Abstract
It is generally assumed that antagonists of Gs-coupled receptors do not activate cAMP signalling, because they do not stimulate cAMP production via Gs-protein/adenylyl cyclase activation. Here, we report a new signalling pathway whereby antagonists of β1-adrenergic receptors (β1ARs) increase cAMP levels locally without stimulating cAMP production directly. Binding of antagonists causes dissociation of a preformed complex between β1ARs and Type-4 cyclic nucleotide phosphodiesterases (PDE4s). This reduces the local concentration of cAMP-hydrolytic activity, thereby increasing submembrane cAMP and PKA activity. Our study identifies receptor/PDE4 complex dissociation as a novel mechanism of antagonist action that contributes to the pharmacological properties of β1AR antagonists and might be shared by other receptor subtypes.
Keywords: cAMP, cyclic nucleotide phosphodiesterase, PDE, β-adrenergic receptor, antagonist
Introduction
In the classical model of Gs protein-coupled receptor (GsPCR) activation, the unoccupied receptor resides in an ‘inactive’ state that does not induce G protein activation. Binding of an agonist causes conformational changes that stabilize the receptor in the ‘active’ state that promotes interaction with, and activate, Gαs. In this two-state model, an antagonist is defined as a ligand that can bind to the receptor without promoting the conformation required for Gs activation. Therefore, an antagonist might prevent binding and signalling of an agonist, but does not trigger a biological response per se. However, it is now well established that the two-state model is an oversimplification that does not accurately describe GsPCR function [1, 2]. GPCRs might interact with more than one type of G protein and might induce G protein-independent signalling events. The β2AR, for example, induces biological effects via activation of Gs protein, Gi protein as well as via β-arrestin [1, 2, 3]. Importantly, it is now established that specific receptor conformations show distinct efficacies in the activation of the different signalling mechanisms. As an extension of this concept, receptor ligands can be biased in that they stabilize conformations of the receptors that show distinct activities towards downstream signalling pathways [2, 3]. A βAR ligand might function antagonistically on receptor signalling via Gs but as an agonist of β-arrestin signalling. Despite this complexity, antagonists to the βAR receptors are generally thought to reduce cAMP signalling, because they do not induce Gs coupling of the receptor per se, but might lower basal and agonist-stimulated activity of the receptor.
We have shown previously that the β1-adrenergic receptor (β1AR) forms a signalling complex with a Type-4 cyclic nucleotide phosphodiesterase (PDE4) and that occupancy of the receptor by an agonist induces dissociation of the β1AR/PDE4 complex [4]. This regulation of the β1AR/PDE4 complex differs from that of the related β2AR, which is characterized by a β-arrestin-dependent recruitment of PDE4 to the occupied receptor [5], and differential interaction with PDEs might contribute to the distinct physiological roles of β1AR and β2AR, for instance in the regulation of cardiac excitation–contraction coupling.
Here, using several independent approaches, we provide evidence that antagonist occupancy of the β1AR receptor triggers a previously undetected signal at the cell membrane by promoting the release of a PDE4. This release results in a local increase in cAMP levels and activation of PKA in the proximity of the receptor without a concurrent stimulation of cAMP synthesis via the classical Gs/adenylyl cyclase (AC) pathway by the antagonist-occupied β1AR itself. These local effects are in opposition to the dampening effect of β1AR antagonists on bulk cellular cAMP and global PKA activation. Our findings might explain some observations on the pharmacological effects of β1AR antagonists.
Results and discussion
Antagonists dissociate β1AR/PDE4 complexes
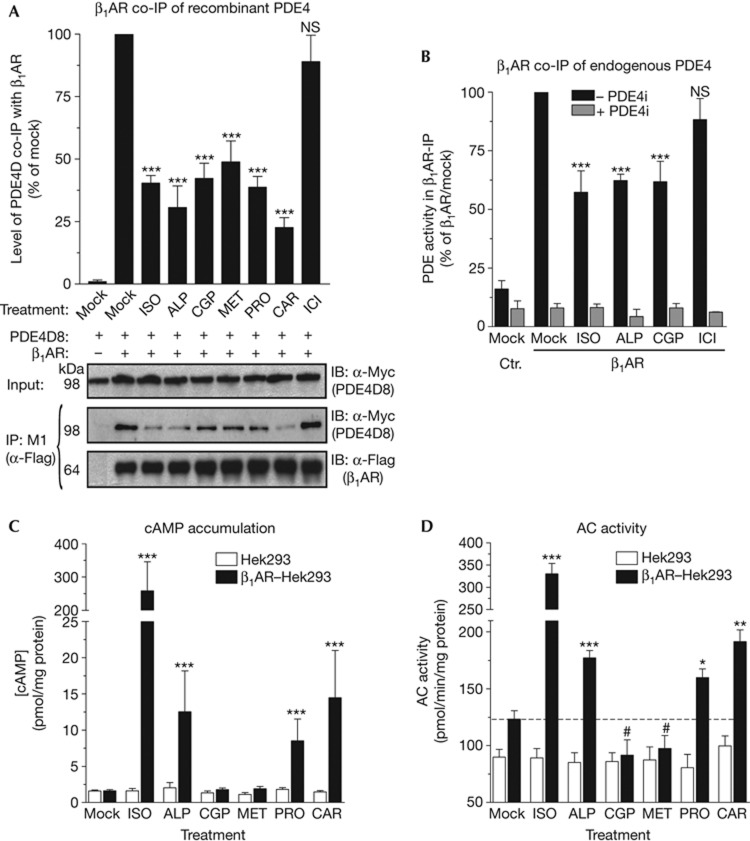
To study the dynamics of β1AR/PDE4 association/dissociation, we tested a series of β-adrenergic ligands for their effect on the interaction between recombinant PDE4D8 and β1AR. Unexpectedly, we found that not only agonists, but also treatment with various β1AR antagonists, including alprenolol (ALP), CGP-20712A (CGP), metoprolol (MET), propranolol (PRO) and carvedilol (CAR) (Fig 1A), induced β1AR/PDE4 complex dissociation. Conversely, the β2AR-selective antagonist ICI-118551 (ICI) had no effect.
Figure 1.
β1AR antagonists dissociate the β1AR/PDE4 complex. (A) Hek293 cells expressing Flag-tagged β1AR and Myc-tagged PDE4D8 were pretreated for 5 min with the βAR agonist ISO (10 μM), the β1AR antagonists ALP, CGP, MET, PRO and CAR, or the β2AR-antagonist ICI (1 μM each) and then lysed and subjected to IP of β1AR. The amount of exogenous PDE4D8 recovered in the IP pellets is quantified. (B) Parental or Flag-β1AR-expressing Hek293 cells were treated for 5 min with ISO (10 μM), ALP and CGP or ICI (each at 1 μM) before cell lysis and β1AR-IP. Shown is the amount of endogenous cAMP-PDE activity recovered in β1AR-IP pellets measured in the absence (− PDE4i) or presence (+ PDE4i) of the PDE4-selective inhibitor rolipram (10 μM). (C) cAMP accumulation by parental or β1AR-expressing Hek293 cells treated for 5 min with ISO (100 nM), or with various β1AR antagonists (each at 1 μM) measured by RIA. (D) Adenylyl cyclase (AC) activity in membrane preparations from parental or β1AR-expressing Hek293 cells measured in the presence or absence of β1AR antagonists (each at 1 μM) or ISO (100 nM). Experiments were performed in the presence of the β2AR-selective antagonist ICI (1 μM) to block signalling of endogenous β2ARs. Data are the mean±s.e.m. of at least three experiments. NS (not significant; P>0.05); *(P<0.05); **(P<0.01); ***(P<0.001); #(significantly decreased; P<0.05). ALP, alprenolol; CAR, carvedilol; CGP, CGP-20712A; ICI, ICI-118551; IP, immunoprecipitation; ISO, isoproterenol; MET, metoprolol; NS, not significant; PDE4, type-4 cyclic nucleotide phosphodiesterase; PRO, propranolol; RIA, radioimmunoassay; β1AR, β1-adrenergic receptor.
When expressed in Hek293 cells, recombinant β1ARs also form complexes with endogenous PDE4 (Fig 1B). Treatment of β1AR-Hek293 cells with βAR-antagonists ALP, CGP or ICI did not affect total PDE or PDE4 activity in cell extracts (supplementary Fig S1 online). Conversely, treatment with ALP, CGP or the β1AR-agonist isoproterenol (ISO), but not the β2AR antagonist ICI, significantly reduced the amount of PDE4 activity associated with the β1AR. This finding confirms a ligand-dependent dissociation of β1AR complexes with endogenous PDE4 similar to that observed with the β1AR/PDE4D8 overexpression system (Fig 1A). The efficacy of antagonists in this signalling mechanism suggests that β1AR/PDE4 complex dissociation is induced regardless of whether or not β1AR ligands promote receptor interaction with a Gs protein. In addition, antagonist-dependent β1AR/PDE4 complex dissociation does not require β-arrestin recruitment as shown in supplementary Fig S2 online.
CGP and MET do not stimulate cAMP production
Next, we probed the effect of antagonist-dependent β1AR/PDE4 complex dissociation on cAMP signalling. Treatment of β1AR-Hek293 cells with PRO, ALP and CAR triggered a significant increase in global cellular cAMP as measured by radioimmunoassay (RIA) (Fig 1C). This increase is due to partial agonist activity of these compounds at this concentration as confirmed by their ability to stimulate AC activity (Fig 1D). Conversely, CGP and MET did not stimulate global cAMP levels (Fig 1C), suggesting that they do not promote receptor coupling to Gs. Indeed, and in line with previous reports [6], CGP and MET act as inverse agonists and lower basal activity of the receptor as reflected by reduced basal AC activity (Fig 1D). Thus, CGP and MET are suitable β1AR-selective ligands to probe the effect of PDE4 displacement on cAMP levels because they do not stimulate cAMP production via Gs protein/AC activation. Fig 1C also demonstrates that dissociation of β1AR/PDE4 signalling complexes does not affect global cAMP levels because CGP and MET do not increase total cAMP.
Complex dissociation augments local cAMP levels
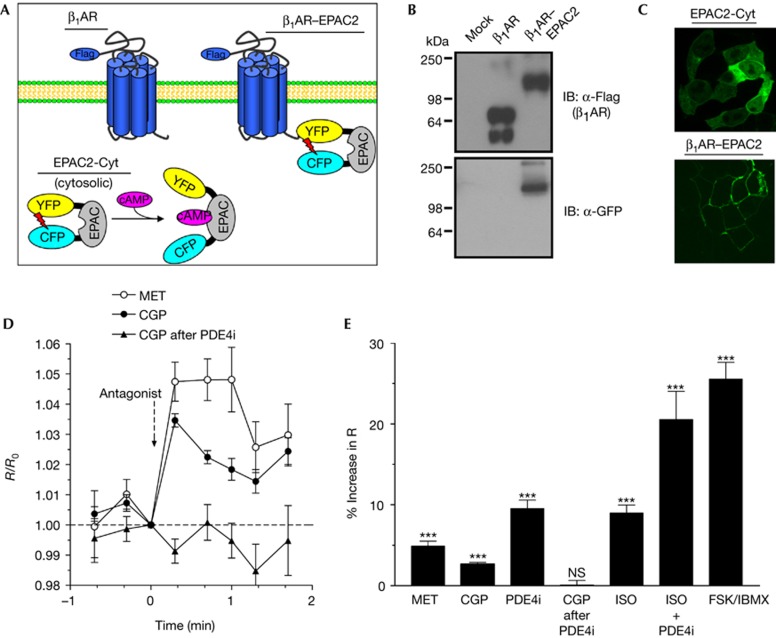
To determine whether antagonist-dependent complex dissociation affects local cAMP levels in the vicinity of the receptors, we generated a live-cell sensor that detects cAMP levels in this specific compartment (Fig 2A). To this end, we fused a FRET-based cAMP sensor (EPAC2-Cyt), which has been characterized previously [7], to the C-terminus of the β1AR. The β1AR–EPAC2 chimera was expressed at similar levels as the wild-type β1AR (Fig 2B). Fusion with the receptor causes membrane localization of the EPAC2 sensor, which by itself distributes throughout the cytosol (Fig 2C). Importantly, fusion of β1AR and the EPAC2 probe does not impair the function of the receptor (supplementary Figs S3,S4 online) nor, as shown below, the function of the EPAC2 sensor.
Figure 2.
Antagonist treatment increases cAMP levels in the vicinity of the β1AR. (A) Scheme illustrating the design of the cytosolic EPAC2-Cyt sensor and the β1AR–EPAC2 chimera. (B) Representative western blots showing the expression of Flag-tagged β1AR and β1AR–EPAC2 in Hek293 cells. (C) The images show YFP emissions in Hek293A cells expressing the cytosolic EPAC2-Cyt or the β1AR–EPAC2 sensor. (D,E) Live-cell cAMP measurements in Hek293 cells expressing the β1AR–EPAC2 sensor. Shown are average traces of R/R0, defined as the CFP/FRET emission ratio (R) at each time point divided by the CFP/FRET ratio at time 0 min (R0), for cells treated with MET (1 μM; n=15), CGP (1 μM; n=54) or for cells treated with CGP following a 5-min pretreatment with a PDE4 inhibitor (PDE4i; rolipram; 10 μM; n=30). An increase in R/R0 indicates an increase in intracellular cAMP levels. (E) Percentage increase in R (CFP/FRET) after treatment with β1AR antagonists MET (n=15) or CGP (n=54), rolipram (PDE4i, 10 μM; n=30), CGP after pretreatment with PDE4i (n=30), ISO (100 nM; n=19), combined treatment with ISO and PDE4i (n=16) or combined treatment with the non-selective PDE inhibitor IBMX (100 μM) and the adenylyl cyclase activator FSK (50 μm) (n=37). NS (not significant; P>0.05); ***(P<0.001). CGP, CGP-20712A; FSK, forskolin; IB, immunoblotting; ISO, isoproterenol; MET, metoprolol; PDE4, type-4 cyclic nucleotide phosphodiesterase; β1AR, β1-adrenergic receptor.
Next, we used the β1AR–EPAC2 sensor to determine whether antagonist-dependent β1AR/PDE4 complex dissociation affects local cAMP levels. As shown in Fig 2D, treatment with β1AR antagonists CGP or MET induced a significant increase in R (the ratio of CFP emission over FRET), which indicates an increase in cAMP levels in the vicinity of the sensor. Treatment with a PDE4-selective inhibitor also induced a significant increase in local cAMP levels (Fig 2E). On pretreatment with PDE4 inhibitor, β1AR antagonists have no further effect on cAMP levels in the vicinity of the β1AR–EPAC2 sensor suggesting that the antagonist-dependent increase in local cAMP is due to reduced PDE4 activity in this compartment. The antagonist-dependent increase in cAMP levels is spatially restricted to the vicinity of the receptor and is not detected with cAMP sensors that are widely distributed throughout the cell membrane or in the bulk cytosol (supplementary Fig S5 online). Whereas treatment with MET and/or CGP does not stimulate cAMP production (Fig 1D), basal constitutive cAMP production is required to detect the effect of antagonist-dependent β1AR/PDE4 complex dissociation as demonstrated by AC inhibition (supplementary Fig S6 online). This finding also confirms that the antagonist-induced changes in CFP/FRET emissions reflect changes in local cAMP levels and not an artifact resulting from conformational changes of the β1AR–EPAC2 chimera upon ligand binding.
Complex dissociation increases PKA activity locally
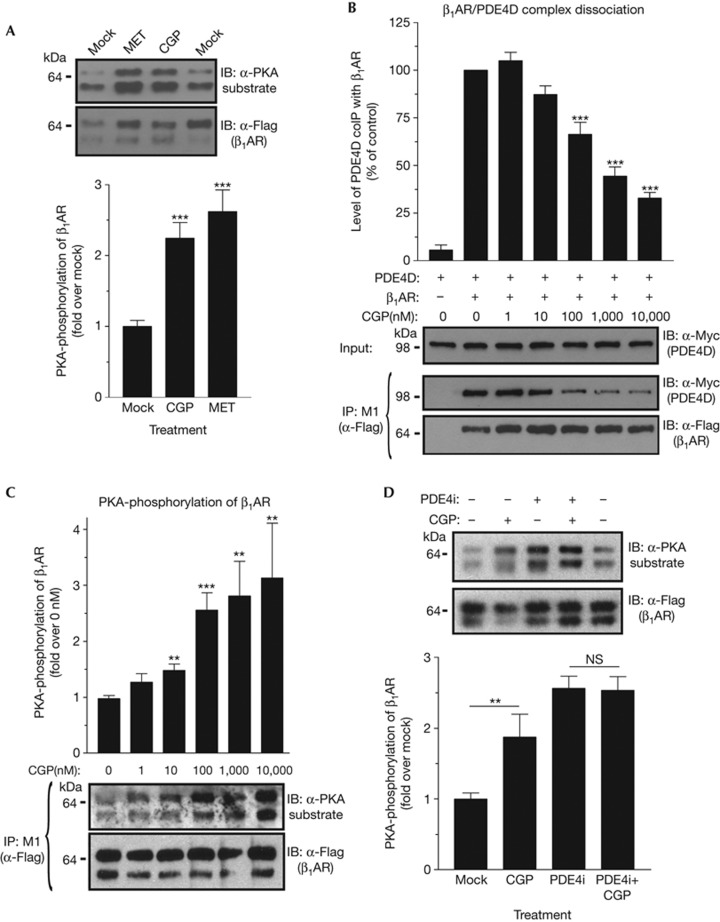
To determine whether the local increase in cAMP levels measured with the β1AR–EPAC2 probe is mirrored by local increases in PKA activity, we determined the effect of antagonist-dependent β1AR/PDE4 complex dissociation on PKA phosphorylation of the receptor itself. Treatment with antagonists induced a significant increase in β1AR phosphorylation by PKA (Fig 3A; supplementary Fig S7 online) in spite of their inverse agonist activity that lowers basal AC activity (Fig 1D). Several observations indicate that the increased PKA phosphorylation of the receptor is due to antagonist-dependent β1AR/PDE4 complex dissociation. First, there is a positive correlation between the time course of antagonist-dependent complex dissociation and that of increased PKA phosphorylation of the receptor. Both are induced within the first minute of treatment (supplementary Fig S8 online). Similarly, the dose–response curve for the antagonist-dependent β1AR/PDE4 complex dissociation compares well with that of increased PKA phosphorylation of the receptor (Fig 3B,C). Finally, treatment with a PDE4 inhibitor also increases PKA phosphorylation of the receptor in line with the idea that PDE4 controls local PKA activity (Fig 3D). Importantly, upon pretreatment with a PDE4 inhibitor, β1AR antagonists have no further effect on PKA phosphorylation levels of β1AR (Fig 3D) confirming that the effect of antagonists is mediated via altered PDE4 activity in the vicinity of the β1AR.
Figure 3.
Antagonist-dependent β1AR/PDE4 complex dissociation increases local PKA activity. (A) Hek293 cells expressing Flag-tagged β1AR were treated for 5 min with β1AR antagonists CGP or MET (each 1 μM). Detergent extracts prepared from these cells were subjected to α-Flag(M1)-IP and the phosphorylation levels of the β1AR recovered in IP pellets was determined by western blot using a PKA-substrate antibody. (B) Hek293 cells expressing Flag-tagged β1AR and Myc-tagged PDE4D8 were treated for 5 min with different concentrations of CGP, before the cells were lysed and subjected to β1AR IP. The amount of exogenous PDE4D8 recovered in the IP pellets is quantified. (C) Dose-dependent increase in PKA phosphorylation of β1AR expressed in Hek293 cells in response to a 5-min treatment with different concentrations of CGP. (D) Hek293 cells expressing Flag-tagged β1AR were treated for 5 min with CGP (1 μM) and/or rolipram (PDE4i; 10 μM). Detergent extracts were subjected to α-Flag(M1)-IP and the phosphorylation levels of the β1AR recovered in IP pellets was determined by western blot with a PKA-substrate antibody. Data are the mean±s.e.m. of at least three experiments. **(P<0.01); ***(P<0.001); NS (not significant; P>0.05). CGP, CGP-20712A; IB, immunoblotting; IP, immunoprecipitation; MET, metoprolol; PDE4, type-4 cyclic nucleotide phosphodiesterase; β1AR, β1-adrenergic receptor.
Antagonists alter subcellular cAMP distribution
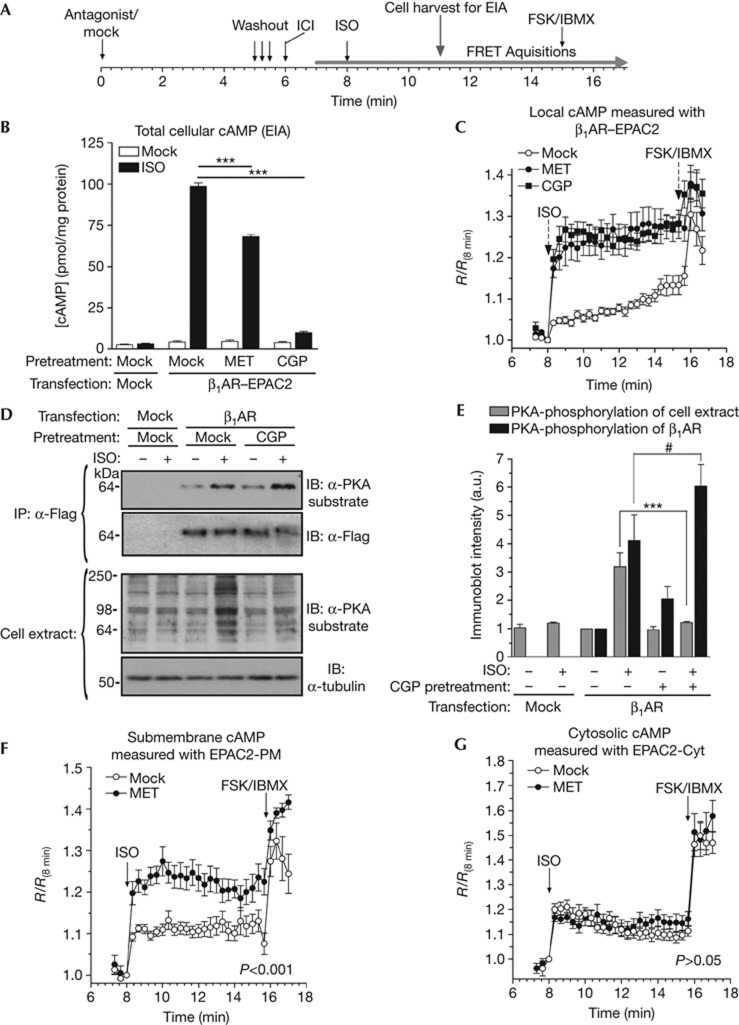
Although antagonist-dependent β1AR/PDE4 complex dissociation is rapid, reassociation of the complex after antagonist washout is relatively slow. Only a fraction of β1AR/PDE4 complexes reformed at 10 min after MET washout (supplementary Figs S9, S10 online). This allowed us to compare agonist signalling of receptors associated with different amounts of PDE4, using the experimental protocol outlined in Fig 4A. As shown in Fig 4B, pretreatment and subsequent washout of β1AR–EPAC2-expressing Hek293 cells with MET or CGP results in a significant reduction in ISO (10 nM)-stimulated global cAMP production compared with mock-treated cells. Incomplete washout of CGP (see supplementary Fig S9 online) is likely responsible for the more severe suppression of ISO-induced cAMP accumulation after washout of CGP compared with MET. In contrast to the suppressed global cAMP signals, cAMP levels in the vicinity of the receptor, as measured by changes in FRET, are significantly elevated upon antagonist pretreatment (Fig 4C), confirming receptor-associated PDE4 activity as the main regulator of cAMP levels in this compartment. The distinct effect of antagonist pretreatment on local and global cAMP levels is mirrored by experiments with the wild-type β1AR. Total cellular cAMP accumulation as measured by enzyme immunoassay (EIA) was reduced by antagonist pretreatment (supplementary Fig S11A online). The reduced global cAMP accumulation was associated with significantly reduced global PKA phosphorylation levels upon antagonist pretreatment (Fig 4D,E; supplementary Fig S11B online). Conversely, basal and ISO-induced PKA phosphorylation of the receptor itself was increased following antagonist pretreatment and washout. These data are consistent with the hypothesis that antagonist pretreatment causes an increase in cAMP in the vicinity of the receptors via PDE4 dissociation.
Figure 4.
Antagonist-dependent β1AR/PDE4 complex dissociation differentially affects submembrane and global cytosolic cAMP levels. (A) Scheme of the treatment protocol for experiments shown in (B–G). Cells were pretreated for 5 min with MET or CGP (1 μM each) or solvent. Cell monolayers were then rapidly rinsed three times to wash out the antagonists, treated with ICI (1 μM) to block signalling of endogenous β2ARs and then stimulated with 10 nM ISO. For measurement of global cAMP levels (B) and PKA-phosphorylation patterns (D,E), cells were harvested 3 min after addition of ISO. For FRET measurements, images were acquired every 20 s for ∼7 min, after which cells were treated with FSK (50 μM) and IBMX (100 μM). (B) Total cellular cAMP accumulation in Hek293 cells expressing β1AR–EPAC2 in response to ISO as measured by EIA. (C) Average traces of FRET from β1AR–EPAC2-expressing cells stimulated with ISO after pretreatment with MET (n=28), CGP (n=19) or solvent (mock; n=21). Data are expressed as R (ratio of CFP/FRET emissions at each time point) divided by the R value acquired before ISO addition (R(8 min)). (D,E) Detergent extracts prepared from control or β1AR-expressing Hek293 cells were subjected to α-Flag IP. Total cell extracts and β1ARs recovered in Flag-IP pellets were subjected to immunoblot analysis using a PKA substrate antibody. (F) Average traces of FRET from EPAC2-PM-expressing neonatal cardiac myocytes stimulated with ISO (10 nM) after pretreatment and washout of MET (n=19) or solvent (mock; n=20). (G) Pretreatment with MET does not affect ISO-induced cAMP levels in the bulk cytosol measured with the EPAC2-Cyt probe (nmock=15; nMET=20). Data represent the means±s.e.m. of at least three experiments. ***(significantly reduced; P<0.001); # (significantly increased; P<0.05). CGP, CGP-20712A; EIA, enzyme immunoassay; FSK, forskolin; ICI, ICI-118551; IP, immunoprecipitation; ISO, isoproterenol; MET, metoprolol; PDE4, type-4 cyclic nucleotide phosphodiesterase; β1AR, β1-adrenergic receptor.
Complex dissociation affects antagonist action
To determine whether antagonist pretreatment augments responses of endogenous β1ARs, we measured its effect in neonatal mouse cardiac myocytes expressing the plasma membrane-targeted EPAC2 cAMP sensor (EPAC2-PM; Fig 4F; supplementary Fig S12 online; [8, 9]). Pretreatment followed by washout of MET potentiated ISO-induced cAMP levels in this compartment, whereas cAMP levels in the bulk cytosol measured with the cytosolic EPAC2-Cyt sensor (Fig 4G) were not affected. Consistent with the hypothesis that the antagonist-dependent increase in submembrane cAMP levels is due to PDE4 translocation, treatment with a PDE4-selective inhibitor also potentiated submembrane cAMP signals (supplementary Fig S12 online) and MET pretreatment had no further effect in the presence of this inhibitor. Taken together, these data suggest that antagonist-dependent β1AR/PDE4 complex dissociation also affects signalling of endogenous β1ARs in cardiac myocytes. An increase in cAMP after antagonist treatment is detected only with the β1AR–EPAC2 sensor, suggesting a restricted effect in proximity of the receptor (supplementary Fig S5 online), whereas the response to an agonist after antagonist pretreatment/washout can be detected with both membrane probes (β1AR–EPAC2 as well as EPAC2-PM) but not with the cytoplasmic sensor (Fig 4). We explain this broader effect of the agonist as a consequence of the large increase in cAMP owing to the activation of cAMP synthesis and decreased local degradation owing to dissociation of the PDE4/β1AR complex. These two events synergize to cause spillover of cAMP from a compartment close to the receptor to other membrane domains.
To determine whether antagonist-dependent β1AR/PDE4 complex dissociation might occur at therapeutic concentrations, dose–response curves for two compounds used in the treatment of heart failure, MET and CAR, were determined in Hek293 cells. β1AR/PDE4 complex dissociation is induced by MET concentrations >100 nM, whereas CAR induces β1AR/PDE4 dissociation at concentrations of as little as 10 nM (supplementary Fig S13 online). Reported peak plasma concentrations for MET and CAR range from 100–1,000 nM and from 20–400 nM, respectively, depending on dosage and preparation (see supplementary Fig S13 online). Thus, MET is more likely to signal via β1AR/PDE4 complex dissociation in individuals receiving high doses of the drug (that is, ⩾200 mg/dose) compared with individuals receiving low doses. Conversely, we predict a significant and long-lasting effect of CAR on β1AR/PDE4 complexes in all patients, because it induces complex dissociation at concentrations lower than therapeutic doses (supplementary Fig S13 online). In addition, the drug has a very slow dissociation constant resulting in quasi-irreversible binding to the β1AR [10] (supplementary Fig S9 online). Thus, individual antagonists likely show distinct efficacies in signalling via β1AR/PDE4 complex dissociation in human heart.
Taken together, our studies reveal the presence of a novel intracellular signal generated when a neutral antagonist or an inverse agonist interacts with the β1AR (supplementary Fig S14 online). By inducing the dissociation of a β1AR/PDE4 complex, antagonist binding results in a local increase in cAMP sufficient to activate PKA locally. These local effects are opposite to the conventional effects of antagonists on the bulk cytoplasmic cAMP where they lower cAMP levels and do not require increased cAMP production via the classical Gs/AC pathway. Thus, these findings provide a new mechanism of antagonist action that might explain some pharmacological properties attributed to this class of β-adrenergic ligands. The related β2AR has also been shown to sequester a PDE4, although via distinct modes of interaction. Nevertheless, this example raises the possibility that other GsPCRs or non-GPCR receptors dynamically interact with PDEs, and that formation and dissociation of these complexes and thereby submembrane cAMP/PKA levels might be regulated by receptor occupancy.
Methods
Immunoprecipitation of Flag-tagged β1ARs from cell lysates. Flag-tagged β1ARs were imunoprecipitated as described previously [4]. In brief, cells were lysed in buffer containing 20 mM HEPES, 150 mM NaCl, 2 mM EDTA, 10% glycerol, 1% n-dodecyl-β-D-maltopyranoside (Anatrace), 1 μM microcystin-LR (Calbiochem) and Complete protease inhibitor cocktail (Roche Applied Sciences). Lysates were cleared by centrifugation at 20,000g and 4 °C, and preincubation with ProteinG Sepharose. Flag-tagged receptors were then immunoprecipitated using M1-affinity resin (α-Flag antibody resin; Sigma-Aldrich).
PDE assay. PDE activity was measured as described previously [11]. In brief, samples were assayed in a reaction mixture containing 40 mM Tris–HCl (pH 8.0), 1 mM MgCl2, 1.4 mM β-mercaptoethanol, 1 μM cAMP, 0.75 mg/ml bovine serum albumin and 0.1 μCi of [3H]cAMP for 10 min at 33 °C. The reaction was terminated by boiling for 1 min. The PDE reaction product 5′-AMP was then hydrolysed by incubation of the assay mixture with 50 μg of Crotalus atrox snake venom (Sigma-Aldrich) for 20 min at 33 °C, and the resulting adenosine was separated by anion exchange chromatography using 1 ml of AG1-X8 resin (BioRad, Hercules, CA) and quantitated by scintillation counting.
Measurement of global cellular cAMP levels. Global intracellular cAMP levels were measured by EIA using a kit from Cayman Chemicals following the manufacturer’s protocol or by RIA as described previously [12].
Measurement of cAMP levels with EPAC2 sensors. Cells grown on collagen-coated glass coverslips were transfected with plasmids or infected with adenoviruses encoding EPAC2 sensors. After overnight culture, cells were serum-starved for 2 h. FRET microscopy was then perfomed as described previously [12]. In brief, coverslips were placed in a modified Sykes–Moore Chamber and kept in 500 μl of Locke’s medium (5 mM HEPES (pH 7.4), 154 mM NaCl, 5.6 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 3.6 mM NaHCO3, 5 mM glucose and 0.05% bovine serum albumin) at 37 °C. Images were acquired with a Nikon TE2000 inverted fluorescence microscope using a × 60 fluorescence objective. CFP (donor) fluorescence was viewed by exciting at 430–455 nm and measuring emission at 470–490 nm. YFP (acceptor) fluorescence was viewed by exciting at 500–520 nm and measuring emission at 535–565 nm. FRET was viewed by exciting at 430–455 nm (donor excitation) and measuring fluorescence at 535–565 nm (acceptor emission).
AC activity assay. AC activity was measured as described [13]. In brief, samples were assayed in a reaction mixture containing 40 mM Tris–HCl (pH 7.4), 5 mM MgCl2, 0.2 mM cAMP, 10 mM phosphoenol pyruvate, three units of pyruvate kinase, 10 μM GTP, 1 mM ATP and 2 μCi of [α32P]-ATP for 15 min at 37 °C. The reaction was terminated by boiling for 2 min. Cyclic AMP was then separated from the substrate ATP by column chromatography using 2.5 cm3 Alumina WN-6. The column was eluted into scintillation vials with 5 ml of 0.1 M ammonium acetate (pH 6.5), the eluate was mixed with 12 ml of Aquasol-2 scintillation fluid (PerkinElmer, Waltham, MA) and the eluted cAMP quantified by scintillation counting.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We are indebted to Drs Brian Kobilka (Stanford University) and Michael Bristow (University of Denver) for critical advice. We thank Dr Robert Lefkowitz (Duke University) for the kind gift of MEFs deficient in β-arrestin 1 and 2 and Drs David Zuckerman and Carolyn Machamer (Johns Hopkins University) for providing the mCherry-β1AR construct. This research was supported by grants from Fondation Leducq (06CVD02 cycAMP) and the National Institutes of Health (HL0927088 and HL107960).
Author contributions: W.R., D.M., P.D. and E.B. performed the experiments and analysed the data. W.R. and M.C. designed the study and wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Kobilka BK, Deupi X (2007) Conformational complexity of G-protein-coupled receptors. Trends Pharmacol Sci 28: 397–406 [DOI] [PubMed] [Google Scholar]
- Reiter E, Ahn S, Shukla AK, Lefkowitz RJ (2012) Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol 52: 179–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahsai AW, Xiao K, Rajagopal S, Ahn S, Shukla AK, Sun J, Oas TG, Lefkowitz RJ (2011) Multiple ligand-specific conformations of the beta2-adrenergic receptor. Nat Chem Biol 7: 692–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter W et al. (2008) Signaling from beta1- and beta2-adrenergic receptors is defined by differential interactions with PDE4. EMBO J 27: 384–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SJ et al. (2002) Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science 298: 834–836 [DOI] [PubMed] [Google Scholar]
- Engelhardt S, Grimmer Y, Fan GH, Lohse MJ (2001) Constitutive activity of the human beta(1)-adrenergic receptor in beta(1)-receptor transgenic mice. Mol Pharmacol 60: 712–717 [PubMed] [Google Scholar]
- Nikolaev VO, Bunemann M, Hein L, Hannawacker A, Lohse MJ (2004) Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem 279: 37215–37218 [DOI] [PubMed] [Google Scholar]
- Blackman BE, Horner K, Heidmann J, Wang D, Richter W, Rich TC, Conti M (2011) PDE4D and PDE4B function in distinct subcellular compartments in mouse embryonic fibroblasts. J Biol Chem 286: 12590–12601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachten S, Schlenstedt J, Gauss R, Baumann A (2006) Molecular identification and functional characterization of an adenylyl cyclase from the honeybee. J Neurochem 96: 1580–1590 [DOI] [PubMed] [Google Scholar]
- Kindermann M, Maack C, Schaller S, Finkler N, Schmidt KI, Laer S, Wuttke H, Schafers HJ, Bohm M (2004) Carvedilol but not metoprolol reduces beta-adrenergic responsiveness after complete elimination from plasma in vivo. Circulation 109: 3182–3190 [DOI] [PubMed] [Google Scholar]
- Richter W, Conti M (2002) Dimerization of the type 4 cAMP-specific phosphodiesterases is mediated by the upstream conserved regions (UCRs). J Biol Chem 277: 40212–40221 [DOI] [PubMed] [Google Scholar]
- Xie M, Rich TC, Scheitrum C, Conti M, Richter W (2011) Inactivation of multidrug resistance proteins disrupts both cellular extrusion and intracellular degradation of cAMP. Mol Pharmacol 80: 281–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter W, Xie M, Scheitrum C, Krall J, Movsesian MA, Conti M (2011) Conserved expression and functions of PDE4 in rodent and human heart. Basic Res Cardiol 106: 249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.