Abstract
Background
The current therapeutic strategy in breast cancer is to identify a target, such as estrogen receptor (ER) status, for tailoring treatments.
Methods
We investigated the patterns of recurrence with respect to ER status for patients treated in two randomized trials with 25 years' median follow-up.
Results
In the ER-negative subpopulations most breast cancer events occurred within the first 5–7 years after randomization, while in the ER-positive subpopulations breast cancer events were spread through 10 years. In the ER-positive subpopulation, 1 year endocrine treatment alone significantly prolonged disease-free survival (DFS) with no additional benefit observed by adding one year of chemotherapy. In the small ER-negative subpopulation chemo-endocrine therapy had a significantly better DFS than endocrine alone or no treatment.
Conclusions
Despite small numbers of patients, “old-fashioned” treatments, and competing causes of treatment failure, the value of ER status as a target for response to adjuvant treatment is evident through prolonged follow-up.
Keywords: breast cancer, chemotherapy, estrogen receptor, hormonal therapy
Introduction
What can we learn from the earliest breast cancer adjuvant trials? Unquestionably the early trials provided essential information that formed the basis of what we know—or what we think we know—today, but does reporting on old treatments, old designs, and even old patients have any value to the modern oncology community? Trials conducted 25 to 30 years ago give us the benefit of hindsight as carefully-collected long-term follow-up reveals different patterns of recurrence within subpopulations defined by estrogen receptor status, a therapeutic target for the past quarter century.
A current method in choosing adjuvant treatments for patients with breast cancer starts with identifying targets indicating responsiveness to available systemic therapies [1]. The estrogen receptor (ER) status of the primary tumor used as indicator of endocrine responsiveness is certainly not new as such. The International Breast Cancer Study Group (IBCSG, formerly Ludwig Breast Cancer Study Group) has been collecting and studying estrogen receptors since its first trials started in 1978 [2,3]. During the last three decades a positive ER status of the primary tumor determined the indication to prescribe endocrine therapy, while the question of whether to add chemotherapy to endocrine treatment is still a matter of debate, especially for patients with node-positive breast cancer [4]. For postmenopausal women with some ER positive breast cancer, the role of chemo-endocrine therapy is difficult to determine due to low numbers of patients enrolled in randomized clinical trials specifically testing this question [5].
The IBCSG conducted two complementary trials to investigate the role of chemo-endocrine therapy, endocrine therapy alone or no adjuvant treatment in the population of postmenopausal patients with lymph node positive breast cancer treated between 1978 and 1981 with mastectomy and axillary lymph node dissection [6–10]. We present the patterns of recurrence for these trials within subpopulations defined according estrogen receptor status at 25 years median follow-up.
PATIENTS AND METHODS
Study Design
Trials III and IV are randomized multicenter clinical trials designed and conducted by IBCSG. In Trial III postmenopausal patients 65 years old or younger were randomized to one of three treatment groups: no adjuvant therapy (Obs), a year of concurrent low dose prednisone and tamoxifen (p+T: tamoxifen 20 mg per day p.o., prednisone 7.5 mg per day p.o.), or a year (12 courses) of concurrent classical cyclophosphamide, methotrexate, and 5-fluorouracil (CMF: Cyclophosphamide 100 mg/m2 p.o. days 1 through 14, Methotrexate 40 mg/m2 i.v. days 1 and 8, 5-Fluorouracil 600 mg/m2 i.v. days 1 and 8; every 28 days), low dose prednisone and tamoxifen (CMFp+T).
In Trial IV patients aged 66 to 80 years were randomized to Obs or p+T. Patients in the observation arm were seen at the same scheduled intervals as those treated with p+T. Patients have been followed life-long, with updates of disease and survival status required yearly.
Patient Characteristics
Between July 1978 and August 1981, 783 eligible patients were enrolled in Trial III (463) and Trial IV (320). Patients with clinical stages T1 to T3 N1 M0 (UICC Classification 2nd Ed. 1974) were considered eligible. Estrogen receptor (ER) assays of the primary tumor were considered positive if levels of cytosol protein were ≥ 10 fmol/mg. ER status was known for about half of the patients (382), and these patients form the study cohort that is the subject of this paper. Most patients in the study cohort had ER-positive (69%) disease with a somewhat high tumor burden based on number of positive nodes (46% 4 or more), and tumor size (60% > 2 cm).
Statistical Methods
Disease-free survival (DFS) was defined as the length of time from the date of randomization to any relapse (including ipsilateral chest wall recurrence), the appearance of a second primary cancer (including contralateral breast cancer), or death, whichever occurred first. The type and site of first failure was recorded for each patient. The Kaplan-Meier [11] method was used to estimate survival distributions for DFS and overall survival (OS). The two-sided log-rank procedure was used to assess the statistical significance of treatment differences between the survival distributions. Cumulative incidence functions were estimated for each of the competing causes of failure (breast cancer recurrence, second (non breast) primary malignancy, or death without prior cancer event), and tests for differences between treatments were conducted [12, 13].
RESULTS
In Trial III, for the ER-positive subpopulation, CMFp+T and p+T had a similar impact on DFS, with both superior to no treatment (p=0.02 and p=0.05, respectively) (Table 1). In the ER-negative subpopulation, the small number of patients treated with CMFp+T had a DFS superior to the p+T and observation groups (p=0.02 and p=0.04, respectively) (Table 1).
Table 1.
Trials III–IV DFS and OS pairwise comparisons according to ER status (median follow-up of 25 years)
| Disease-Free Survival | Overall Survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | HR | 95%CI | P * | 5-Yr DFS% ± SE | 25-yr DFS% ± SE | HR | 95%CI | P * | |
| Trial III [p+T:Obs] | |||||||||
| ER+ | |||||||||
| p+T | 45 | 0.65 | 0.42 to 1.00 | 0.05 | 56± 7 | 15± 5 | 0.78 | 0.50 to 1.23 | 0.29 |
| Obs | 53 | 30± 6 | 7± 4 | ||||||
| ER− | |||||||||
| p+T | 30 | 1.08 | 0.63 to 1.85 | 0.77 | 20± 7 | 17± 7 | 1.22 | 0.70 to 2.12 | 0.48 |
| Obs | 33 | 30± 8 | 11± 6 | ||||||
| Trial III [CMFp+T:Obs] | |||||||||
| ER+ | |||||||||
| CMFp+T | 58 | 0.62 | 0.41 to 0.92 | 0.02 | 58± 7 | 10± 5 | 0.82 | 0.54 to 1.26 | 0.37 |
| Obs | 53 | 30± 6 | 7± 4 | ||||||
| ER− | |||||||||
| CMFp+T | 19 | 0.49 | 0.25 to 0.97 | 0.04 | 63±11 | 35±11 | 0.68 | 0.34 to 1.38 | 0.29 |
| Obs | 33 | 30± 8 | 11± 6 | ||||||
| Trial III [CMFp+T:p+T] | |||||||||
| ER+ | |||||||||
| CMFp+T | 58 | 0.96 | 0.63 to 1.47 | 0.85 | 58± 7 | 10± 5 | 1.07 | 0.67 to 1.69 | 0.78 |
| P+T | 45 | 56± 7 | 15± 5 | ||||||
| ER− | |||||||||
| CMFp+T | 19 | 0.44 | 0.22 to 0.89 | 0.02 | 63± 11 | 35±11 | 0.55 | 0.27 to 1.13 | 0.11 |
| P+T | 30 | 20± 7 | 17± 7 | ||||||
| Trial III–IV [p+T:Obs] * | |||||||||
| ER+ | |||||||||
| p+T | 104 | 0.64 | 0.48 to 0.86 | 0.003 | 52± 5 | 11± 3 | 0.72 | 0.53 to 0.98 | 0.04 |
| Obs | 100 | 35± 5 | 4± 2 | ||||||
| ER− | |||||||||
| p+T | 51 | 1.18 | 0.78 to 1.79 | 0.44 | 22± 6 | 10± 4 | 1.30 | 0.84 to 2.01 | 0.23 |
| Obs | 50 | 30± 6 | 13± 5 | ||||||
stratified by Trial.
Abbreviations: p+T: low-dose prednisone and tamoxifen for one year; CMF: classical cyclophosphamide, methotrexate, 5-fluorouracil for one year; Obs: observation: no adjuvant systemic therapy; ER: estrogen receptor status, HR: hazard ratio, CI: confidence interval, DFS: disease-free survival, OS: overall survival, SE: standard error
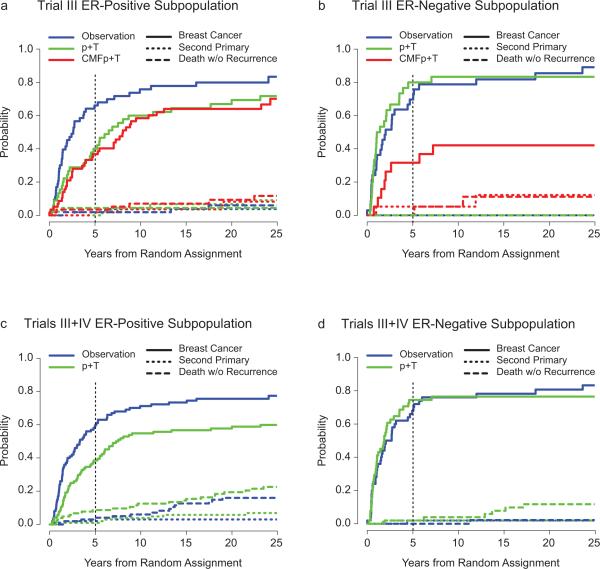
When data from the common arms of Trial III and IV were combined, in the ER-positive subpopulation, one year of endocrine therapy provided significantly longer DFS and OS compared with no adjuvant treatment, while no such differences were observed in the ER-negative subpopulation (Table 1). In postmenopausal patients the non-breast cancer competing DFS related events (second, non-breast malignancies, and deaths without prior cancer event) were more likely to have had an impact on DFS comparisons. Figure 1 presents competing risk cumulative incidence plots that separate breast cancer events, second (non-breast) malignancies, and deaths without prior cancer event. Overall, in the ER-negative subpopulations, most breast cancer events occurred within the first 5–7 years after randomization (Figures 1b and 1d), while in the ER-positive subpopulations breast cancer events were spread through 10 years (Figures 1a and 1c). The significant impact of one year of endocrine therapy in the control of breast cancer events in the ER-positive subpopulation is evident in Figure 1C (P=0.001). By contrast the lack of endocrine treatment effect in the ER-negative subpopulation is evident in Figure 1d.
Fig 1.
Cumulative incidence for competing causes of failure according to estrogen receptor subpopulations for Trial III (a,b) and Trials III plus IV combined (c,d).
DISCUSSION
After a median follow-up of 25 years, distinct patterns of recurrence emerged for the ER-positive subpopulation compared with the ER-negative subpopulation (Figure 1). In the ER-negative subpopulation, most breast cancer events happen in the first five years, while in the ER-positive subpopulation breast cancer events occur more gradually over the first 10–15 years [14]. The distinctive tumor latency between endocrine responsive and unresponsive breast cancer suggests a different behavior of persistent, dormant micrometastatic disease. Clarification of the various pathways by which clinical dormancy can occur should allow new research and clinical approaches, through strategies to induce and/or maintain dormancy and/or kill dormant cells [15].
These long term results indicate the importance of estrogen-receptor status as a primary therapeutic target for selecting proper systemic treatment as well as individualized follow-up procedures [16].
Based on the long-term pattern of recurrence of endocrine-responsive breast cancer, extending treatment duration beyond 5 years of tamoxifen has been the focus of several trials. Overall, sustained therapy with aromatase inhibitors is associated with a significant reduction in disease relapse, while the value of extended tamoxifen therapy is still controversial [17].
On the other hand, prolonged treatment with antihormonal therapy can induce cell drug resistance. Recent evidence suggests that endocrine-resistant breast cancer cells undergo apoptosis when exposed to low concentrations of estrogen, resuming tumor sensitivity to the reintroduction of prolonged anti-hormonal therapy [18]. The SOLE trial, conducted by the IBCSG within the Breast International Group (BIG), investigates this innovative therapeutic approach in postmenopausal women with endocrine-responsive node-positive operable breast cancer by comparing extended continuous treatment with letrozole with intermittent therapy, after completion of 4–6 years of adjuvant endocrine treatment.
The patients on these trials presented with high risk factors (i.e. large tumors and positive axillary nodes), indicating for many the presence of subclinical disease at the time of randomization. After 25 years of follow-up we observed the early occurrence of events in the ER-negative subpopulation, for which the use of chemotherapy halved the risk of relapse. In the ER-positive subpopulation, the addition of CMF chemotherapy to endocrine treatment had no impact on recurrence. These results further question the actual additional benefit from combination chemotherapy for patients in a risk group defined as intermediate [1], endocrine-responsive breast cancer as recently suggested by several randomized trials [19–21].
Two large trials, MINDACT [22] and TAILORx [23], are currently investigating the benefit of chemotherapy for patients with a genetic signature indicating a sufficient risk of relapse. TAILORx, unfortunately, is designed for patients with node-negative disease only, thus diluting the focus on the target and missing the chance to extend findings to the clinically intermediate risk cohort. The recent extension of MINDACT also to patients with node-positive disease will ensure a more accurate definition of the subset of patients most likely to take advantage from adjuvant chemotherapy.
The 25-year results of the two IBCSG trials for postmenopausal women give us the opportunity to look for subpopulations defined by estrogen receptor status determined with ligand-binding assays. Even with the small numbers of patients, the treatments are clearly affecting the disease according to the therapeutic targets. These long term results should encourage the design of clinical trials in which selection of patients to participate is target-related rather than risk-related. Tailored treatment investigations testing treatments in targeted subpopulations across risk groups is the approach most likely to yield progress in the future.
Acknowledgment
We thank the patients, physicians, nurses, and data managers who participate in the International Breast Cancer Study Group trials. We thank Rita Hinkle for data management and IBCSG patients and participants who have submitted yearly trial patient data for 30 years: West Swedish Breast Cancer Group; Institute of Oncology, Ljubljana, Slovenia; Swiss Group for Clinical Cancer Research (SAKK); Australian New Zealand Breast Cancer Trials Group (ANZ BCTG); Groote Shuur Hospital, Cape Town, South Africa.
Funding (in addition to above) provided by: Ludwig Institute for Cancer Research, Swedish Cancer Society, The Cancer Council Australia, National Health Medical Research Council of Australia, Frontier Science and Technology Research Foundation, US-National Cancer Institute (CA-75362), Cancer Association of South Africa (CANSA), and Foundation of Clinical Cancer Research of Eastern Switzerland (OSKK).
REFERENCES
- 1.Goldhirsch A, Wood W, Gelber R, et al. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer. Ann Oncol. 2007;18:1133–44. doi: 10.1093/annonc/mdm271. [DOI] [PubMed] [Google Scholar]
- 2.Zava DT, Wyler-Von Ballmoos A, Goldhirsch A, et al. A quality control study to assess the inter-laboratory variability of routine estrogen and progesterone receptor assays. Eur J Cancer Clin Oncol. 1982;18:713–21. doi: 10.1016/0277-5379(82)90068-2. [DOI] [PubMed] [Google Scholar]
- 3.Jordan VC, Zava DT, Eppenberger U, et al. Reliability of steroid hormone receptor assays: an international study. Eur J Cancer Clin Oncol. 1983;19:357–363. doi: 10.1016/0277-5379(83)90133-5. [DOI] [PubMed] [Google Scholar]
- 4.Goldhirsch A, Coates AS, Gelber RD, et al. First select the target: better choice of adjuvant treatments for breast cancer patients. Ann Oncol. 2006;17:1772–1776. doi: 10.1093/annonc/mdl398. [DOI] [PubMed] [Google Scholar]
- 5.Early Breast Cancer Trialists' Collaborative Group Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 6.Ludwig Breast Cancer Study Group Randomized trial of chemoendocrine therapy, endocrine therapy, and mastectomy alone in postmenopausal patients with operable breast cancer and axillary node metastasis. Lancet. 1984;1:1256–1260. [PubMed] [Google Scholar]
- 7.Goldhirsch A, Gelber RD, Simes RJ, et al. Costs and benefits of adjuvant therapy in breast cancer: A quality-adjusted survival analysis. J Clin Oncol. 1989;7:36–44. doi: 10.1200/JCO.1989.7.1.36. [DOI] [PubMed] [Google Scholar]
- 8.Castiglione M, Gelber RD, Goldhirsch A. Adjuvant systemic therapy for breast cancer in the elderly: competing causes of mortality. J Clin Oncol. 1990;8:519–526. doi: 10.1200/JCO.1990.8.3.519. [DOI] [PubMed] [Google Scholar]
- 9.Castiglione-Gertsch M, Johnsen C, Goldhirsch A, et al. The International (Ludwig) Breast Cancer Study Group Trials I–IV: 15 years follow-up. Ann Oncol. 1994;5:717–724. doi: 10.1093/oxfordjournals.annonc.a058976. [DOI] [PubMed] [Google Scholar]
- 10.Crivellari D, Price K, Gelber RD, et al. Adjuvant endocrine therapy compared with no systemic therapy for elderly women with early breast cancer: 21-year results of International Breast Cancer Study Group Trial IV. J Clin Oncol. 2003;21:4517–4523. doi: 10.1200/JCO.2003.03.559. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 12.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 13.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. Wiley; New York: 1980. p. 169. [Google Scholar]
- 14.Saphner T, Tormey DC, Gray R. Annual Hazard Rates of Recurrence for Breast Cancer After Primary Therapy. J Clin Oncol. 1996;14:2738–2746. doi: 10.1200/JCO.1996.14.10.2738. [DOI] [PubMed] [Google Scholar]
- 15.Goss P, Allan AL, Rodenhiser DI, et al. New clinical and experimental approaches for studying tumour dormancy: does tumour dormancy offer a therapeutic target? APMIS. 2008;116:552–568. doi: 10.1111/j.1600-0463.2008.001059.x. [DOI] [PubMed] [Google Scholar]
- 16.Clough-Gorr KM, Fink AK, Silliman RA. Challenges associated with longitudinal survivorship research: attrition and a novel approach of reenrollment in a 6-year follow-up study of older breast cancer survivors. J Cancer Surviv. 2008;2(2):95–103. doi: 10.1007/s11764-008-0049-y. [DOI] [PubMed] [Google Scholar]
- 17.Cufer T. Reducing the risk for breast cancer recurrence after completion of tamoxifen treatment in postmenopausal women. Ann Oncol Suppl. 2007;8:viii8–17. doi: 10.1016/j.clinthera.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Swaby RF, Jordan VC. Low-dose estrogen therapy to reverse acquired antihormonal resistance in the treatment of breast cancer. Clin Breast Cancer. 2008;8(2):124–133. doi: 10.3816/CBC.2008.n.012. [DOI] [PubMed] [Google Scholar]
- 19.Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295:1658–1667. doi: 10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albain K, Barlow W, O'Malley F, et al. Mature outcomes and new biologic correlates on phase III Intergroup trial 0100 (INT-0100, SWOG-8814): Concurrent (CAFT) vs sequential (CAF-T) chemohormonal therapy (cyclophosphamide, doxorubicin, 5-fluorouracil, tamoxifen) vs T alone for postmenopausal, node positive, estrogen (ER) and/or progesterone PgR receptor-positive breast cancer. Proc San Antonio Breast Cancer Symposium; 2004. Abstr 37. [Google Scholar]
- 21.Hamilton A, Hortobagyi G. Chemotherapy: what progress in the last 5 years? J Clin Oncol. 2005;23:1760–1775. doi: 10.1200/JCO.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 22.Breast International Group [Accessed October 3, 2008];MINDACT (Microarray In Node negative Disease may Avoid ChemoTherapy) trial. http://www.breastinternationalgroup.org/TransBIG/Mindact.aspx .
- 23.National Cancer Institute US National Institutes of Health. [Accessed October 3, 2008];The TAILORx Breast Cancer Trial. http://www.cancer.gov/clinicaltrials/digestpage/TAILORx.