Abstract
Contact between telomeres and the fission yeast spindle pole body during meiotic prophase is crucial for subsequent spindle assembly, but the feature of telomeres that confers their ability to promote spindle formation remains mysterious. Here we show that while strains harbouring circular chromosomes devoid of telomere repeat tracts undergo aberrant meiosis with defective spindles, the insertion of a single internal telomere repeat stretch rescues the spindle defects. Moreover, the telomeric overhang-binding protein Pot1 is dispensable for rescue of spindle formation. Hence, an inherent feature of the double-strand telomeric region endows telomeres with the capacity to promote spindle formation.
Keywords: telomere, meiosis, spindle, spindle pole body, fission yeast
INTRODUCTION
Although the classically recognized roles for telomeres centre around their ability to protect chromosome ends from degradation and fusion, telomeres have further and dramatically different roles in meiotic cell cycles. During the early stages of meiosis, all the telomeres in the nucleus cluster within a limited region of the nuclear membrane (NM) to form the ‘bouquet’ structure [1]. This highly conserved chromosomal configuration promotes meiotic homologue pairing by directing the chromosome movements that promote homologue alignment [2], [3,4,5,6,7,8,9]. However, the severity of the meiotic defects precipitated by bouquet disruption far exceeds that conferred by the associated reduction in homologue pairing [10]. The fission yeast bouquet associates with the spindle pole body (SPB; the fungal centrosome-equivalent) via a trans-NM linkage comprising a SUN–KASH protein pair and persists throughout meiotic prophase, a period in which the SPB is pulled back and forth across the cell by cytoplasmic dynein motor-associated microtubules [11,12,13,14]. During this period, the SPB-associated telomeres pull the chromosomes back and forth, generating the elongated nuclear shape coined the ‘horsetail’. At the end of meiotic prophase, horsetail nuclear movement ceases, the telomeres are released and the SPB divides into two spindle organizing centres that insert into the NM before meiosis I (MI). When bouquet formation is compromised, SPB division, NM insertion and spindle formation are aberrant, resulting in monopolar, multiple or unstable spindles and chromosome missegregation at both MI and meiosis II (MII) [10]. Hence, contact between the bouquet and the SPB during meiotic prophase seems to be crucial for the subsequent ability of the SPB to nucleate the spindle.
Telomeric DNA consists of tandemly arranged G/C-rich repeats that stretch for hundreds (in yeasts) to thousands (in mammals) of base pairs out to the ends of chromosomes; a 3′ overhang of the G-rich telomere strand comprises the extreme chromosome end. Telomere proteins, known collectively as shelterin, include factors that bind the double-strand (ds) telomeric repeats or the single-strand (ss) 3′ overhang, as well as bridging proteins that link these two categories. Telomere function during mitotic cell cycles requires an interplay between the ds and ss telomere-binding complexes [15, 16]. This is illustrated by the consequences of disrupting specific components of fission yeast shelterin. Taz1 binds ds telomere repeats while Pot1 binds both the ss overhang and bridging proteins that contact the Taz1 complex; both Taz1 and Pot1 are conserved in mammals. In the absence of Taz1, telomere length control goes awry and protection from the nonhomologous end-joining pathway is lost, leading to lethal telomere fusions if cells are arrested in the G1 phase in which nonhomologous end-joining pathway levels are high enough to constitute a threat [17, 18]. In these taz1Δ cells, Pot1 recruitment via the ds telomere-binding complex is gone but Pot1 still binds telomeres, presumably via the 3′ overhang, which persists in the absence of Taz1. Loss of Pot1 leads to a different fate-rampant degradation of the 5′ telomeric strand and in turn, loss of the ds telomere-binding complex and loss of the entire telomere; survival of pot1+ deletion occurs only through circularization of each of the three telomere-less chromosomes [19, 20].
During mitotic interphase, centromeres cluster at the SPB while telomeres localize to 2–4 clusters at the NM distal to the SPB [21]. On induction of meiosis, the meiosis-specific Bqt1/Bqt2 complex recruits the SUN-domain inner NM protein Sad1 (and the associated KASH-domain outer NM protein Kms1) to telomeres by binding the Taz1-interacting protein Rap1 [22,23,24]. Formation of this Taz1–Rap1–Bqt1/2–Sad1–Kms1 linkage triggers the movement of Sad1-associated telomeres to the SPB and instigates bouquet formation. Deletion of taz1+, rap1+, bqt1+ or bqt2+ disrupts this process, destroying the bouquet and along with it, meiotic spindle formation [10].
The observation that telomeric contact has such a prominent influence on meiotic spindle formation raises several fundamental questions; for instance, what aspect of the clustered telomeres bestows their ability to control spindle formation? The telomere bouquet has several features that might be important, including the associated complexes of shelterin proteins, the unique position of telomeres at chromosome ends or their association in a cluster. Moreover, the bouquet could be envisioned to transduce the frictional drag of the attached chromosomes into a powerful mechanical force on the SPB. A clue regarding the bouquet feature relevant to promoting spindle formation was provided by examining cells in which Taz1 is replaced by a mutant protein (Taz1-A606V) that fails to bind DNA [3] (A. Deshpande and JPC, unpublished data). During meiotic prophase, Taz1-A606V is recruited to the SPB but telomeres themselves are left behind. In such cells, SPB division and spindle formation fail in a manner reminiscent of the defects in cells lacking the bouquet [10]; hence, contact with telomere proteins unconnected to telomeric chromatin cannot trigger proper SPB and spindle behaviour, refining our conception of the relevant telomeric property to include some feature(s) of the telomeric nucleoprotein assemblage. Here, we exploit the unusual ability of fission yeast lacking telomerase to survive through chromosome circularization to strip cells of telomeres, rebuild specific partial telomeric features and further pinpoint the requirements for control of spindle formation.
RESULTS AND DISCUSSION
To address which feature(s) of the bouquet confers its impact on meiotic spindle assembly, we utilized fission yeast strains in which each chromosome has lost its telomeres and circularized [25]. Such strains provide a unique baseline condition for bouquet disruption, as all the components required for bouquet formation (telomere-binding proteins and proteins that link them to the SPB) are present except the telomeres themselves. To obtain these strains, we deleted the gene encoding the catalytic subunit of telomerase, Trt1, and raised survivors under conditions in which the relatively sick circular chromosome-containing survivors (hereafter referred to as ‘circular strains’) can grow without being overtaken by alternative faster-growing trt1Δ survivor types [26].
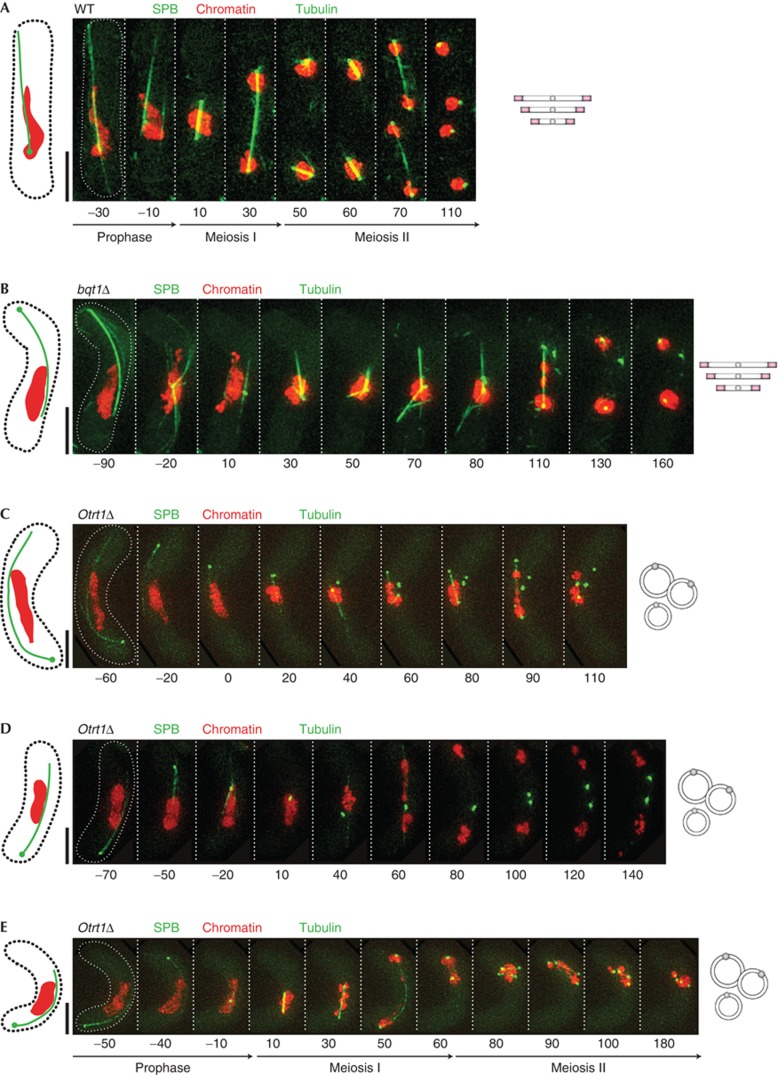
Although haploid circular strains are fully capable of mating to form diploid zygotes, they show meiotic abnormalities and poor spore viability [27]. Such meiotic problems might be expected on the basis of at least two conspicuous defects, the lack of bouquet formation and the likelihood that recombination between two circular chromosomes will result in a lethally dicentric di-chromosome circle. Live analysis of meiosis reveals that the SPB traverses the zygote repeatedly in strains containing circular chromosomes just as it does in wild-type (wt) linear chromosome-containing strains (hereafter referred to as ‘linear strains’) as well as bqt1Δ linear strains (Fig 1A–E); hence, the bouquet is dispensable for SPB oscillations. Together with the absence of a linkage between chromosomes and the SPB, the elongated horsetail chromatin configuration fails to materialize despite the continued oscillation of the SPB. Correspondingly, circular strains encounter defects in SPB division and spindle formation during MI and MII that mirror those seen in linear strains lacking the bouquet. In wt cells, the SPB divides neatly into two equally intense SPB signals at MI and four in MII, in each case separating in a symmetric manner and nucleating spindles that span the MI and MII nuclei (Fig 1A). In contrast, the SPB of circular strains often fails to show this symmetric separation and appears to dislodge from the nucleus; in this scenario, monopolar, unstable or multiple spindles are observed (Fig 1C,D and data not shown). The SPB and spindle phenotypes are incompletely penetrant (see, for example, Fig 1E, in which bipolar meiotic spindle formation occurs properly in a circular strain), being seen in only ∼55% of meioses in the circular chromosome setting, again recapitulating the effects of bouquet abolition in linear strains [10].
Figure 1.
Defective meiotic spindle formation in circular strains. Series of frames from films of strains carrying endogenously tagged Sid4 (SPB) and Hht1 (chromatin) along with expressed tagged Atb2 (tubulin). (A) Wt meiosis. (B) In bqt1Δ cells, SPBs fail to separate and a monopolar spindle forms at MI; at MII, only one spindle forms. (C–E) Examples of meiosis in circular trt1Δ cells, showing instances of monopolar spindle formation at MI (C), spindle formation failure (D) and fairly normal bipolar spindle formation (E). Quantitation is shown in supplementary Fig S3 online and Fig 2D. MI, meiosis I; MII, meiosis II; SPB, spindle pole body; Wt, wild-type.
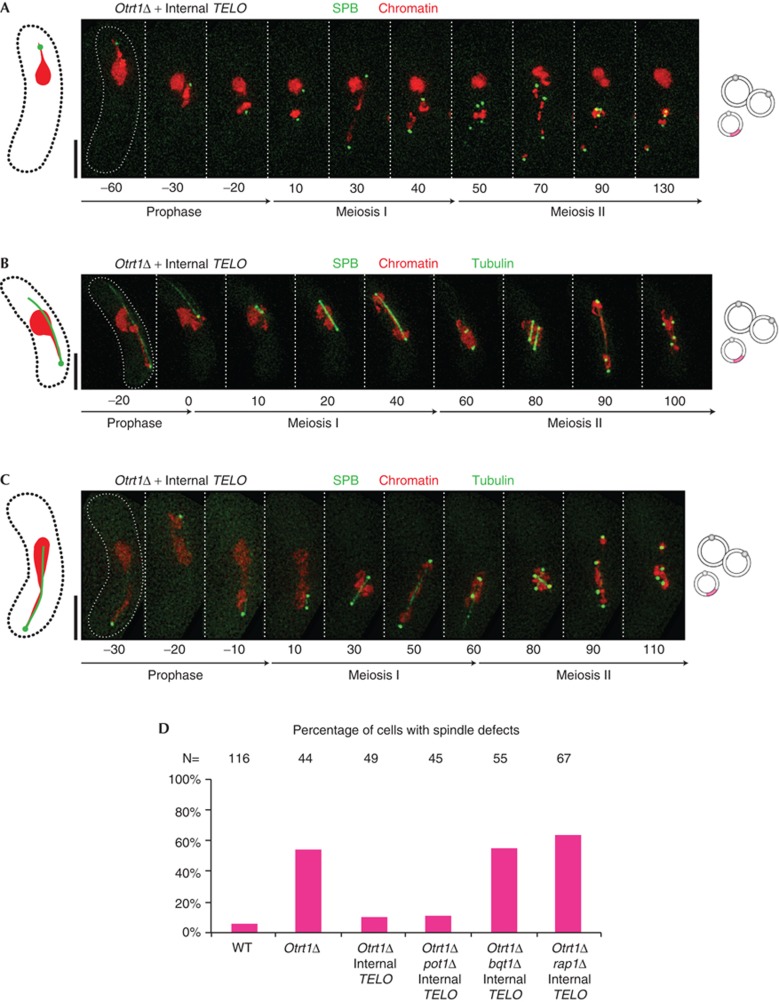
These circular strains lacking the bouquet provide an opportunity to re-create specific features of telomeres and ask whether these features confer the ability to promote proper SPB division and spindle formation. To embark on such an analysis, we introduced a single synthetic internal telomere stretch of 500 bp at the ura4+ locus on Chromosome III (Chr III) [28]. Although no specific sites of Taz1 localization (as viewed in live cells with a carboxy-terminal YFP tag inserted at the endogenous taz1+ locus) can be detected in mitotically growing circular strains, those harbouring the internal telomere stretch show a single clear Taz1 focus in each nucleus (supplementary Fig S1 online), confirming that these internal telomere tracts recruit the ds telomere-binding complex. Moreover, the internally placed telomere stretches successfully associate with the SPB during meiosis (supplementary Fig S2A online). Likewise, the oscillating SPB can be seen to pull a fraction of the chromatin back and forth during the horsetail stage by means of a Taz1–SPB association (Fig 2A–C). Remarkably, SPB division and separation (Fig 2A; supplementary Fig S3 online) as well as spindle formation (Fig 2B–D) appear normal in nearly all instances of meiosis in circular strains harbouring the internal telomere stretch. The SPBs divide into two and then four clearly discernable foci at MI and MII, respectively, and each pair of separated SPBs organizes a stable spindle that pulls chromatin to the respective poles (Fig 2B,C). Notably, the spindles frequently fail to capture all the chromosomes, indicating that although the single internal telomere rescues the ability of the bouquet to confer bipolar spindle formation, it does not rescue the defects of bouquet-deficient cells in achieving perfect meiotic chromosome–spindle attachment (M. Klutstein, A. Fennell and JPC, in preparation). Examples of prophase nuclei in which the chromosome harbouring the internal telomere becomes entangled with other chromosomes are seen (Fig 2B,C), as are examples in which the SPB-associated chromosome remains largely unassociated with other chromosomes (Fig 2A). As expected, the ability of the internal telomere stretch to rescue spindle formation depends completely on factors known to be required for bouquet formation, Bqt1 and Rap1 (Fig 2D; supplementary Figs S2D,S4 online).
Figure 2.
Meiotic SPB and spindle defects in circular strains are rescued by introduction of a single internal telomere stretch. Colour designations are as in Fig 1; additional pink patches on chromosome diagrams represent inserted telomere repeat stretches. (A) Example showing SPB and chromatin. One chromosome (most probably that carrying the internal telomere; see supplementary Figs S1,S2 online) contacts the SPB. In this case, only the SPB-contacting chromosome segregates; the others fail to segregate despite proper SPB division. (B,C) Examples showing SPB, spindle and chromatin. Bipolar spindles form at both MI and MII; see text for discussion of their parallel and perpendicular arrangements at MII. (D) Quantitation of spindle defects. A cell is scored as having proper spindle formation if bipolar spindles, flanked by separating SPBs and pulling chromatin towards the respective poles, are seen at both MI and MII. MI, meiosis I; MII, meiosis II; SPB, spindle pole body.
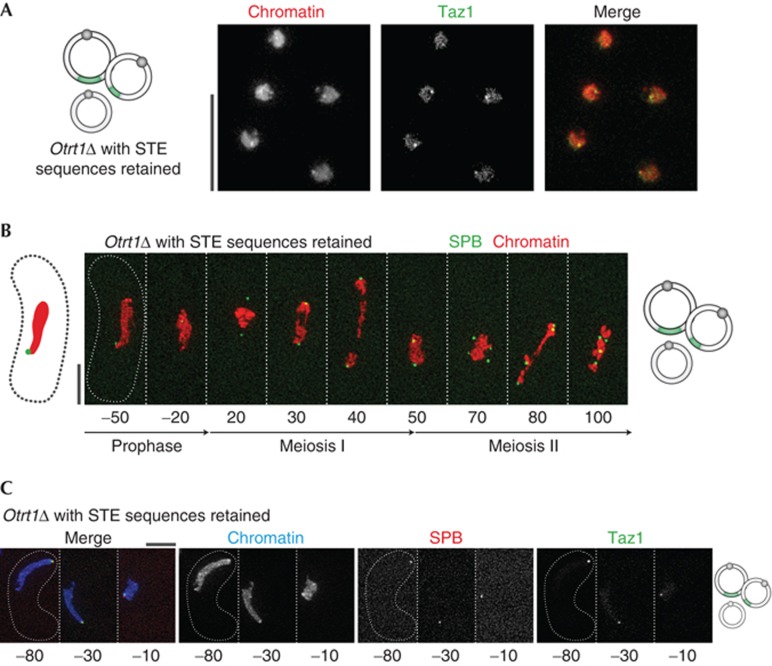
Two classes of circular strains have been characterized, Types A and B, in which subtelomeric sequences located ∼5 kb proximal to wt telomeres are retained or deleted in the circularized chromosomes, respectively; the former sustain Taz1 binding to the residual subtelomeric sequences, putatively through epigenetic maintenance of a Taz1 complex derived from the corresponding wt telomeres preceding trt1+ deletion [29]. These Taz1-bound subtelomeres associate with meiotic SPBs, while Type B circulars appear devoid of bound Taz1 and fail to confer subtelomere–SPB association ([29], Fig 3). Accordingly, bipolar spindle formation occurs properly in Type A, but not Type B, circular strains (Fig 3). Hence, like the contact between a single internal telomere stretch (which is inserted into Chr III of a Type B survivor) and the SPB, the residual bouquet formation in Type A survivors is sufficient to confer proper SPB behaviour and spindle formation even in the absence of canonical telomere repeat sequences. These data also indicate that Trt1 is dispensable for meiotic spindle regulation.
Figure 3.
Circular strains retaining subtelomeric sequences that bind Taz1 show proper meiotic SPB behaviour. Green patches on chromosome diagrams represent retained subtelomeric sequences. (A) When extensive subtelomeric sequences are retained at the fusion points of circular chromosomes [29], Taz1 binding, meiotic bouquet formation and proper meiotic SPB division and separation occur (B,C). Chromatin is viewed through endogenous tagging of one copy of the gene encoding histone H3 (see Methods). SPB, spindle pole body.
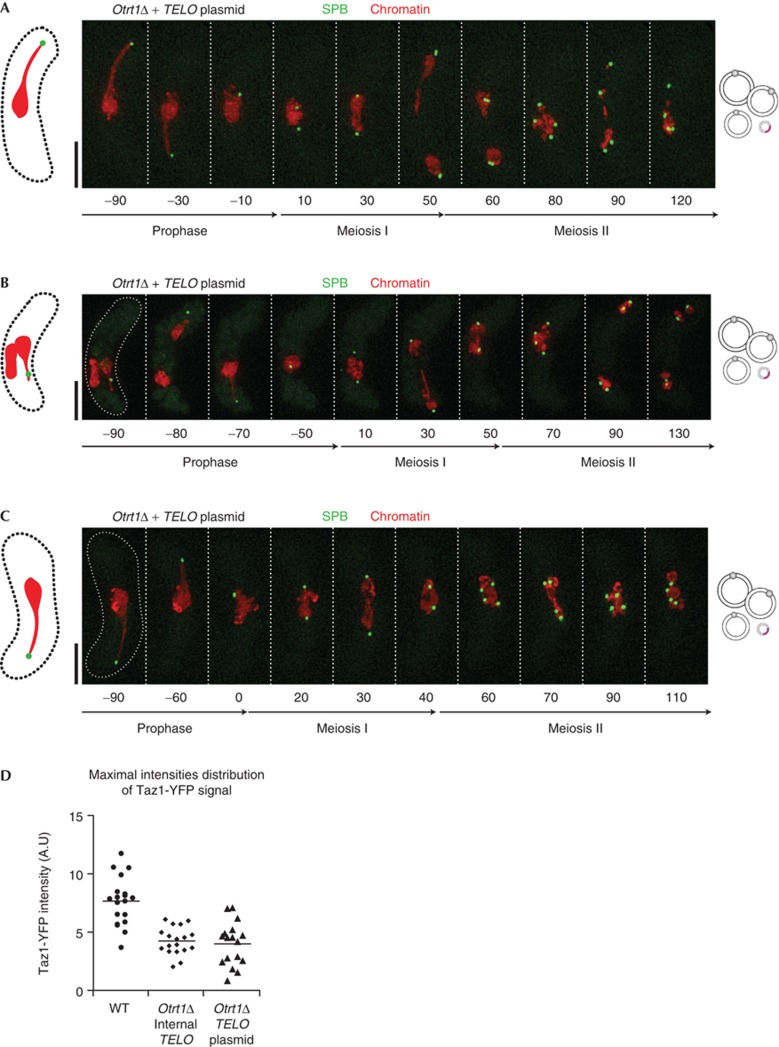
The ability of a telomere stretch inserted within a circular chromosome to confer proper SPB division and spindle formation argues against the idea that SPB regulation requires the immense mechanical force that might be transduced by the simultaneous end-on attachment of all chromosomal telomeres to the SPB. Nonetheless, the internal telomere does connect the SPB with an entire 3.5-Mb chromosome. Hence, the foregoing observations prompted us to wonder whether a telomere stretch embedded within a small (6.9-kb) plasmid would be endowed with SPB regulatory function. To investigate this, we introduced such a plasmid into circular strains devoid of any chromosomal telomeric repeat stretches. As expected, Taz1 localizes to the telomere stretch on the plasmid and a small fraction of cellular chromatin can be seen to traverse the cell along with the SPB during the horsetail stage (Fig 4A–C; supplementary Fig S2 online). Notably, the intensities of the Taz1-YFP foci that appear at the single internal chromosomal telomere stretch are similar to those seen at the plasmid-borne telomeres and considerably lower than those generated by the bona fide bouquets of wt cells (Fig 4D). Note that the telomere stretches inserted in either Chr III or the plasmid are longer than endogenous telomeres and comprise sequences optimized for Taz1 binding (Miller et al [28]; see Methods); therefore, the Taz1-YFP intensity at each inserted telomere stretch exceeds that at a single natural telomere. Hence, the copy number of the telomere-containing plasmid appears to be roughly one per haploid genome content in these circular strains. Remarkably, all zygotes harbouring this telomere-containing plasmid show full competence in SPB division and separation, recapitulating the full rescue of SPB/spindle behaviour that emerges from insertion of a telomere stretch within a bona fide chromosome (Fig 4A–C; supplementary Fig S3 online).
Figure 4.
Introduction of a plasmid containing a telomere stretch rescues meiotic SPB and spindle defects in Otrt1Δ cells. Colour designations are as in Fig 1. (A–C) Examples of meiosis in cells lacking chromosomal telomeres but harbouring a 6.9-kb plasmid with a 500-bp internal telomere stretch. Although the level of chromosomal entanglement varies between films, the SPB divides and separates into two and then four foci of equal intensity at MI and II, respectively, in all cases. Quantitation is shown in supplementary Fig S3 online. (D) Telomere densities at the SPB were monitored through the intensity of Taz1-YFP foci. The near equivalence of Taz1-YFP intensity in circular strains harbouring a chromosomally inserted or plasmid-borne telomere stretch suggests that the plasmid is present at approximately one copy per haploid genome. MI, meiosis I; MII, meiosis II; SPB, spindle pole body.
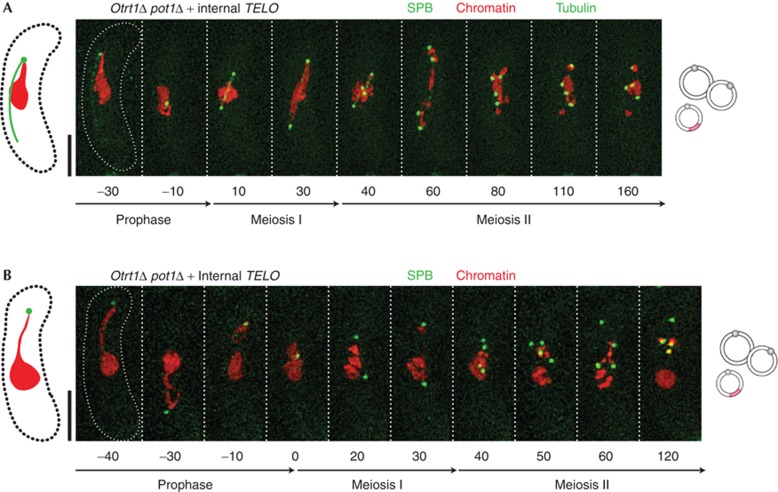
The foregoing results show that the telomere repeats contacting the SPB need not encompass any feature unique to a chromosome end (for example, a 3′ ss overhang) to confer proper SPB division and spindle formation. At first glance this suggests that unlike the chromosome end-protection functions of telomeres, their SPB regulatory function does not require the interplay of ss and ds telomere-binding complexes. However, the ss overhang-binding protein Pot1 is also known to localize to telomeres through interactions with the Taz1 complex and therefore associates with internal telomere stretches [30], leaving open the possibility that Pot1 participates in meiotic SPB control. The role of Pot1 in bouquet formation and meiosis cannot be readily assessed in linear chromosome-containing strains as pot1+ deletion leads to immediate telomere loss and chromosome circularization [20], abolishing the cell’s ability to form a bouquet. Against this backdrop, our observation that the SPB regulatory function of the bouquet can be fully realized by a single internal telomere provides a unique opportunity for assessing Pot1 function in bouquet formation and SPB regulation. Accordingly, we deleted pot1+ in circular strains harbouring the internal telomere and induced mating and meiosis. The resulting pot1Δ/Δzygotes show proper SPB separation and spindle formation indistinguishable from their pot1+/+ counterparts (Fig 5; supplementary Fig S2 online), indicating that Pot1 is dispensable for bouquet formation and the SPB regulatory function of telomeres.
Figure 5.
Pot1 is dispensable for meiotic telomere–SPB association and spindle formation. Colour designations are as in Fig 1. Rescue of SPB division (A,B) and spindle formation (A) by internal telomere stretches is independent of Pot1. Quantitation is shown in Fig 2D and supplementary Fig S3 online. SPB, spindle pole body.
The robust rescue of spindle formation by internal telomere stretches afforded the chance to consider wider questions of why meiosis generally fails in cells harbouring circular chromosomes. In those bouquet-deficient linear strains that manage to form proper spindles, chromosome segregation generally proceeds with high fidelity [10]. In contrast, the aberrant meiotic chromosome segregation patterns seen in circular strains are retained even when spindle formation is rescued by the internal telomere (Figs 2, 3, 4, 5). Striking and common phenotypes include the formation of two MII spindles at approximately right angles to each other resulting in a crossed-spindle appearance (Fig 2C), or two MII spindles that remain parallel but closely apposed within the nuclear mass. In both cases, the spindles elongate during anaphase, stretching the chromatin mass, but the SPBs suddenly pop back within the collapsed chromatin mass on spindle disassembly at telophase. Both of these patterns probably stem from entangled circular chromosomes, the bulk of which remain entwined despite the attachment of their respective kinetochores to spindles. We surmised that the severity of this chromosome entanglement would be a result of meiotic recombination; if so, abolition of recombination should confer a nuclear division pattern more closely resembling that of linear strains. To test this idea, we deleted the gene encoding Rec12, the Type II DNA topoisomerase responsible for the DNA ds breaks that trigger all meiotic recombination events [31]. Inspection of rec12Δ meiosis in those circular chromosome-containing zygotes that form bipolar spindles reveals substantial rescue of the chromosome segregation defects. Although the lack of meiotic recombination in the absence of Rec12 confers unequal chromosome segregation, entangled masses of chromatin are much less apparent in rec12Δ circulars than in rec12+ circulars, as are crossed and parallel spindles (supplementary Fig S5 online). Hence, meiotic recombination is indeed an important instigator of aberrant chromosome segregation patterns in circular strains. These observations speak to the ability of elongating spindles to stretch the entangled circular chromosomes and conversely, to the force exerted by these entangled chromosomes on the SPB and NM once the spindle microtubules depolymerize.
The ability of single internal telomere stretches to confer proper spindle formation puts useful constraints on models for how telomeric contact influences the SPB’s ability to nucleate spindles. We can dispense with the idea that telomere clustering per se, that is, the collection of multiple telomeres with their associated proteins at a single site, is required for SPB control. We can also rule out the possibility that the collective frictional drag produced by simultaneous end-on association of all chromosomes to the SPB is the relevant bouquet feature for SPB control, as the drag exerted by anchoring an internal segment of a 6.9-kb plasmid should be vanishingly small compared with that of the bona fide bouquet. Indeed, the ability of internal telomere stretches to rescue spindle formation despite the pulling forces generated by entangled circular chromosomes (evinced by the inward SPB propulsion seen on spindle disassembly; see Figs 2B,C, 4A–C) suggests that SPB/spindle defects in the absence of the bouquet might not be a result of these pulling forces. It is also worth noting that centromeres are absent from the plasmids harbouring internal telomere stretches, rendering untenable a model involving pulling forces generated by two points of chromosome attachment to fixed structures—the telomeres at the SPB and the centromeres elsewhere on the NM. Finally, we note that the SPB remains associated with the NM throughout the horsetail stage with or without the bouquet; only at the onset of MI does the SPB–NM association become tenuous in a bouquet-defective setting [10]. Rather than a purely mechanical effect on the SPB, contact with the bouquet or with the internal telomere stretch might trigger a chemical or conformational alteration in the SPB or in NM factors that control the SPB. Such alterations are clearly generated by telomeres in a Pot1-independent manner. The device of manipulating the properties of this internal telomere stretch—for instance, its size or chromatin composition—should yield further insights into the mechanism by which chromosomes regulate their own transport vehicles during nuclear division.
METHODS
Taz1-YFP quantitation was performed with Volocity software (Improvision) on deconvolved 3D movies projected in 2D images using the Sum Intensity setting of SoftWoRx (Applied Precision). Microscope and experimental settings were kept rigorously identical for each experiment compared. During each prophase time point, the intensity of the area containing Taz1-YFP signal at the SPB was quantified, as was a signal-free region of equal dimensions within the same cell (‘area background’). To normalize for the inherent variability between cells and experiments, the average intensity for one pixel of background outside the cell (average background) was calculated for every time point. Taz1-YFP signal intensity for each time point is calculated as (Taz1-YFP signal area–area background)/average background.
All scale bars (to the left of each row, or atop the −10 panel in the merged image in Fig 3C) represent 5 μm.
Further methods and reagents are described in supplementary information online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank Mike Dresser, Ofer Rog and our lab members for discussions. We are also grateful to Yuan Zhao for help with pot1Δ strain construction. This work was supported by the European Research Council and Cancer Research UK.
Footnotes
The authors declare that they have no conflict of interest.
References
- Scherthan H (2006) Meiotic telomeres. In Telomeres de Lange T, Lundblad V, Blackburn EH (eds), 2nd edn, pp 225–259Cold Spring Harbor: Cold Spring Harbor Laboratory Press, [Google Scholar]
- Ding DQ, Yamamoto A, Haraguchi T, Hiraoka Y (2004) Dynamics of homologous chromosome pairing during meiotic prophase in fission yeast. Dev Cell 6: 329–341 [DOI] [PubMed] [Google Scholar]
- Nimmo ER, Pidoux AL, Perry PE, Allshire RC (1998) Defective meiosis in telomere-silencing mutants of Schizosaccharomyces pombe. Nature 392: 825–828 [DOI] [PubMed] [Google Scholar]
- Cooper JP, Watanabe Y, Nurse P (1998) Fission yeast Taz1 protein is required for meiotic telomere clustering and recombination. Nature 392: 828–831 [DOI] [PubMed] [Google Scholar]
- Niwa O, Shimanuki M, Miki F (2000) Telomere-led bouquet formation facilitates homologous chromosome pairing and restricts ectopic interaction in fission yeast meiosis. Embo J 19: 3831–3840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat JJ, Kim KP, Koszul R, Zanders S, Weiner B, Kleckner N, Alani E (2008) Csm4, in collaboration with Ndj1, mediates telomere-led chromosome dynamics and recombination during yeast meiosis. PLoS Genet 4: e1000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad MN, Dominguez AM, Dresser ME (1997) Ndj1p, a meiotic telomere protein required for normal chromosome synapsis and segregation in yeast. Science 276: 1252–1255 [DOI] [PubMed] [Google Scholar]
- Lee CY, Conrad MN, Dresser ME (2012) Meiotic chromosome pairing is promoted by telomere-led chromosome movements independent of bouquet formation. PLoS Genet 8: e1002730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua PR, Roeder GS (1997) Tam1, a telomere-associated meiotic protein, functions in chromosome synapsis and crossover interference. Genes Dev 11: 1786–1800 [DOI] [PubMed] [Google Scholar]
- Tomita K, Cooper JP (2007) The telomere bouquet controls the meiotic spindle. Cell 130: 113–126 [DOI] [PubMed] [Google Scholar]
- Chikashige Y, Ding DQ, Funabiki H, Haraguchi T, Mashiko S, Yanagida M, Hiraoka Y (1994) Telomere-led premeiotic chromosome movement in fission yeast. Science 264: 270–273 [DOI] [PubMed] [Google Scholar]
- Miki F, Kurabayashi A, Tange Y, Okazaki K, Shimanuki M, Niwa O (2004) Two-hybrid search for proteins that interact with Sad1 and Kms1, two membrane-bound components of the spindle pole body in fission yeast. Mol Genet Genomics 270: 449–461 [DOI] [PubMed] [Google Scholar]
- Ding DQ, Chikashige Y, Haraguchi T, Hiraoka Y (1998) Oscillatory nuclear movement in fission yeast meiotic prophase is driven by astral microtubules, as revealed by continuous observation of chromosomes and microtubules in living cells. J Cell Sci 111: Part 6701–712 [DOI] [PubMed] [Google Scholar]
- Vogel SK, Pavin N, Maghelli N, Jülicher F, Tolic-Nørrelykke IM (2009) Self-organization of dynein motors generates meiotic nuclear oscillations. PLoS Biol 7: e1000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehe PM, Cooper JP (2010) Fission yeast telomeres forecast the end of the crisis. FEBS Lett 584: 3725–3733 [DOI] [PubMed] [Google Scholar]
- Jain D, Cooper JP (2010) Telomeric strategies: means to an end. Annu Rev Genet 44: 243–269 [DOI] [PubMed] [Google Scholar]
- Ferreira MG, Cooper JP (2001) The fission yeast Taz1 protein protects chromosomes from Ku-dependent end-to-end fusions. Mol Cell 7: 55–63 [DOI] [PubMed] [Google Scholar]
- Ferreira MG, Cooper JP (2004) Two modes of DNA double-strand break repair are reciprocally regulated through the fission yeast cell cycle. Genes Dev 18: 2249–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt CW, Cooper JP (2010) Pot1 inactivation leads to rampant telomere resection and loss in one cell cycle. Nucleic Acids Res 38: 6968–6975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P, Cech TR (2001) Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292: 1171–1175 [DOI] [PubMed] [Google Scholar]
- Funabiki H, Hagan I, Uzawa S, Yanagida M (1993) Cell cycle-dependent specific positioning and clustering of centromeres and telomeres in fission yeast. J Cell Biol 121: 961–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikashige Y, Hiraoka Y (2001) Telomere binding of the Rap1 protein is required for meiosis in fission yeast. Curr Biol 11: 1618–1623 [DOI] [PubMed] [Google Scholar]
- Kanoh J, Ishikawa F (2001) spRap1 and spRif1, recruited to telomeres by Taz1, are essential for telomere function in fission yeast. Curr Biol 11: 1624–1630 [DOI] [PubMed] [Google Scholar]
- Chikashige Y, Tsutsumi C, Yamane M, Okamasa K, Haraguchi T, Hiraoka Y (2006) Meiotic proteins bqt1 and bqt2 tether telomeres to form the bouquet arrangement of chromosomes. Cell 125: 59–69 [DOI] [PubMed] [Google Scholar]
- Nakamura TM, Cooper JP, Cech TR (1998) Two modes of survival of fission yeast without telomerase. Science 282: 493–496 [DOI] [PubMed] [Google Scholar]
- Jain D, Hebden AK, Nakamura TM, Miller KM, Cooper JP (2010) HAATI survivors replace canonical telomeres with blocks of generic heterochromatin. Nature 467: 223–227 [DOI] [PubMed] [Google Scholar]
- Naito T, Matsuura A, Ishikawa F (1998) Circular chromosome formation in a fission yeast mutant defective in two ATM homologues. Nat Genet 20: 203–206 [DOI] [PubMed] [Google Scholar]
- Miller KM, Rog O, Cooper JP (2006) Semi-conservative DNA replication through telomeres requires Taz1. Nature 440: 824–828 [DOI] [PubMed] [Google Scholar]
- Sadaie M, Naito T, Ishikawa F (2003) Stable inheritance of telomere chromatin structure and function in the absence of telomeric repeats. Genes Dev 17: 2271–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi T, Kanoh J, Saito M, Ishikawa F (2008) Fission yeast Pot1-Tpp1 protects telomeres and regulates telomere length. Science 320: 1341–1344 [DOI] [PubMed] [Google Scholar]
- Sharif WD, Glick GG, Davidson MK, Wahls WP (2002) Distinct functions of S. pombe Rec12 (Spo11) protein and Rec12-dependent crossover recombination (chiasmata) in meiosis I; and a requirement for Rec12 in meiosis II. Cell Chromosome 1: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.