Abstract
Purpose
Currently there is no effective medicinal treatment for uterine fibroids (UFs), a common health disorder that affects women of reproductive age. Identification of modifiable risk factors such as vitamin D (Vit D) deficiency could help develop novel strategies for the prevention and/or treatment of UFs. The purpose of this study was to identify whether low serum Vit D3 levels correlate with increased risk of UFs.
Methods
A total of 154 premenopausal women were recruited for this cross-sectional study. The control group comprised 50 subjects with a normal, fibroid-free uterine structure, confirmed by transvaginal ultrasonography. The 104 case subjects had at least one fibroid lesion that was 2 cm3 in volume or larger, confirmed by transvaginal ultrasonography. For each case subject, total uterine volume and total volume of all existing fibroids were measured in three perpendicular planes, with volume determined according to the prolate ellipse formula (a × b × c × 0.523), where a is height, b is width, and c is depth. Serum Vit D [25(OH) D3] levels were measured by radioimmunoassay. The independent t-test was used to compare serum Vit D levels across groups. Correlations were assessed by Spearman’s rank correlation test.
Results
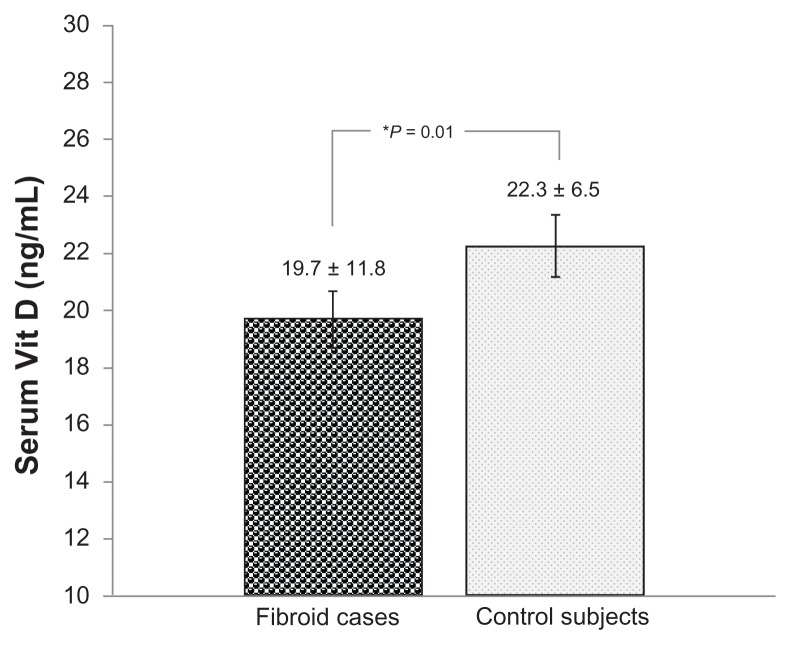
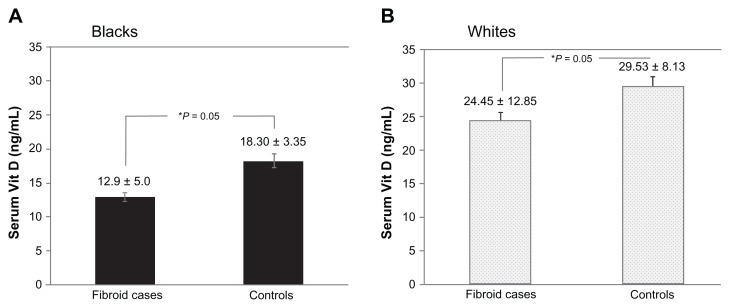
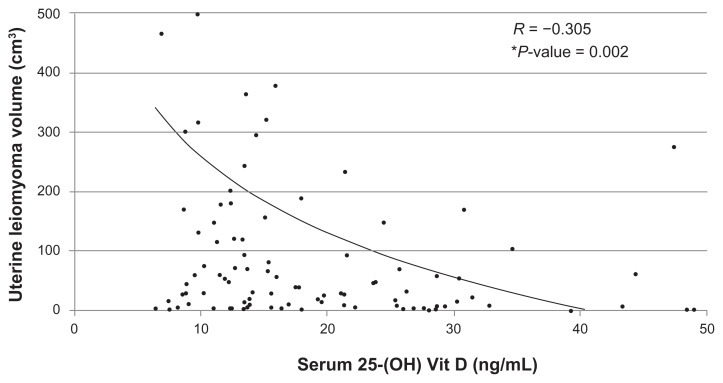
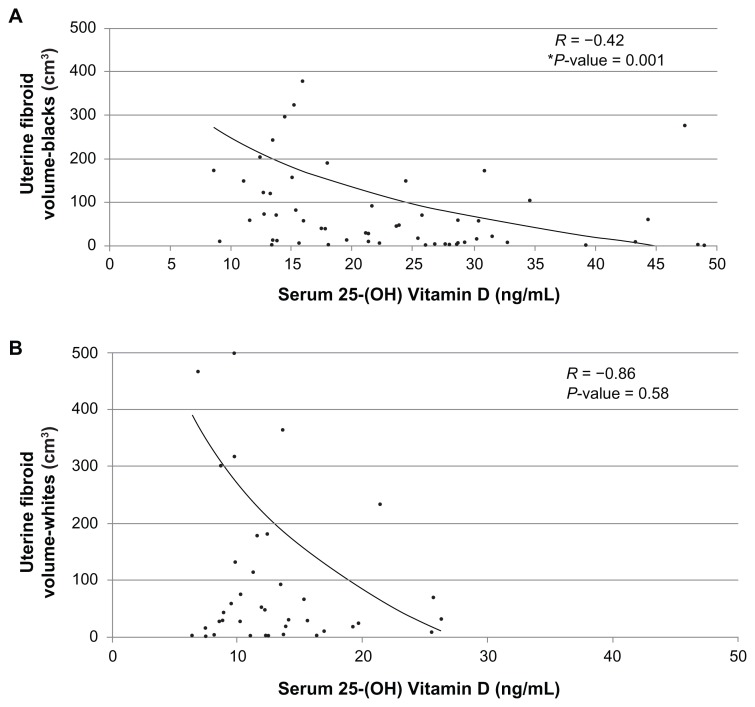
Lower serum 25-(OH) Vit D levels were significantly associated with the occurrence of UFs (P = 0.01). A statistically significant inverse correlation was also observed between serum 25-(OH) Vit D levels and total UF volume (r = −0.31; P = 0.002) within the case cohort. Subjects with larger fibroid volumes had lower serum Vit D levels and vice versa. Data stratified for ethnicity showed a statistically significant inverse correlation between serum 25-(OH) Vit D levels and total fibroid volume in black subjects (r = −0.42; P = 0.001). An inverse correlation was also evident in white subjects (r = −0.86; P = 0.58) but this did not reach statistical significance.
Conclusion
Lower serum Vit D levels are inversely correlated with UF burden in different ethnic groups. Vit D deficiency is a possible risk factor for the occurrence of UFs.
Keywords: vitamin D deficiency, race/ethnicity, risk factor, premenopausal women
Introduction
Uterine fibroids (UFs) are the most common benign tumors affecting the health of women of reproductive age.1 The prevalence of UFs is reported to be as high as 77%.2–5 In addition, women of color suffer a much higher incidence, at least two times higher odds, than white women of developing this serious disease.2,3 Many UF patients suffer pelvic pain, menorrhagia, dysmenorrhea/dyspareunia, pressure-related symptoms, miscarriage, and subfertility.6 Currently, there is no effective medicinal treatment for UFs, and the enormous cost burden of this common disease on the US health care system is estimated to be about $34.4 billion per year.7 There are a range of current management options: removal of the uterus (hysterectomy), removal of the fibroid lesions (myomectomy), uterine artery embolization, image-guided focused ultrasound thermal therapy, and, in mild cases, reassurance and observation.6,8,9 Currently, novel noninvasive treatment options for UFs – such as localized gene therapy, oral green tea extracts, and selective progesterone receptor modulators – are being explored by the authors’ laboratory and by others.8,10–13 A safe and effective oral treatment option for UFs would be a major advance in the field and it would have an immense impact on women’s health worldwide.
Vitamin D (Vit D) is a prohormone produced in the skin via a sunlight-initiated reaction and metabolically converted to the active metabolite 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], mainly in the liver and kidneys.14 Vit D exerts its effects via activation of its cellular receptor (vitamin D receptor [VDR]), which in turn alters the transcription rates of target genes responsible for various biological responses.15–17 Local production of 1,25(OH)2D3 is dependent on circulating precursor levels, which may explain the association of serum Vit D deficiency with various diseases.18 Diverse functions for Vit D have been confirmed by the presence of VDR in a wide range of human tissues, including skin, colon, brain, pancreas, and breast, as well as activated T and B lymphocytes, monocytes, and macrophages.14 The authors and others have recently demonstrated VDR expression in both the myometrium and the endometrium of the human uterus, throughout the menstrual cycle, in addition to UF tissues.19–21 The ability of 1,25(OH)2D3 to inhibit growth and promote differentiation of a variety of cell types has suggested diverse functions in preventing cancers, modulating the immune system, and controlling various endocrine systems.22 This effect is mediated predominantly through a G1/S (gap 1/synthesis) phase block of the cell cycle. The 1,25(OH)2D3 regulates many of the cell cycle regulatory genes and modulates activities of cyclin-dependent kinases, leading to a decreased number of cells in the S phase and an accumulation of cells in the G0–G1 phase.23,24 The authors have recently shown that Vit D inhibited extracellular signal–regulated kinase activation and downregulated the expression of anti-apoptotic (BCL-2, BCL-w), cell cycle–regulating (cyclin-dependent kinase 1), and cell-proliferating (proliferating cell nuclear antigen) genes in human fibroid cells.25 The authors have also demonstrated that Vit D is an antifibrotic factor that inhibits the growth of human fibroid cells in a dose-dependent fashion by significantly reducing many of the transforming growth factor beta 3 (TGF-β3)–mediated effects such as the TGF-β3 induction of fibronectin and collagen type 1 protein expression, the induction of protein expression of plasminogen activator inhibitor-1, and the phosphorylation of Smad2 as well as nuclear translocation of Smad2 and Smad3.15 Furthermore, the authors have recently demonstrated the ability of Vit D to safely shrink UF lesions in Eker rats, an authentic orthotopic animal model for UFs.26 Taken together, these findings suggest that Vit D may be a potentially useful therapeutic agent for the nonsurgical management of UFs.15 This report evaluates the possible correlation between serum Vit D3 levels and the tumor burden of UF disease in a diverse population of women of reproductive age.
Methods
Subjects
This cross-sectional observational study was performed at the outpatient gynecology clinic of Sohag University’s Faculty of Medicine, (Sohag, Egypt), which serves more than 9 million people as a tertiary care center. This study did not involve any treatment or intervention, and the Sohag Medical School Ethical Committee approved this study. Written informed consent was obtained from eligible candidates before participation in the study, and the racial assignment of these subjects was by self-identification in clinic records.
Women between 18 and 50 years of age who were in a premenopausal status (menstrual cycle day 3 serum follicle-stimulating hormone < 10 mIU/mL) were considered eligible to participate in the study. The final 154 women recruited for this study were between 22 and 45 years of age. They included 50 participants with a normal, fibroid-free uterine structure, based on transvaginal ultrasonography (TVU), who were used as the control group. The remaining 104 participants were case subjects and had at least one fibroid lesion with a mean volume of 2 cm3 or greater, as confirmed by TVU. For each case subject, both total uterine volume and total volume of all existing fibroids (total fibroid volume) were measured. The case subjects were recruited from women presenting to university clinics with various UF-related symptoms. The control subjects were recruited from asymptomatic women seen at these clinics for routine well women exams. Women with one or more of the following conditions were excluded: prior myomectomy or hysterectomy; current pregnancy, or a pregnancy within the 6 months prior to the start of the study; currently lactating, or lactating within the 6 months prior to the start of the study; and candidates who had experienced an abortion or miscarriage within the 6 months prior to the start of the study. Women currently using a vitamin supplement or any hormonal treatment or women who had used a vitamin supplement or any hormonal treatment within the 6 months prior to enrollment were also excluded from the study.
Study methodology
The study procedure involved a brief history and physical examination including biometric measurement, pelvic screening, and bimanual examination. Blood samples were also collected from each participant during the study visit. Serum Vit D [25(OH) D3] levels were measured using radioimmunoassay techniques described previously.27–29 Briefly, the DiaSorin Liaison® 25-OH Vitamin D Total assay (DiaSorin Inc, Stillwater, MN, USA), which uses direct competitive chemiluminescence immunoassay technology (approved by the US Food and Drug Administration), was employed for the quantitative determination of 25-hydroxyvitamin D at Heartland Assays Inc® (Ames, IA, USA).27,28 The sensitivity for this assay is 2.5 ng/mL, and the inter- and intra-assay coefficients of variation are 11.2% and 8.1%, respectively. Recovery of endogenous 25(OH)D is 100%.29 Ultrasound evaluations were performed by TVU on all consenting subjects, while transabdominal ultrasonography was performed as needed for some subjects for whom TVU was not sufficient to evaluate the fibroid lesions in their entirety – particularly, large fundal fibroids. The ultrasonography assessment was performed using an Acuson 128 XP10® ultrasound machine – fitted with a 3.5/5.0 MHz endovaginal probe for the transvaginal scan and a 2.5/3.5 MHz convex probe for the abdominal scan. One physician (a certified ultrasonographer) who was not aware of the study objectives or group assignments performed all of the ultrasound scans.
The following parameters were evaluated by TVU:
total uterine size, as measured in three perpendicular planes
number of fibroid lesions
volume of all fibroid lesions, determined according to the prolate ellipse formula (a × b × c × 0.523), where a is height, b is width, and c is depth
position/location of each fibroid lesion within the uterus (uterine fundus, lower uterine segment, cervix, extrauterine) charted on a standardized anatomical sketch of the uterus and numbered to ease identification of each fibroid lesion over the course of the study
unusual characteristics (echogenicity, presence of calcifications, presence of central necrosis, etc) of each fibroid lesion.
Statistical analysis
Data entry, screening, and analysis were carried out using statistical software (SPSS, v 19.0; IBM Corporation, Armonk, NY, USA). Descriptive analysis was performed using means, frequencies, standard deviations, and percentages. The independent t-test was used to compare serum Vit D levels across groups. Correlations were assessed by Spearman’s rank correlation test. A P-value ≤ 0.05 was considered significant.
Results
Study population demographics
Of the 154 women who participated in the study, 87 were black and 67 were white. Of these participants, 104 women were confirmed by TVU to have at least one UF – with a volume of 2 cm3 or greater – and so served as case subjects (Table 1). The remaining 50 participants showed normal uterine structure with no UFs and so served as healthy controls (case-to-control ratio of 2:1).
Table 1.
Demographics of the study cohorts (n = 154)
| Participantsa | Ethnicityb | Age [years]c (P-value)d | BMIb,c (P-value)d | |
|---|---|---|---|---|
|
| ||||
| Black | White | |||
| Cases (n = 104)e | 61 | 43 | 37.1 ± 2.9 (NS) | 29.2 ± 4.3 (NS) |
| Controls (n = 50)f | 26 | 24 | 36.8 ± 3.4 (NS) | 29.2 ± 4.3 (NS) |
| Total | 87 | 67 | – | – |
Notes:
The inclusion criteria for participants were premenopausal (menstrual cycle day 3 serum follicle-stimulating hormone < 10 mIU/mL) women aged between 18 and 50 years;
ethnicity of the participants was determined by self-report in clinical charts and BMI was evaluated upon participation in the study;
data presented as mean plus or minus standard deviation;
a P-value ≤ 0.05 was considered statistically significant;
cases were participants who had at least one fibroid (minimum volume of 2 cm3), confirmed by transvaginal ultrasonography;
controls were participants who did not have any fibroids, confirmed by transvaginal ultrasonography.
Abbreviations: BMI, body mass index; NS, not significant.
The age of participants ranged from 22 to 45 years (mean age, 36.9 ± 6.3 years) (Table 1). The mean age of the white subjects (38 ± 5.7 years) was slightly higher than but not significantly different to that of the black subjects (36.2 ± 6.7 years; P = 0.28). On the other hand, the mean body mass index (BMI) of the black subjects (30.8 ± 7.7) was significantly higher than that of the white subjects (28.0 ± 6.1; P = 0.05). There were no significant differences between case and control cohorts in age, BMI (Table 1), religious affiliation, or gynecological and obstetrical history.
Lower serum Vit D levels are associated with increased risk of UFs in different ethnic groups
To identify a potential association between serum Vit D levels and risk of UFs, the authors evaluated the serum levels in subjects with symptomatic UFs and in well-matched healthy controls (as described in the Methods section) (Table 1). As expected, the mean level of serum 25-OH Vit D, plus or minus standard deviation, was found to be significantly lower in the black subjects (14.2 ± 5.2 ng/mL) than in the white subjects (25.5 ± 12.2 ng/mL), irrespective of disease status (P = 0.03). Interestingly, the mean level of serum 25-OH Vit D, plus or minus standard deviation, was found to be significantly lower in cases with UFs (19.7 ± 11.8 ng/mL) than in healthy controls (22.3 ± 6.5 ng/mL) (P = 0.01) (Figure 1 and Table 2). Furthermore, after stratifying the data to black and white subjects, the authors observed a statistically significant difference in serum 25 (OH) Vit D levels between cases and controls within each ethnic group. Black subjects diagnosed with symptomatic UFs had lower serum 25 (OH) Vit D levels (12.9 ± 5.0 ng/mL) than black healthy controls (18.30 ± 3.35 ng/mL; P = 0.05) (Figure 2A and Table 2). Similarly, white subjects diagnosed with UFs had lower serum 25 (OH) Vit D levels (24.45 ± 12.85 ng/mL) than white healthy controls (29.53 ± 8.13 ng/mL; P = 0.052) (Figure 2B and Table 2).
Figure 1.
Comparison of serum 25 (OH) vitamin D (Vit D) levels in uterine fibroid cases and healthy controls.
Notes: *Statistically significant (P ≤ 0.05). Total uterine fibroid cases, n = 104 (blacks = 61; whites = 43); total controls, n = 50 (blacks = 26; whites = 24).
Table 2.
Differences in serum vitamin D (Vit D) levelsa among study participants: blacks versus whitesb and casesc versus controlsd
| Participants | Serum Vit D levels (ng/mL)e according to ethnicity | ||
|---|---|---|---|
|
|
|||
| Blacksf | Whitesg | Total (n = 154)h | |
| Cases | 12.9 ± 5.0 (n = 61) | 24.45 ± 12.85 (n = 43) | 19.7 ± 11.8 (n = 104) |
| Controls | 18.30 ± 3.35 (n = 26) | 29.53 ± 8.13 (n = 24) | 22.3 ± 6.5 (n = 50) |
| Total (n = 154)i | 14.2 ± 5.2 (n = 87) | 25.5 ± 12.2 (n = 67) | – |
Notes:
Serum Vit D levels were measured in blood samples of all consenting participants, by radioimmunoassay;
ethnicity of the participants was determined by self-identification in clinic records;
cases were participants who had at least one fibroid (minimum volume of 2 cm3), confirmed by transvaginal ultrasonography;
controls were participants who did not have any fibroids, confirmed by transvaginal ultrasonography;
data presented as mean plus or minus standard deviation;
P = 0.05 (statistically significant);
P = 0.052;
P = 0.01 (statistically significant);
P = 0.03 (statistically significant).
Figure 2.
Comparison of serum 25-(OH) vitamin D (Vit D) levels between cases and controls in different ethnic groups: Vit D levels in (A) blacksa and (B) whites.b
Notes:aUterine fibroid cases, n = 61; healthy controls, n = 26; buterine fibroid cases, n = 43; healthy controls, n = 24; *statistically significant (P ≤ 0.05).
Serum Vit D levels inversely correlate with the severity of UF disease
Next, the authors wanted to assess if a dose-response effect exists between serum Vit D levels and the severity of fibroid disease in the case cohort. Interestingly, a statistically significant inverse correlation (r = −0.31; P = 0.002) was observed between serum 25-(OH) Vit D levels and total volume of UFs (Figure 3) within the case cohort. In other words, for participants with UFs, the lower the serum Vit D levels, the larger the total fibroid volume, and vice versa.
Figure 3.
Correlation of serum 25-(OH) vitamin D (Vit D) levels to uterine fibroid volume given as a logarithmic trend line.
Notes: Total fibroid cases, n = 104; *statistically significant (P ≤ 0.05).
On stratifying the data based on ethnicity into blacks and whites, a statistically significant inverse correlation (r = −0.305; P = 0.001) was observed between serum 25-(OH) Vit D levels and total fibroid volume in the black subjects (Figure 4A). In the white subjects (Figure 4B) the inverse correlation was evident (r = −0.86; P = 0.58), but this did not reach statistical significance.
Figure 4.
Evaluating racial trend in the correlation between serum 25-(OH) vitamin D levels to uterine fibroid volume: logarithmic trend lines given for (A) blacka and (B) whiteb participants.
Notes:aFibroid cases, n = 61; bfibroid cases, n = 43; *statistically significant (P ≤ 0.05).
Discussion
The authors have recently reported, for the first time, in vitro data describing a potential central role for Vit D in the biology of UFs.15,30 Additionally, the authors’ recent in vivo data from the Eker rat model of UFs confirmed subcutaneously infused Vit D’s ability to shrink UF lesions via induction of apoptosis and inhibition of proliferation.26 These encouraging results, and the recent reports on the prevalence of hypovitaminosis D in developing countries,31 triggered the present authors to explore – especially in a population comprising both blacks and whites – the possible correlation between serum Vit D and the occurrence and severity of UFs.
The participants in this study are from a mixed population. As Egypt is part of Mediterranean North Africa, Egyptians are often grouped with Near Eastern populations because of similar genotypic signatures and phenotypic attributes such as lighter skin pigmentation. However, recent genomic studies32,33 have also documented significant signatures of sub-Saharan African ancestry with black races introduced into North African Egyptian populations about 750 years ago. Thus, the Egyptian population, with its mix of Africans (blacks, originating mostly from southern Egypt) and Caucasians (whites, from northern Egypt), is an ideal population to test the authors’ hypothesis. Interestingly, the level of serum 25-OH Vit D was found to be significantly lower in the black subjects than in the white subjects, irrespective of disease status; this is in agreement with prior reports from the United States and suggests similar Vit D biosynthesis and biological processes irrespective of global geographic location.34
The authors’ data show that, in women afflicted with UFs, there is a statistically significant negative correlation between serum Vit D levels and UF volume. The largest fibroid tumor burden was observed in women with the lowest serum Vit D levels. This indeed establishes a dose-effect paradigm and provides strong evidence for a biological role for Vit D in the pathogenesis of UFs. Several studies in the last decade have suggested a role for Vit D in muscle strength, cardiovascular health, neurological diseases, insulin-resistance, malignancies, autoimmune diseases, and infections.35 The present authors’ data extend that role to include UFs, an important global disease of the female reproductive tract. The authors’ recently published preclinical work suggests a dose-dependent inhibitory effect of Vit D on human fibroid cell growth in vitro.15,25 The authors have also reported that such effect is mediated through the downregulation of proliferating cell nuclear antigen, cyclin-dependent kinase 1, and TGF-β3-induced fibronectin and collagen type 1 protein expression, among others.15 In addition, the authors have demonstrated that Vit D suppresses catechol O-methyl transferase expression and activity in human fibroid cells.25 Such an emerging role of Vit D in UF biology is intriguing, as it presents a possible explanation for the high prevalence of such disease in the black population.36,37 It is well documented that UFs are at least two to four times more common in blacks than in whites.2,3,36.37 It is also a well-established observation that the incidence of Vit D deficiency is more common in blacks than in whites.38,39 A possible etiologic factor for such disparity in serum Vit D levels is deep skin pigmentation, which makes Vit D photosynthesis inefficient in dark-skinned individuals.40 A unifying model is emerging where a lifelong Vit D–deficiency status would be consistent with an earlier onset of UFs and a higher tumor burden,41 which is indeed what the authors have observed in black subjects, as compared with white subjects and as reported in this study. Recently, the authors have reported that Vit D deficiency has also been associated with higher estrogen receptor and progesterone receptor expression and enhanced estrogen and progesterone signaling in the myometrium.42,43 Thus, chronic Vit D deficiency in black adolescent females would lead to chronic heightened estrogen and progesterone signaling in the myometrium, and this could then conceivably lead to the initiation and progression of UF in the presence of other triggering factors such as obesity,44 certain genetic variants, and possibly also epigenetic alterations.10,36 Interestingly, BMI has been implicated in an increase of the risk of fibroid incidence. Studies have reported that when two BMIs of ≥30.0 and <20.0 were adjusted for age, race, or ethnicity and compared, the BMI ≥ 30.0 was associated with a 2.3-fold increase in the odds for having fibroids.45,46 While the present authors see a slight difference in BMI between black and white subjects, reports indicate that there is no correlation between Vit D levels and BMI, especially in black women.47
Another important translational application of the authors’ data is the possible use of Vit D or its potent hypocalcemic analogues as a potential treatment and/or preventive option for UFs. Indeed, the authors’ published data using the subcutaneous supplement of Vit D in the Eker rat, a preclinical model of spontaneous UF development, showed a dramatic shrinkage of UFs in a 3-week trial.26 The authors have also observed similar effect of paricalcitol48 on human fibroid cells in vitro (unpublished observation). Another attractive feature of Vit D as an antifibroid therapy is that the safety range for Vit D is wide, and only chronic overdosage with Vit D supplements in the presence of markedly impaired renal function may lead to levels considered to be toxic.49
Conclusion
The authors have documented the safety of Vit D in the Eker rat model of UFs, where no side effects and no negative effects on liver function tests were observed.26 Further laboratory and clinical investigations of the role of Vit D in the biology of UFs are warranted. Further investigations could establish Vit D, or its analogues, as a novel oral noninvasive therapeutic/preventive agent for this common disease; this in turn would have a major positive impact on women’s health worldwide.
Acknowledgments
This work was supported by grants from the National Institute of Child Health and Human Development, National Institutes of Health (grant 1 R01 HD046228-01), and Research Centers in Minority Institutions (grant 2 G12 RR003032-26) to SKH. This work was also supported in part by the Vanderbilt Clinical and Translational Science Awards grant UL1 RR024975 from the National Center for Research Resources, National Institutes of Health to SKH.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Buttram VC., Jr Uterine leiomyomata: aetiology, symptomatology and management. Prog Clin Biol Res. 1986;225:275–296. [PubMed] [Google Scholar]
- 2.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 3.Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90(6):967–973. doi: 10.1016/s0029-7844(97)00534-6. [DOI] [PubMed] [Google Scholar]
- 4.Stewart EA. Uterine fibroids. Lancet. 2001;357(9252):293–298. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- 5.Csatlos E, Rigó J, Jr, Szabó I, Nagy Z, Joó JG. Uterine leiomyoma. Orv Hetil. 2010;151(42):1734–1741. doi: 10.1556/OH.2010.28977. Hungarian. [DOI] [PubMed] [Google Scholar]
- 6.Shen SH, Fennessy F, McDannold N, Jolesz F, Tempany C. Image-guided thermal therapy of uterine fibroids. Semin Ultrasound CT MR. 2009;30(2):91–104. doi: 10.1053/j.sult.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):211. e1–9. doi: 10.1016/j.ajog.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Hendy A, Salama S. Gene therapy and uterine leiomyoma: a review. Hum Reprod Update. 2006;12(4):385–400. doi: 10.1093/humupd/dml015. [DOI] [PubMed] [Google Scholar]
- 9.Ezzati M, Norian JM, Segars JH. Management of uterine fibroids in the patient pursuing assisted reproductive technologies. Womens Health (Lond Engl) 2009;5(4):413–421. doi: 10.2217/whe.09.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassan MH, Othman EE, Hornung D, Al-Hendy A. Gene therapy of benign gynecological diseases. Adv Drug Deliv Rev. 2009;61(10):822–835. doi: 10.1016/j.addr.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassan MH, Salama SA, Zhang D, et al. Gene therapy targeting leiomyoma: adenovirus-mediated delivery of dominant-negative estrogen receptor gene shrinks uterine tumors in Eker rat model. Fertil Steril. 2010;93(1):239–250. doi: 10.1016/j.fertnstert.2008.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang D, Al-Hendy M, Richard-Davis G, et al. Green tea extract inhibits proliferation of uterine leiomyoma cells in vitro and in nude mice. Am J Obstet Gynecol. 2010;202(3):289. e1–9. doi: 10.1016/j.ajog.2009.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassan MH, Fouad H, Bahashwan S, Al-Hendy A. Towards non-surgical therapy for uterine fibroids: catechol-O-methyl transferase inhibitor shrinks uterine fibroid lesions in the Eker rat model. Hum Reprod. 2011;26(11):3008–3018. doi: 10.1093/humrep/der280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norman AW, Bouillon R. Vitamin D nutritional policy needs a vision for the future. Exp Biol Med (Maywood) 2010;235(9):1034–1045. doi: 10.1258/ebm.2010.010014. [DOI] [PubMed] [Google Scholar]
- 15.Halder SK, Goodwin JS, Al-Hendy A. 1,25-Dihydroxyvitamin D3 reduces TGF-beta3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J Clin Endocrinol Metab. 2011;96(4):E754–E762. doi: 10.1210/jc.2010-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bikle DD. Vitamin D: an ancient hormone. Exp Dermatol. 2011;20(1):7–13. doi: 10.1111/j.1600-0625.2010.01202.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Nicosia SV, Bai W. Vitamin D receptor is a novel drug target for ovarian cancer treatment. Curr Cancer Drug Targets. 2006;6(3):229–244. doi: 10.2174/156800906776842939. [DOI] [PubMed] [Google Scholar]
- 18.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289(1):F8–F28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 19.McDonnell DP, Mangelsdorf DJ, Pike JW, Haussler MR, O’Malley BW. Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science. 1987;235(4793):1214–1217. doi: 10.1126/science.3029866. [DOI] [PubMed] [Google Scholar]
- 20.Vienonen A, Miettinen S, Blauer M, et al. Expression of nuclear receptors and cofactors in human endometrium and myometrium. J Soc Gynecol Investig. 2004;11(2):104–112. doi: 10.1016/j.jsgi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Feng L, Jayes FL, Jung SH, Leppert PC. Vitamin D receptor (VDR) is over-expressed in the center of uterine fibroids. Fertil Steril. 2010;94(4):S75. [Google Scholar]
- 22.Ylikomi T, Laaksi I, Lou YR, et al. Antiproliferative action of vitamin D. Vitam Horm. 2002;64:357–406. doi: 10.1016/s0083-6729(02)64010-5. [DOI] [PubMed] [Google Scholar]
- 23.Wang QM, Jones JB, Studzinski GP. Cyclin-dependent kinase inhibitor p27 as a mediator of the G1-S phase block induced by 1,25-dihydroxyvitamin D3 in HL60 cells. Cancer Res. 1996;56(2):264–267. [PubMed] [Google Scholar]
- 24.Zhuang SH, Burnstein KL. Antiproliferative effect of 1alpha, 25-dihydroxyvitamin D3 in human prostate cancer cell line LNCaP involves reduction of cyclin-dependent kinase 2 activity and persistent G1 accumulation. Endocrinology. 1998;139(3):1197–1207. doi: 10.1210/endo.139.3.5770. [DOI] [PubMed] [Google Scholar]
- 25.Sharan C, Halder SK, Thota C, Jaleel T, Nair S, Al-Hendy A. Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-O-methyltransferase. Fertil Steril. 2011;95(1):247–253. doi: 10.1016/j.fertnstert.2010.07.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halder SK, Sharan C, Al-Hendy A. Vitamin D treatment induces dramatic shrinkage of uterine leiomyomas growth in the Eker rat model. Biol Reprod. doi: 10.1095/biolreprod.111.098145.. In press 2012; BOR papers in press. Online Feb 2012. [DOI] [Google Scholar]
- 27.Ersfeld DL, Rao DS, Body JJ, et al. Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON automated analyzer. Clin Biochem. 2004;37(10):867–874. doi: 10.1016/j.clinbiochem.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Wagner D, Hanwell HE, Vieth R. An evaluation of automated methods for measurement of serum 25-hydroxyvitamin D. Clin Biochem. 2009;42(15):1549–1556. doi: 10.1016/j.clinbiochem.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Horst RL. Exogenous versus endogenous recovery of 25-hydroxyvitamins D2 and D3 in human samples using high-performance liquid chromatography and the DiaSorin LIAISON Total-D Assay. J Steroid Biochem Mol Biol. 2010;121(1–2):180–182. doi: 10.1016/j.jsbmb.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Bläuer M, Rovio PH, Ylikomi T, Heinonen PK. Vitamin D inhibits myometrial and leiomyoma cell proliferation in vitro. Fertil Steril. 2009;91(5):1919–1925. doi: 10.1016/j.fertnstert.2008.02.136. [DOI] [PubMed] [Google Scholar]
- 31.Arabi A, Rassi RE, El-Hajj Fuleihan G. Hypovitaminosis in developing countries. Nat Rev Endocrinol. 2010;6(10):550–561. doi: 10.1038/nrendo.2010.146. [DOI] [PubMed] [Google Scholar]
- 32.Henn BM, Botigué LR, Gravel S, et al. Genomic ancestry of North Africans supports back-to-Africa migrations. PLoS Genet. 2012;8(1):e1002397. doi: 10.1371/journal.pgen.1002397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sánchez-Quinto F, Botigué LR, Civit S, et al. North African populations carry the signature of admixture with Neandertals. PLoS One. 2012;7(10):e47765. doi: 10.1371/journal.pone.0047765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167(11):1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 35.Lauretani F, Maggio M, Valenti G, Dall’aglio E, Ceda GP. Vitamin D in older population: new roles for this ‘classic actor’? Aging Male. 2010;13(4):215–232. doi: 10.3109/13685538.2010.487551. [DOI] [PubMed] [Google Scholar]
- 36.Al-Hendy A, Salama SA. Ethnic distribution of estrogen receptor-alpha polymorphism is associated with a higher prevalence of uterine leiomyomas in black Americans. Fertil Steril. 2006;86(3):686–693. doi: 10.1016/j.fertnstert.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 37.Csokmay JM, Hill MJ, Maguire M, Payson MD, Fujimoto VY, Armstrong AY. Are there ethnic differences in pregnancy rates in African-American versus white women undergoing frozen blastocyst transfers? Fertil Steril. 2011;95(1):89–93. doi: 10.1016/j.fertnstert.2010.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peiris AN, Bailey BA, Peiris P, Copeland RJ, Manning T. Race and vitamin D status and monitoring in male veterans. J Natl Med Assoc. 2011;103(6):492–497. doi: 10.1016/s0027-9684(15)30363-1. [DOI] [PubMed] [Google Scholar]
- 39.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31(1):48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Rostand SG. Vitamin D, blood pressure, and African Americans: toward a unifying hypothesis. Clin J Am Soc Nephrol. 2010;5(9):1697–1703. doi: 10.2215/CJN.02960410. [DOI] [PubMed] [Google Scholar]
- 41.Grant WB, Peiris AN. Possible role of serum 25-hydroxyvitamin D in black-white health disparities in the United States. J Am Med Dir Assoc. 2010;11(9):617–628. doi: 10.1016/j.jamda.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 42.Halder SK, Goodwin S, Al-Hendy A. Vitamin D exhibits antiestrogenic effects in human uterine leiomyoma cells. Fertil Steril. 2010;94(4):S219–S220. [Google Scholar]
- 43.Halder SK, Osteen KG, Al-Hendy A. 1,25-dihydroxyvitamin D3 regulates steroid hormone functions in human uterine leiomyoma cells. Fertil Steril. 2011;96(3):S149. [Google Scholar]
- 44.Nair S, Al-Hendy A. Adipocytes enhance the proliferation of human leiomyoma cells via TNF-α proinflammatory cytokine. Reprod Sci. 2011;18(12):1186–1192. doi: 10.1177/1933719111408111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marshall LM, Spiegelman D, Manson JE, et al. Risk of uterine leiomyomata among premenopausal women in relation to body size and cigarette smoking. Epidemiology. 1998;9(5):511–517. [PubMed] [Google Scholar]
- 46.Faerstein E, Szklo M, Rosenshein N. Risk factors for uterine leiomyoma: a practice-based case-control study. I. African-American heritage, reproductive history, body size, and smoking. Am J Epidemiol. 2001;153(1):1–10. doi: 10.1093/aje/153.1.1. [DOI] [PubMed] [Google Scholar]
- 47.Nesby-O’Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76(1):187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 48.Meems LM, Cannon MV, Mahmud H, et al. The vitamin D receptor activator paricalcitol prevents fibrosis and diastolic dysfunction in a murine model of pressure overload. J Steroid Biochem Mol Biol. 2012;132(3–5):282–289. doi: 10.1016/j.jsbmb.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Shroff R, Knott C, Rees L. The virtues of vitamin D: but how much is too much? Pediatr Nephrol. 2010;25(9):1607–1620. doi: 10.1007/s00467-010-1499-9. [DOI] [PubMed] [Google Scholar]