month-old non-tg mice, α-syn knock-out mice, mThy1-α-syn tg, and control and DLB human samples. E: Immunoblot analysis on a native (nondenaturing) PAGE gel with the SYN105 antibody of TBS-soluble protein extracts from the CPu and hippocampus (HPc) regions of 6-month-old non-tg (co) and mThy1-α-syn tg (tg). F: Samples from recombinant α-syn incubated with calpain-1 for 0, 10, and 60 minutes, and with recombinant α-syn incubated with calpain-1 + calpeptin (inhibitor, I), analyzed with the SYN105 antibody. G: Recombinant α-syn was subjected to gel electrophoresis and short blotting before silver staining to document slice sides corresponding to SYN105 immunoreactivity of CT α-syn. Gel slices between 10 and 12 kDa were excised (red asterisk). H: Tryptic peptides (black asterisks) of liquid chromatography coupled with tandem mass spectrometry spectra were analyzed using Mascot (Matrix Science, London, UK) and detected peptides (in bold) corresponding to α-syn spanning aa 13 to 122.
Next, we tested the specificity of the SYN105 antibody in brain homogenates. For this purpose, TBS-soluble fractions of caudate putamen or hippocampi from 6-month-old mThy1-α-syn tg mice, non-tg, and α-syn knockout controls were analyzed and compared with striatal and hippocampal samples from DLB cases. These samples initially were analyzed with the SYN105 antibody, which robustly detected both the 14-kDa and 12-kDa species of α-syn in the mThy1-α-syn tg mice and in the DLB samples. The 12-kDa fragment of α-syn was detected at around equal levels to monomeric α-syn and was strongly visible in caudate putamen and to a minor extent in the hippocampi of mThy1-α-syn tg mice (Figure 1D). The 12-kDa fragment of α-syn was detected prominently in the caudate putamen and hippocampal samples from the DLB cases (Figure 1D).
In contrast, immunoblot with an antibody against FL α-syn (Figure 1D) (aa 91 to 99), showed predominant detection of monomeric mouse and human α-syn (14 kDa) in the caudate putamen and hippocampus of mThy1-α-syn tg mice and in the human samples. An additional minor signal of human α-syn was detected at a lower molecular weight (12 kDa, Figure 1D). This lower band was not seen in protein extracts from non-tg mice using the FL α-syn antibody or other antibodies against the CT of α-syn (aa 121 to 125; SYN211; data not shown).
To evaluate the specific of SYN105 to α-syn oligomers, TBS-soluble fractions of caudate putamen or hippocampi from mThy1-α-syn tg mice and non-tg controls were run on a nondenaturing native gel (Figure 1E). SYN105 detected higher-molecular-weight α-syn species in samples from the caudate putamen or hippocampus of mThy1-α-syn tg mice (Figure 1E). No signal was observed in the samples from non-tg mice.
To further investigate and corroborate the specificity of the SYN105 antibody to calpain-cleaved α-syn, recombinant α-syn incubated with calpain-1 for 0, 10, and 60 minutes was analyzed by immunoblot. Recombinant α-syn also was incubated with calpain-1 in the presence of calpeptin, an inhibitor (I, Figure 1F) of calpain. Immunoblot analysis of recombinant α-syn incubated with calpain-1 for 10 minutes with the SYN105 antibody detected a band at 12 kDa representing the calpain-1 cleavage product. This band was more prominent after 60 minutes of incubation, as was a much higher molecular weight band (Figure 1G). SYN105 only detected the FL α-syn when recombinant α-syn was incubated with calpain in the presence of calpeptin (Figure 1F).
To further characterize the specificity of the SYN105 antibody for CT α-syn bands co-migrating with calpain-cleaved recombinant α-syn as identified by immunoblotting using SYN105 and silver staining (Figure 1G), signals were analyzed by reverse-phase chromatography. Peaks were collected and peptide masses were determined by sequencing of tryptic peptides using liquid chromatography coupled with tandem mass spectroscopy. This analysis detected peptides corresponding to α-syn spanning aa 13 to 122 (Figure 1H).
Collectively, these results show that the SYN105 antibody has a high affinity for calpain-cleaved CT α-syn and suggest that CT α-syn truncation may be a specific characteristic of human α-syn.
The mThy1-α-Syn Tg Mice Display Extensive Accumulation of SYN105-Reactive CT α-Syn in Dystrophic Neurites
Immunohistochemical analysis with the SYN105 antibody against CT α-syn showed strong immunolabeling of the neuropil, throughout cortical and subcortical regions of the mThy1-α-syn tg mice (Figure 2A). A more detailed analysis showed that the SYN105 antibody immunostained punctuate-like structures, varying in size between 1 and 4 μm diameter in the neocortical neuropil. The larger dot-like structures were arranged in clusters and had the appearance of dystrophic neurites (Figure 2B). In contrast to antibodies recognizing FL α-syn, only minimal labeling of neuronal cell bodies was detected. In hippocampal regions, the SYN105 antibody immunolabeled abundant dot-like structures averaging 1 to 2 microns in diameter, distributed throughout the cornu ammonis 1 (CA1) and dentate gyrus neuropil (Figure 2B). In addition, in the molecular layer of the dentate gyrus and in the stratum lacunosum, the SYN105 antibody immunostained dystrophic neurites arranged in clusters (Figure 2B). Similar structures were detected in the neuropil of the basal ganglia, thalamus, and substantia nigra (Figure 2C). In the cerebellum, immunostaining was present in punctate structures in both the molecular and granular cell layers (Figure 2, A and C). The patterns of immunostaining with the SYN105 antibody were compared with those obtained with an antibody that recognizes FL α-syn. This antibody displayed extensive puncta-like immunolabeling of the neuropil in neocortical, limbic, striatal, and subcortical regions (Figure 2D). At higher magnification, in the neocortex, hippocampus, basal ganglia, and substantia nigra, the antibody against FL α-syn immunostained neuronal cell bodies as well as dystrophic neurites (Figure 2, E and F). However, the dystrophic neurites were immunolabeled more prominently with the SYN105 antibody.
Figure 2.
Immunohistochemical characterization of the SYN105 CT α-syn. A: Low-magnification image of immunoreactivity in a representative mThy1-α-syn tg mouse using the SYN105 antibody. B: Representative high-magnification images of SYN105 signal in the frontal cortex and molecular layer (ML) of the dentate gyrus (DG), stratum lacunosum, and cornu ammonis 1 (CA1) regions of the hippocampus (hip) of mThy1-α-syn tg mice. Arrows indicate dystrophic neuritis containing alpha-synuclein accumulation. C: Representative high-magnification images of SYN105 signal in the basal ganglia, thalamus, substantia nigra (s. nigra), and cerebellum of mThy1-α-syn tg mice. Insets are presented at higher magnification in the lower panels. D: Low-magnification image of immunoreactivity in a representative mThy1-α-syn tg mouse using the FL α-syn antibody. E: Representative high-magnification images of FL α-syn immunoreactivity in the frontal cortex and molecular layer (ML) of the dentate gyrus (DG), stratum lacunosum, and CA1 regions of the hippocampus of mThy1-α-syn tg mice. F: Representative high-magnification images of FL α-syn immunoreactivity in the basal ganglia, thalamus, substantia nigra, and cerebellum of mThy1-α-syn tg mice. Arrows indicate dystrophic neuritis containing alpha-synuclein accumulation. Scale bars: 30 μm (B); 40 μm (E).
To evaluate gender differences in SYN105 immunoreactivity, the TBS-soluble fractions from frontal cortex homogenates of 6-month-old male and female mThy1-α-syn tg mice and both male and female non-tg mice were analyzed. Immunoblot with the SYN105 antibody strongly detected α-syn bands at both 14 and 12 kDa, and at higher molecular weights in both male and female mThy1-α-syn tg mice (Figure 3A). The 14- and 12-kDa bands were more prominent in male mThy1-α-syn tg mice in comparison with female mThy1-α-syn tg mice (Figure 3, A and B). SYN105 did not detect specific bands at 14 or 12 kDa or higher in the non-tg mice (Figure 3A). Immunoblot with an antibody against FL α-syn strongly detected α-syn bands at 14 kDa with only minimal detection at 12 kDa and at higher molecular weights in both male and female mThy1-α-syn tg mice (Figure 3A). Similar to SYN105, immunoblot with the antibody against FL α-syn showed that the 14-kDa band was significantly more prominent in male mThy1-α-syn tg mice in comparison with female mThy1-α-syn tg mice (Figure 3, A and C). The antibody against FL α-syn did not detect specific bands at 14 or 12 kDa or higher in the non-tg mice (Figure 3A). Immunohistochemical analysis of α-syn expression in both male and female mThy1-α-syn tg mice in the neocortex (Figure 3D), basal ganglia (Figure 3E), and substantia nigra (Figure 3F) using the SYN105 antibody detected synapses and neurites. Likewise, male and female mThy1-α-syn tg mice displayed similar patterns of immunoreactivity in the brain regions examined (Figure 3, D–F).
Figure 3.
Comparative immunostaining pattern in male and female mThy1-α-syn tg mice. A: Immunoblot of α-syn levels in brain samples from 6-month-old male and female mThy1-α-syn tg mice and non-tg mice using the SYN105 antibody and the FL α-syn antibody. B: Analysis (means ± SEM) of α-syn levels in mThy1-α-syn tg mice and non-tg mice using the SYN105 antibody. C: Analysis (means ± SEM) of α-syn levels in mThy1-α-syn tg mice and non-tg mice using the FL α-syn antibody. D: Immunohistochemical analysis of SYN105 and FL α-syn immunoreactivity in the neocortex of 6-month-old male and female mThy1-α-syn tg mice. E: Immunohistochemical analysis of SYN105 and FL α-syn immunoreactivity in the basal ganglia of 6-month-old male and female mThy1-α-syn tg mice. F: Immunohistochemical analysis of SYN105 and FL α-syn immunoreactivity in the substantia nigra (s. nigra) of 6-month-old male and female mThy1-α-syn tg mice. *P < 0.05 between male and female mice. Scale bars: 30 μm (D–F).
Comparative Analysis of Mouse and Human Tissue with SYN105 and Other Common α-Syn Antibodies
To comparatively evaluate the pattern of immunoreactivity observed with SYN105 to other antibodies against pathologic forms of α-syn found in Lewy bodies (as detected by LB509), phosphorylated α-syn (as detected by an antibody against pSer129) and FL α-syn sections from the putamen (Figure 4A) and hippocampus (Figure 4B) from DLB cases and mThy1-α-syn tg mice were examined.
Figure 4.
Comparative analysis of α-syn immunoreactivity between mThy1-α-syn tg mice and human DLB using varied antibodies. A: Representative image of α-syn immunoreactivity in the putamen of a DLB case and a mThy1-α-syn tg mouse immunostained with antibodies against CT α-syn (SYN105), α-syn found in Lewy bodies (LB509), α-syn phosphorylated at serine 129 (pSer129), and FL α-syn. B: Representative image of α-syn immunoreactivity in the hippocampus of a DLB case and a mThy1-α-syn tg mouse immunostained with antibodies against CT α-syn (SYN105), α-syn found in Lewy bodies (LB509), α-syn phosphorylated at serine 129 (pSer129), and FL α-syn. Scale bar = 50 μm.
Immunohistochemical analysis with SYN105 in the putamen in DLB and tg mice showed the presence of abundant punctate-like structures corresponding to dystrophic neurites (Figure 4A). With the LB509 and pSer129 antibodies, similar dystrophic neurites were immunostained in the putamen of the DLB cases and tg mice (Figure 4A). In contrast, the antibody against full-length α-syn reacted with the neuropil and only detected very few of the dystrophic neurites (Figure 4A), these structures had an average diameter of 2 to 4 microns, whereas the smaller punctae associated with presynaptic boutons measure an average of 1 micron in diameter.
Immunohistochemical analysis with SYN105 in the hippocampus of the DLB cases and in tg mice showed the presence of abundant fusiform and punctate-like structures (Figure 4B). The LB509 and pSer129 antibodies detected similar dystrophic neurites in the hippocampus of the DLB cases and tg mice (Figure 4B). The antibody against FL α-syn reacted with the neuropil and only detected sparse dystrophic neurites (Figure 4B).
These results show that the immunoreactivity pattern observed with the SYN105 antibody is comparable with that observed with other antibodies aimed at pathologic forms of α-syn such as the LB509 and pSer129 antibodies.
Co-localization of CT α-Syn with Axonal Markers in the mThy1-α-Syn Tg
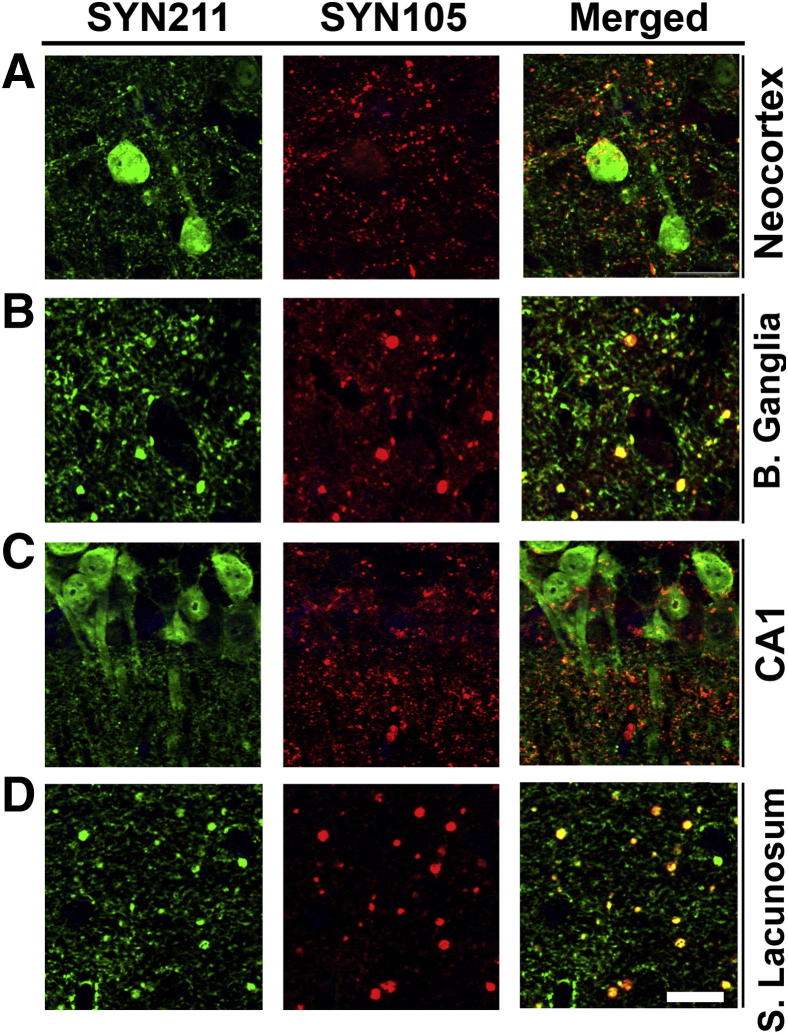
Double-labeling studies with a monoclonal antibody against human α-syn (SYN211) and SYN105 in the neocortex (Figure 5A), basal ganglia (Figure 5B), CA1 (Figure 5C), and stratum lacunosum (Figure 5D) regions of the hippocampus showed extensive co-localization in dystrophic neurites but with only minimal co-localization in neuronal cell bodies.
Figure 5.
Double-labeling characterization of dystrophic neurites in mThy1-α-syn tg mice with the SYN105 antibody. Co-localization of dystrophic neurites with human α-syn detected with the SYN211 antibody and the SYN105 antibody in the neocortex (A), basal ganglia (B), CA1 (C), and stratum lacunosum (S. Lacunosum) (D) of the hippocampus in mThy1-α-syn tg mice with. B. ganglia, basal ganglia. Scale bar = 20 μm.
No immunostaining with the SYN105 antibody was observed in sections from non-tg and α-syn knockout mice (data not shown). Control experiments in which sections from the mThy1-α-syn tg mice were incubated with SYN105 that had been pre-adsorbed with a 20-fold excess of CT α-syn peptide showed a complete abolition of immunostaining (data not shown).
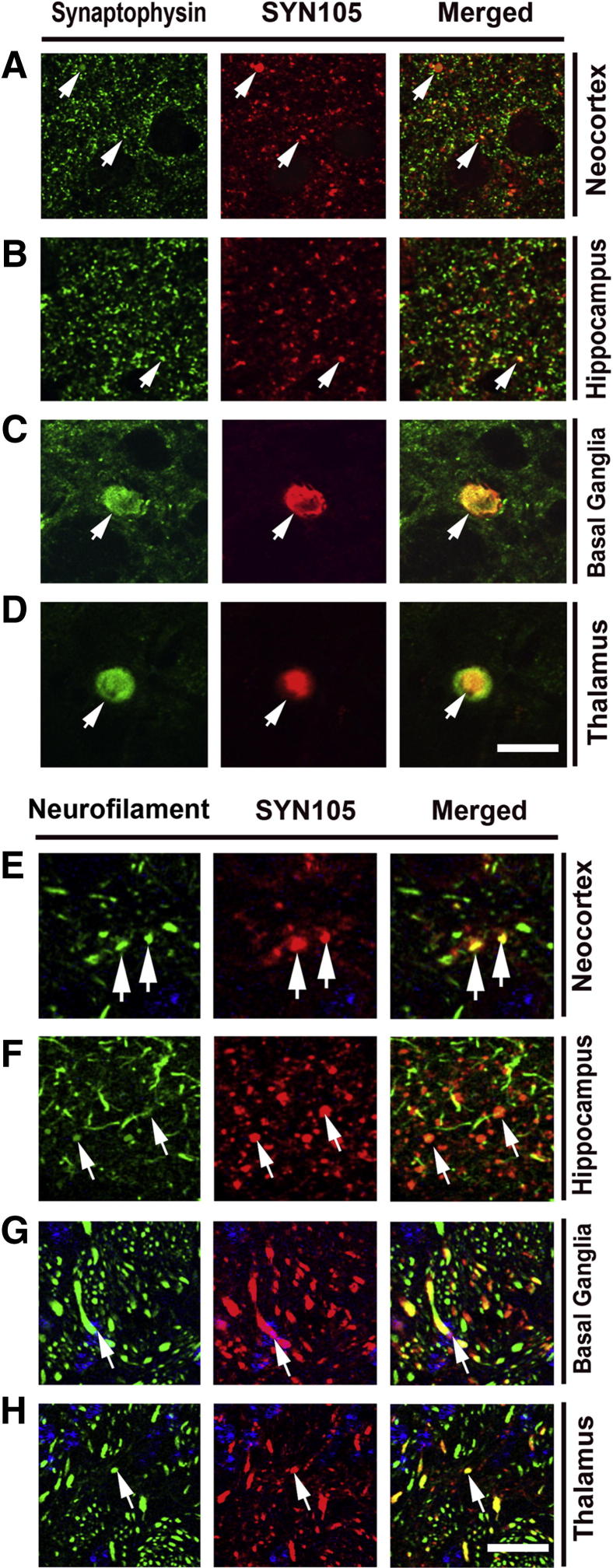
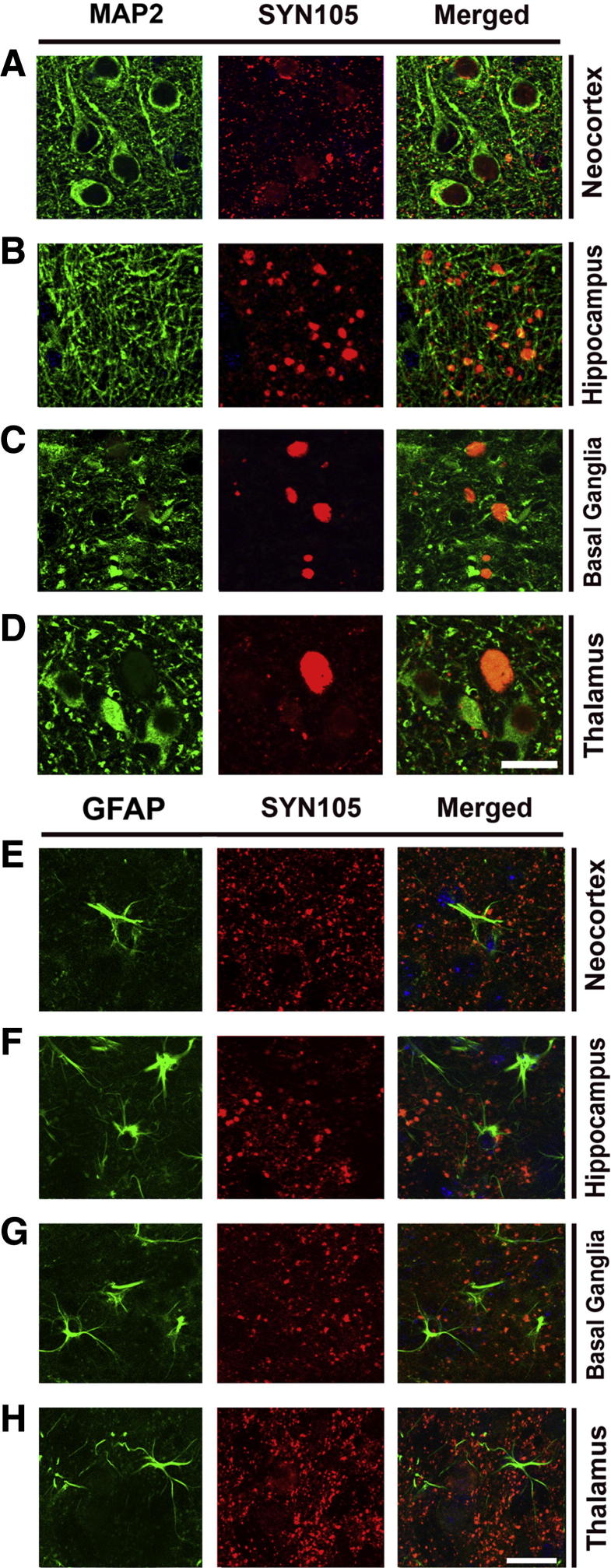
To further corroborate the neuronal localization of the CT α-syn fragments in the mThy1-α-syn tg mice, double-labeling studies were performed with axonal and dendritic markers. In the neocortex and hippocampus, approximately 50% of the finer SYN105-positive dot-like structures co-localized with an antibody against the presynaptic marker synaptophysin (Figure 6, A and B). Moreover, 35% of the larger SYN105-positive dystrophic neurites displayed synaptophysin immunoreactivity (Figure 6, A and B). In the basal ganglia and thalamus, more than 50% of the SYN105-immunoreactive dystrophic neurites displayed synaptophysin immunoreactivity (Figure 6, C and D). Similarly, analysis with an antibody against phosphorylated neurofilaments (SMI312, axonal marker) showed that the SYN105-positive dystrophic neurites in the neocortex, hippocampus, and basal ganglia displayed SMI312 immunoreactivity (Figure 6, E–H). In contrast, there was only minimal co-localization between SYN105-positive dystrophic neurites and an antibody against the dendritic marker microtubule-associated protein-2 or glial fibrillary acidic protein in any of the regions examined (Figure 7).
Figure 6.
Double-labeling characterization of presynaptic and axonal specificity of the SYN105 antibody in mThy1-α-syn tg mice. Arrows indicate co-localization of SYN105 immunoreactivity with the presynaptic marker, synaptophysin, in the neocortex (A), hippocampus (B), basal ganglia (C), and thalamus (D) of mThy1-α-syn tg mice. Arrows indicate co-localization of SYN105 immunoreactivity with the axonal neurofilament marker SMI312 in the neocortex (E), hippocampus (F), basal ganglia (G), and thalamus (H) of mThy1-α-syn tg mice. Scale bar = 20 μm.
Figure 7.
Double-labeling characterization of specificity of the SYN105 antibody in mThy1-α-syn tg mice. Co-localization of the dendritic marker, microtubule-associated protein-2 (MAP2), with SYN105 immunoreactivity in the neocortex (A), hippocampus (B), basal ganglia (C), and thalamus (D) of mThy1-α-syn tg mice. Co-localization of glial fibrillary acidic protein (GFAP) immunoreactivity with SYN105 immunoreactivity in the neocortex (E), hippocampus (F), basal ganglia (G), and thalamus (H) of mThy1-α-syn tg mice. Scale bars: 10 μm (A–D); 10 μm (E–H).
Consistent with studies in the mThy1-α-syn tg mice, co-localization studies in hippocampal samples from DLB cases showed co-localization between SYN105 and synaptophysin and phosphorylated neurofilaments (SMI312, axonal marker) (Figure 8, A and B) but no co-localization with glial fibrillary acidic protein (Figure 8C). Taken together, these results support the notion that the dystrophic neurites that accumulate CT α-syn epitopes are axonal in origin.
Figure 8.
Double-labeling characterization of specificity of the SYN105 antibody in DLB. Co-localization of SYN105 immunoreactivity with the presynaptic marker synaptophysin (A), the axonal neurofilament marker SMI312 (B), and glial fibrillary acidic protein (GFAP) immunoreactivity (C) in the hippocampus of a DLB patient. Arrowheads in A indicate dystrophic neurites that are co-labeled with alpha-synuclein and synaptophysin. Arrowheads in B indicate axons co-labeled with alpha-synuclein and phosphorylated neurofilaments. Arrows in C are GFAP positive astroglial cells and arrowheads are alpha-synculein positive axons. Scale bar = 10 μm.
Ultrastructural Characteristics of the Dystrophic Neurites in the mThy1-α-syn Tg
To further investigate the subcellular characteristics of the dystrophic neurites immunostained by SYN105, electron microscopy (Figure 9, A and B) and immunogold analysis (Figure 9, C and D) was performed. Transmitted electron microscopy analysis of sections from the neocortex, hippocampus, and basal ganglia of mThy1-α-syn tg mice stained with uranyl acetate showed typical dystrophic neurites between 5 and 10 μm in diameter displaying abundant laminated and electrodense bodies (Figure 9B). Immunoelectron microscopy with the SYN105 antibody showed abundant gold particles in association with the membranes of the multilaminated bodies and in the electrodense bodies (Figure 9D).
Figure 9.
Ultrastructural and immunoelectron characterization of dystrophic neurites and SYN105 immunoreactivity in the mThy1-α-syn tg mice. Ultrastructural analysis of neurite morphology in the neocortex of non-tg mice (A) and the neocortex, basal ganglia, and hippocampus from mThy1-α-syn tg mice (B). Immunogold labeling of membranes of the multilaminated bodies and electrodense bodies in the neocortex of non-tg mice (C) and the neocortex, basal ganglia, and hippocampus of mThy1-α-syn tg mice (D). B. ganglia, basal ganglia. Original magnification, ×25,000. Scale bar = 1 μm.
Discussion
The present study showed that neuritic dystrophy, similar to that observed in patients with DLB and PD, is a prominent feature of the mThy1-α-syn tg model and that these dystrophic neurites accumulate CT α-syn that can be best detected with the novel SYN105 polyclonal antibody. Neuritic dystrophy in the hippocampus, basal ganglia, and subcortical nuclei has been described previously in DLB and PD dementia patients,7,11,45,46 and PD,47,48 suggesting that axonal damage might better correlate behavioral and neurodegenerative pathology than the formation of Lewy bodies. In addition, in DLB brains, the presynaptic presence of α-syn aggregates resulted in significant synaptic pathology, with reduction of postsynaptic marker expression and loss of dendritic spines within postsynaptic areas.49 In vitro studies have reported a negative impact of α-syn on neurite outgrowth and synaptic integration.50 In mouse embryonic stem cells and in a rat neuronal cell line, lenti viral overexpression of α-syn resulted in decreased neurite length and less branching.50,51 Decreased neurite length also was observed in newly generated, double-cortin–positive neuroblasts in the dentate gyrus.52 Thus, identification of axonal pathology using in vivo experimental animal models and in patients with DLB or PD represents both an important target and a biomarker for the disease.
Although it is now recognized that abnormal accumulation of α-syn in synapses and axons (rather than Lewy bodies) plays a critical role in the pathogenesis of DLB and PD, the mechanisms leading to this axonal pathology are less clear. In this study we show that a unique feature of the axonal pathology in the mThy1-α-syn tg is the presence of abundant CT-cleaved α-syn as detected with the SYN105 antibody. CT truncation of α-syn has emerged as a key posttranslational mechanism that regulates the aggregation capacity of α-syn.18,22,28,53 Generation of tg animals carrying a CT deletion of the human construct ranging from aa 1 to 119,21 aa 1 to 120,19 and aa 1 to 13020 induced pathologic alterations specifically in dopaminergic neurons. In PD and DLB, levels of calpain-1 are increased in the substantia nigra and locus coeruleus of PD patients.54 Recent studies have indicated that calpain-1 is responsible for the cleavage of α-syn at the CT site (aa 122), resulting in the formation of toxic fragments.12,22,28,55
Moreover, it has been shown that excitotoxicity56,57 and oxidative stress,58,59 events commonly seen in PD and DLB patients, might be related to increased calcium levels,59 which in turn can activate calpain-1, resulting in cleavage of α-syn at the aa 122 of the C-terminus. Consistently, we recently reported that neuronal accumulation of α-syn is associated with alterations in calcium-buffering capacity in our tg mice.60 The resulting fragments have been proposed to play an important role in α-syn oligomerization and toxic conversion.28,61,62 It is possible that progressive accumulation of these α-syn oligomers containing CT fragments might interfere with axonal transport, resulting in neuritic dystrophy.
Thus, generation of antibodies that specifically detect the aa 122 epitope exposed after calpain-1 digestion in human diseased brains and in respective animal models are important tools to understand the underlying pathogenic processes. Although antibodies against defined α-syn epitopes that detect different α-syn species already have been developed,27,28,55,63,64 these antibodies often detect murine α-syn or cross-react with FL α-syn. By using the aa 122 region of the human α-syn protein, which is a major cleavage site of calpain-1 and also contains a mismatch between murine and human α-syn sequence, we were able to generate an antibody that strongly detects the human truncated α-syn.
Although extensive biochemical evidence points toward the affinity of SYN105 binding to calpain cleaved CT α-syn, an alternative explanation for the lower-molecular-weight band identified by the SYN105 antibody is the fact that this band might represent a splice variant of α-syn. New evidence exists that both wild-type controls and tg mice expressing human α-syn under the mThy1-promoter may express splice variants of the α-syn gene, which are increased in the hippocampal region.65 However, its specific molecular weight is identical in electrophoretic motility assays to the calpain-1 cleaved recombinant α-syn fragment. In addition, owing to the fact that hippocampal protein extracts of non-tg mice did not contain lower-molecular-weight α-syn bands when detecting with the antibody with epitope localized within amino acid residues 91 to 99,66 it appears unlikely that the detected fragment consists of neither the α-syn 112 splice variant, that lacks amino acid residues 41–54 nor the previously (described α-syn 126 variant, which lacks amino acid residue 103–130 owing to a loss of exon 5.). In addition, Muntane et al,64 albeit using specific antibody against the 112 isoform of α-syn, failed to detect this isoform in human brain, suggesting that this isoform might not be expressed at the protein level.
The mechanisms governing CT α-syn's role in selectively accumulating in axons and presynaptic terminals versus the cell body or dendrites is not completely clear. One possibility might be that α-syn–cleaving enzymes such as calpain-1 might be concentrated in these regions; however, immunoelectron microscopy studies have shown that calpain-1 is present both in presynaptic and postsynaptic regions.67 α-Syn is primarily a presynaptic terminal protein2 that is transported via slow axonal mechanisms,68 suggesting that the cleavage occurs at the axonal site and because of alterations in axonal transport, the CT fragments accumulate at the axons. The α-syn CT fragments also may be more difficult to clear and additionally may promote oligomerization and further accumulation that might contribute to axonal transport defects. Previous studies have shown that both in PD and DLB patients and in primary neuronal cultures, α-syn interferes with the transport of other synaptic proteins such as synapsin-I, resulting in synaptic dysfunction.6,69 Moreover, a recent study in tg mice reported that α-syn enhances axonal degeneration after peripheral nerve lesion.70 Thus, growing experimental evidence suggests that α-syn fragments and aggregates are abundant in synapses and axons and may interfere with axonal transport.
Our study indicates that neuritic dystrophy is a prominent feature of the mThy1-α-syn tg model and that CT α-syn might play an important role in the process of axonal damage in the tg model as well as in DLB and PD.
Footnotes
Supported in part by NIH grants (AG 11385, AG 18840, AG 022074, and NS 044233) and in part by Neotope Biosciences.
The NIH had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosures: D.G., P.S., R.B., and D.S. are employees of Neotope Biosciences, which provided the SYN105 antibody to the University of California, San Diego, and were intellectually involved in the conception and execution of experiments.
References
- 1.Weinreb P., Zhen W., Poon A., Conway K., Lansbury P.J. NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 2.Iwai A., Masliah E., Yoshimoto M., Ge N., Flanagan L., de Silva H.A., Kittel A., Saitoh T. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 3.Murphy D.D., Rueter S.M., Trojanowski J.Q., Lee V.M. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20:3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masliah E., Rockenstein E., Mante M., Crews L., Spencer B., Adame A., Patrick C., Trejo M., Ubhi K., Rohn T.T., Mueller-Steiner S., Seubert P., Barbour R., McConlogue L., Buttini M., Games D., Schenk D. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS One. 2011;6:e19338. doi: 10.1371/journal.pone.0019338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Roy S. The paradoxical cell biology of alpha-Synucle. Results Probl Cell Differ. 2009;48:159–172. doi: 10.1007/400_2009_23. [DOI] [PubMed] [Google Scholar]
- 6.Scott D.A., Tabarean I., Tang Y., Cartier A., Masliah E., Roy S. A pathologic cascade leading to synaptic dysfunction in alpha-synuclein-induced neurodegeneration. J Neurosci. 2010;30:8083–8095. doi: 10.1523/JNEUROSCI.1091-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halliday G.M., Holton J.L., Revesz T., Dickson D.W. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol. 2011;122:187–204. doi: 10.1007/s00401-011-0852-9. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto M., Hsu L.J., Xia Y., Takeda A., Sisk A., Sundsmo M., Masliah E. Oxidative stress induces amyloid-like aggregate formation of NACP/alpha-synuclein in vitro. Neuroreport. 1999;10:717–721. doi: 10.1097/00001756-199903170-00011. [DOI] [PubMed] [Google Scholar]
- 9.Iwatsubo T., Yamaguchi H., Fujimuro M., Yokosawa H., Ihara Y., Trojanowski J.Q., Lee V.-M. Purification and characterization of Lewy bodies from brains of patients with diffuse Lewy body disease. Am J Pathol. 1996;148:1517–1529. [PMC free article] [PubMed] [Google Scholar]
- 10.Lansbury P.T., Jr. Evolution of amyloid: what normal protein folding may tell us about fibrillogenesis and disease. Proc Natl Acad Sci U S A. 1999;96:3342–3344. doi: 10.1073/pnas.96.7.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trojanowski J.Q., Lee V.M. Aggregation of neurofilament and alpha-synuclein proteins in Lewy bodies: implications for the pathogenesis of Parkinson disease and Lewy body dementia. Arch Neurol. 1998;55:151–152. doi: 10.1001/archneur.55.2.151. [DOI] [PubMed] [Google Scholar]
- 12.Dufty B.M., Warner L.R., Hou S.T., Jiang S.X., Gomez-Isla T., Leenhouts K.M., Oxford J.T., Feany M.B., Masliah E., Rohn T.T. Calpain-cleavage of alpha-synuclein: connecting proteolytic processing to disease-linked aggregation. Am J Pathol. 2007;170:1725–1738. doi: 10.2353/ajpath.2007.061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sulzer D. Clues to how alpha-synuclein damages neurons in Parkinson’s disease. Mov Disord. 2010;25(Suppl 1):S27–S31. doi: 10.1002/mds.22639. [DOI] [PubMed] [Google Scholar]
- 14.Takeda A., Hashimoto M., Mallory M., Sundsumo M., Hansen L., Masliah E. C-terminal alpha-synuclein immunoreactivity in structures other than Lewy bodies in neurodegenerative disorders. Acta Neuropathol. 2000;99:296–304. doi: 10.1007/pl00007441. [DOI] [PubMed] [Google Scholar]
- 15.Conway K.A., Lee S.J., Rochet J.C., Ding T.T., Williamson R.E., Lansbury P.T., Jr. Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc Natl Acad Sci U S A. 2000;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsigelny I.F., Bar-On P., Sharikov Y., Crews L., Hashimoto M., Miller M.A., Keller S.H., Platoshyn O., Yuan J.X., Masliah E. Dynamics of alpha-synuclein aggregation and inhibition of pore-like oligomer development by beta-synuclein. FEBS J. 2007;274:1862–1877. doi: 10.1111/j.1742-4658.2007.05733.x. [DOI] [PubMed] [Google Scholar]
- 17.Winner B., Jappelli R., Maji S.K., Desplats P.A., Boyer L., Aigner S., Hetzer C., Loher T., Vilar M., Campioni S., Tzitzilonis C., Soragni A., Jessberger S., Mira H., Consiglio A., Pham E., Masliah E., Gage F.H., Riek R. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci U S A. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray I.V., Giasson B.I., Quinn S.M., Koppaka V., Axelsen P.H., Ischiropoulos H., Trojanowski J.Q., Lee V.M. Role of alpha-synuclein carboxy-terminus on fibril formation in vitro. Biochemistry. 2003;42:8530–8540. doi: 10.1021/bi027363r. [DOI] [PubMed] [Google Scholar]
- 19.Tofaris G.K., Garcia Reitbock P., Humby T., Lambourne S.L., O’Connell M., Ghetti B., Gossage H., Emson P.C., Wilkinson L.S., Goedert M., Spillantini M.G. Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1-120): implications for Lewy body disorders. J Neurosci. 2006;26:3942–3950. doi: 10.1523/JNEUROSCI.4965-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wakamatsu M., Ishii A., Iwata S., Sakagami J., Ukai Y., Ono M., Kanbe D., Muramatsu S., Kobayashi K., Iwatsubo T., Yoshimoto M. Selective loss of nigral dopamine neurons induced by overexpression of truncated human alpha-synuclein in mice. Neurobiol Aging. 2008;29:574–585. doi: 10.1016/j.neurobiolaging.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 21.Daher J.P., Ying M., Banerjee R., McDonald R.S., Hahn M.D., Yang L., Flint Beal M., Thomas B., Dawson V.L., Dawson T.M., Moore D.J. Conditional transgenic mice expressing C-terminally truncated human alpha-synuclein (alphaSyn119) exhibit reduced striatal dopamine without loss of nigrostriatal pathway dopaminergic neurons. Mol Neurodegener. 2009;4:34. doi: 10.1186/1750-1326-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C.W., Giasson B.I., Lewis K.A., Lee V.M., Demartino G.N., Thomas P.J. A precipitating role for truncated alpha-synuclein and the proteasome in alpha-synuclein aggregation: implications for pathogenesis of Parkinson disease. J Biol Chem. 2005;280:22670–22678. doi: 10.1074/jbc.M501508200. [DOI] [PubMed] [Google Scholar]
- 23.Sevlever D., Jiang P., Yen S.H. Cathepsin D is the main lysosomal enzyme involved in the degradation of alpha-synuclein and generation of its carboxy-terminally truncated species. Biochemistry. 2008;47:9678–9687. doi: 10.1021/bi800699v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sung J.Y., Park S.M., Lee C.H., Um J.W., Lee H.J., Kim J., Oh Y.J., Lee S.T., Paik S.R., Chung K.C. Proteolytic cleavage of extracellular secreted {alpha}-synuclein via matrix metalloproteinases. J Biol Chem. 2005;280:25216–25224. doi: 10.1074/jbc.M503341200. [DOI] [PubMed] [Google Scholar]
- 25.Levin J., Giese A., Boetzel K., Israel L., Hogen T., Nubling G., Kretzschmar H., Lorenzl S. Increased alpha-synuclein aggregation following limited cleavage by certain matrix metalloproteinases. Exp Neurol. 2009;215:201–208. doi: 10.1016/j.expneurol.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Lee E.J., Woo M.S., Moon P.G., Baek M.C., Choi I.Y., Kim W.K., Junn E., Kim H.S. Alpha-synuclein activates microglia by inducing the expressions of matrix metalloproteinases and the subsequent activation of protease-activated receptor-1. J Immunol. 2010;185:615–623. doi: 10.4049/jimmunol.0903480. [DOI] [PubMed] [Google Scholar]
- 27.Mishizen-Eberz A.J., Guttmann R.P., Giasson B.I., Day G.A., 3rd, Hodara R., Ischiropoulos H., Lee V.M., Trojanowski J.Q., Lynch D.R. Distinct cleavage patterns of normal and pathologic forms of alpha-synuclein by calpain I in vitro. J Neurochem. 2003;86:836–847. doi: 10.1046/j.1471-4159.2003.01878.x. [DOI] [PubMed] [Google Scholar]
- 28.Mishizen-Eberz A.J., Norris E.H., Giasson B.I., Hodara R., Ischiropoulos H., Lee V.M., Trojanowski J.Q., Lynch D.R. Cleavage of alpha-synuclein by calpain: potential role in degradation of fibrillized and nitrated species of alpha-synuclein. Biochemistry. 2005;44:7818–7829. doi: 10.1021/bi047846q. [DOI] [PubMed] [Google Scholar]
- 29.Magen I., Chesselet M.F. Genetic mouse models of Parkinson’s disease. The state of the art. Prog Brain Res. 2010;184:53–87. doi: 10.1016/S0079-6123(10)84004-X. [DOI] [PubMed] [Google Scholar]
- 30.Lam H.A., Wu N., Cely I., Kelly R.L., Hean S., Richter F., Magen I., Cepeda C., Ackerson L.C., Walwyn W., Masliah E., Chesselet M.F., Levine M.S., Maidment N.T. Elevated tonic extracellular dopamine concentration and altered dopamine modulation of synaptic activity precede dopamine loss in the striatum of mice overexpressing human alpha-synuclein. J Neurosci Res. 2011;89:1091–1102. doi: 10.1002/jnr.22611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rockenstein E., Mallory M., Hashimoto M., Song D., Shults C.W., Lang I., Masliah E. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68:568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- 32.Fleming S.M., Salcedo J., Fernagut P.O., Rockenstein E., Masliah E., Levine M.S., Chesselet M.F. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J Neurosci. 2004;24:9434–9440. doi: 10.1523/JNEUROSCI.3080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleming S.M., Salcedo J., Hutson C.B., Rockenstein E., Masliah E., Levine M.S., Chesselet M.F. Behavioral effects of dopaminergic agonists in transgenic mice overexpressing human wildtype alpha-synuclein. Neuroscience. 2006;142:1245–1253. doi: 10.1016/j.neuroscience.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masliah E., Rockenstein E., Adame A., Alford M., Crews L., Hashimoto M., Seubert P., Lee M., Goldstein J., Chilcote T., Games D., Schenk D. Effects of alpha-synuclein immunization in a mouse model of Parkinson’s disease. Neuron. 2005;46:857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 35.Rascovsky K., Salmon D.P., Ho G.J., Galasko D., Peavy G.M., Hansen L.A., Thal L.J. Cognitive profiles differ in autopsy-confirmed frontotemporal dementia and AD. Neurology. 2002;58:1801–1808. doi: 10.1212/wnl.58.12.1801. [DOI] [PubMed] [Google Scholar]
- 36.Terry R.D., Masliah E., Salmon D.P., Butters N., DeTeresa R., Hill R., Hansen L.A., Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 37.Hansen L., Samuel W. Criteria for Alzheimer disease and the nosology of dementia with Lewy bodies. Neurology. 1997;48:126–132. doi: 10.1212/wnl.48.1.126. [DOI] [PubMed] [Google Scholar]
- 38.Galasko D., Katzman R., Salmon D.P., Hansen L. Clinical and neuropathological findings in Lewy body dementias. Brain Cogn. 1996;31:166–175. doi: 10.1006/brcg.1996.0040. [DOI] [PubMed] [Google Scholar]
- 39.McKeith I., Galasko D., Kosaka K., Perry E., Dickson D., Hansen L., Salmon D., Lowe J., Mirra S., Byrne E., Quinn N., Edwardson J., Ince P., Bergeron C., Burns A., Miller B., Lovestone S., Collerton D., Jansen E., de Vos R., Wilcock G., Jellinger K., Perry R. Clinical and pathological diagnosis of dementia with Lewy bodies (DLB): report of the CDLB International Workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 40.Nuber S., Petrasch-Parwez E., Winner B., Winkler J., von Horsten S., Schmidt T., Boy J., Kuhn M., Nguyen H.P., Teismann P., Schulz J.B., Neumann M., Pichler B.J., Reischl G., Holzmann C., Schmitt I., Bornemann A., Kuhn W., Zimmermann F., Servadio A., Riess O. Neurodegeneration and motor dysfunction in a conditional model of Parkinson’s disease. J Neurosci. 2008;28:2471–2484. doi: 10.1523/JNEUROSCI.3040-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shevchenko A., Wilm M., Vorm O., Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 42.Masliah E., Rockenstein E., Veinbergs I., Mallory M., Hashimoto M., Takeda A., Sagara Y., Sisk A., Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 43.Spencer B., Potkar R., Trejo M., Rockenstein E., Patrick C., Gindi R., Adame A., Wyss-Coray T., Masliah E. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. J Neurosci. 2009;29:13578–13588. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rockenstein E., Mallory M., Mante M., Sisk A., Masliah E. Early formation of mature amyloid-b proteins deposits in a mutant APP transgenic model depends on levels of Ab1-42. J Neurosci Res. 2001;66:573–582. doi: 10.1002/jnr.1247. [DOI] [PubMed] [Google Scholar]
- 45.Dickson D.W. Neuropathology of non-Alzheimer degenerative disorders. Int J Clin Exp Pathol. 2009;3:1–23. [PMC free article] [PubMed] [Google Scholar]
- 46.Lippa C.F., Duda J.E., Grossman M., Hurtig H.I., Aarsland D., Boeve B.F., Brooks D.J., Dickson D.W., Dubois B., Emre M., Fahn S., Farmer J.M., Galasko D., Galvin J.E., Goetz C.G., Growdon J.H., Gwinn-Hardy K.A., Hardy J., Heutink P., Iwatsubo T., Kosaka K., Lee V.M., Leverenz J.B., Masliah E., McKeith I.G., Nussbaum R.L., Olanow C.W., Ravina B.M., Singleton A.B., Tanner C.M., Trojanowski J.Q., Wszolek Z.K. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007;68:812–819. doi: 10.1212/01.wnl.0000256715.13907.d3. [DOI] [PubMed] [Google Scholar]
- 47.Duda J.E., Giasson B.I., Lee V.M., Trojanowski J.Q. Is the initial insult in Parkinson’s disease and dementia with Lewy bodies a neuritic dystrophy? Ann N Y Acad Sci. 2003;991:295–297. [Google Scholar]
- 48.Gai W., Blessing W., Blumberg P. Ubiquitin-positive degenerating neurites in the brainstem in Parkinson’s disease. Brain. 1995;118:1447–1459. doi: 10.1093/brain/118.6.1447. [DOI] [PubMed] [Google Scholar]
- 49.Kramer M.L., Schulz-Schaeffer W.J. Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci. 2007;27:1405–1410. doi: 10.1523/JNEUROSCI.4564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takenouchi T., Hashimoto M., Hsu L., Mackowski B., Rockenstein E., Mallory M., Masliah E. Reduced neuritic outgrowth and cell adhesion in neuronal cells transfected with human α-synuclein. Mol Cell Neurosci. 2001;17:141–150. doi: 10.1006/mcne.2000.0923. [DOI] [PubMed] [Google Scholar]
- 51.Crews L., Mizuno H., Desplats P., Rockenstein E., Adame A., Patrick C., Winner B., Winkler J., Masliah E. Alpha-synuclein alters Notch-1 expression and neurogenesis in mouse embryonic stem cells and in the hippocampus of transgenic mice. J Neurosci. 2008;28:4250–4260. doi: 10.1523/JNEUROSCI.0066-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winner B., Lie D.C., Rockenstein E., Aigner R., Aigner L., Masliah E., Kuhn H.G., Winkler J. Human wild-type alpha-synuclein impairs neurogenesis. J Neuropathol Exp Neurol. 2004;63:1155–1166. doi: 10.1093/jnen/63.11.1155. [DOI] [PubMed] [Google Scholar]
- 53.Li W., West N., Colla E., Pletnikova O., Troncoso J.C., Marsh L., Dawson T.M., Jakala P., Hartmann T., Price D.L., Lee M.K. Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson’s disease-linked mutations. Proc Natl Acad Sci U S A. 2005;102:2162–2167. doi: 10.1073/pnas.0406976102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mouatt-Prigent A., Karlsson J.O., Agid Y., Hirsch E.C. Increased M-calpain expression in the mesencephalon of patients with Parkinson’s disease but not in other neurodegenerative disorders involving the mesencephalon: a role in nerve cell death? Neuroscience. 1996;73:979–987. doi: 10.1016/0306-4522(96)00100-5. [DOI] [PubMed] [Google Scholar]
- 55.Anderson J.P., Walker D.E., Goldstein J.M., de Laat R., Banducci K., Caccavello R.J., Barbour R., Huang J., Kling K., Lee M., Diep L., Keim P.S., Shen X., Chataway T., Schlossmacher M.G., Seubert P., Schenk D., Sinha S., Gai W.P., Chilcote T.J. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 56.Price D.L., Rockenstein E., Ubhi K., Phung V., MacLean-Lewis N., Askay D., Cartier A., Spencer B., Patrick C., Desplats P., Ellisman M.H., Masliah E. Alterations in mGluR5 expression and signaling in Lewy body disease and in transgenic models of alpha-synucleinopathy–implications for excitotoxicity. PLoS One. 2010;5:e14020. doi: 10.1371/journal.pone.0014020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Schulz J.B. Mechanisms of neurodegeneration in idiopathic Parkinson’s disease. Parkinsonism Relat Disord. 2007;13(Suppl 3):S306–S308. doi: 10.1016/S1353-8020(08)70021-X. [DOI] [PubMed] [Google Scholar]
- 58.Cali T., Ottolini D., Brini M. Mitochondria, calcium, and endoplasmic reticulum stress in Parkinson’s disease. Biofactors. 2011;37:228–240. doi: 10.1002/biof.159. [DOI] [PubMed] [Google Scholar]
- 59.Mattson M.P. Calcium and neurodegeneration. Aging Cell. 2007;6:337–350. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 60.Reznichenko L., Cheng Q., Nizar K., Gratiy S.L., Saisan P.A., Rockenstein E.M., Gonzalez T., Patrick C., Spencer B., Desplats P., Dale A.M., Devor A., Masliah E. In vivo alterations in calcium buffering capacity in transgenic mouse model of synucleinopathy. J Neurosci. 2012;32:9992–9998. doi: 10.1523/JNEUROSCI.1270-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giasson B.I., Uryu K., Trojanowski J.Q., Lee V.M. Mutant and wild type human alpha-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem. 1999;274:7619–7622. doi: 10.1074/jbc.274.12.7619. [DOI] [PubMed] [Google Scholar]
- 62.Mazzulli J.R., Armakola M., Dumoulin M., Parastatidis I., Ischiropoulos H. Cellular oligomerization of alpha-synuclein is determined by the interaction of oxidized catechols with a C-terminal sequence. J Biol Chem. 2007;282:31621–31630. doi: 10.1074/jbc.M704737200. [DOI] [PubMed] [Google Scholar]
- 63.Huang Z., Xu Z., Wu Y., Zhou Y. Determining nuclear localization of alpha-synuclein in mouse brains. Neuroscience. 2011;199:318–332. doi: 10.1016/j.neuroscience.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muntane G., Ferrer I., Martinez-Vicente M. Alpha-synuclein phosphorylation and truncation are normal events in the adult human brain. Neuroscience. 2012;200:106–119. doi: 10.1016/j.neuroscience.2011.10.042. [DOI] [PubMed] [Google Scholar]
- 65.McLean J.R., Hallett P.J., Cooper O., Stanley M., Isacson O. Transcript expression levels of full-length alpha-synuclein and its three alternatively spliced variants in Parkinson’s disease brain regions and in a transgenic mouse model of alpha-synuclein overexpression. Mol Cell Neurosci. 2012;49:230–239. doi: 10.1016/j.mcn.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perrin R.J., Payton J.E., Barnett D.H., Wraight C.L., Woods W.S., Ye L., George J.M. Epitope mapping and specificity of the anti-alpha-synuclein monoclonal antibody Syn-1 in mouse brain and cultured cell lines. Neurosci Lett. 2003;349:133–135. doi: 10.1016/s0304-3940(03)00781-x. [DOI] [PubMed] [Google Scholar]
- 67.Perlmutter L.S., Siman R., Gall C., Seubert P., Baudry M., Lynch G. The ultrastructural localization of calcium-activated protease “calpain” in rat brain. Synapse. 1988;2:79–88. doi: 10.1002/syn.890020111. [DOI] [PubMed] [Google Scholar]
- 68.Tang Y., Das U., Scott D.A., Roy S. The slow axonal transport of alpha-synuclein-mechanistic commonalities amongst diverse cytosolic cargoes. Cytoskeleton (Hoboken) 2012;69:506–513. doi: 10.1002/cm.21019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Katsuse O., Iseki E., Marui W., Kosaka K. Developmental stages of cortical Lewy bodies and their relation to axonal transport blockage in brains of patients with dementia with Lewy bodies. J Neurol Sci. 2003;211:29–35. doi: 10.1016/s0022-510x(03)00037-6. [DOI] [PubMed] [Google Scholar]
- 70.Siebert H., Kahle P.J., Kramer M.L., Isik T., Schluter O.M., Schulz-Schaeffer W.J., Bruck W. Over-expression of alpha-synuclein in the nervous system enhances axonal degeneration after peripheral nerve lesion in a transgenic mouse strain. J Neurochem. 2010;114:1007–1018. doi: 10.1111/j.1471-4159.2010.06832.x. [DOI] [PubMed] [Google Scholar]