Abstract
The phosphorylation state of Retinoblastoma protein (Rb) plays a role in cell proliferation and apoptosis. Within cells, cyclin dependent kinases (cdks) phosphorylate Rb in response to growth stimulatory signals, whereas protein phosphatase 1 (PP1) dephosphorylates Rb when cells stop proliferating or undergo apoptosis in response to anti-proliferative or stress signals. Stimulation of PP1 activity via siRNA mediated knockdown of its interacting protein PNUTS (Phosphatase Nuclear Targeting Subunit) leads to Rb dephosphorylation and apoptosis in cancer cells. Here we utilize two separate methods to modulate the phosphorylation state of Rb in cancer cells. Kinase activity toward Rb is inhibited by the clinically relevant cdk inhibitor, Roscovitine. In addition, siRNA mediated PNUTS knockdown stimulates phosphatase activity toward Rb. Either of these treatments in cancer cells causes a two-fold stimulation of apoptosis. When activation of phosphatase activity is combined with inhibition of cdk activity toward Rb, however, cells exhibit a 4-fold increase in apoptosis. The mechanism by which PNUTS knockdown mediated PP1 activation leads to apoptosis was determined to be dependent on the activity of the transcription factor E2F1. The Rb phosphorylation profiles resulting from each treatment were analyzed and found to be similar but not identical. In addition, the two treatments differentially effect the expression of bcl-2 family proteins. Thus inhibition of cdk activity and activation of PP1 activity toward pRb are functionally distinct processes that together increase the apoptotic effect in cells.
Keywords: Rb, cdk, roscovitine, PNUTS, E2F1
Introduction
Apoptosis is a highly controlled process utilized by multicellular organisms during development or later within the adult organism to clear damaged cells in a manner that minimizes disruption to nearby cells (1). Many cancer cells acquire the ability to evade apoptosis which results in the persistence of damaged cells which can contribute to tumorigenesis (2). The Rb tumor suppressor protein (Rb), in addition to its functions in cell proliferation, differentiation and senescence, is also an important regulator of apoptosis (3). Evidence for this notion was initially revealed using Rb-null mice which exhibit excessive apoptosis in the nervous system, lens, and skeletal muscles (4,5,6). Subsequently it was shown that expression of exogenous Rb in SAOS-2 Rb-null cells blocked apoptosis in response to radiation exposure (7). Finally it was demonstrated that apoptosis triggered by DNA damaging agents could be blocked in an Rb-dependent manner (8). This data has led to the notion that Rb plays an important role in apoptosis.
The function of Rb in the control of cell division is regulated by phosphorylation on several amino acids (9). In proliferating cells, Rb phosphorylation status is controlled by the Cyclin Dependent Kinases (cdks) which phosphorylate Rb in response to growth stimulatory signals and Protein Phosphatase 1 (PP1) which dephosphorylates Rb at the end of each M phase (9,10). In the absence of proliferative stimuli, cdk activity is inhibited, and Rb is unphosphorylated which leads to cell cycle arrest. The anti-proliferative activity of Rb is mediated via interaction with the E2F family of transcription factors which regulate both the G1 to S phase transition and stimulation of apoptosis (9,11). However, when proliferating cells are treated with apoptotic stimuli, Rb becomes dephosphorylated due to the activation of PP1 which can lead to cell cycle arrest and/or apoptosis (12–14). Activity of PP1 is controlled by association with regulatory subunits responsible for the substrate specificity, localization and activity of the PP1 catalytic subunit (15). PNUTS (Phosphatase Nuclear Targeting Subunit) is a PP1 binding protein that regulates PP1 activity toward Rb (16–18). When PNUTS expression is reduced by siRNA in colon, breast and ovarian cancer cells, apoptosis is induced due to activation of PP1 and dephosphorylation of Rb (19). The effect of PNUTS knockdown is dependent on the expression of Rb. In addition, PNUTS is involved in controlling cell death in response to cell stress such as hypoxia (20).
In this work, in order to examine the effect of targeting both cdk and PP1 activity toward Rb in cancer cells, we combined PNUTS siRNA with cdk inhibition using the clinically relevant cdk inhibitor, Roscovitine (21). Roscovitine (CYC 202, Seliciclib) is a purine analog that competes with ATP for binding to the active site of cdks and exhibits potent in vitro activity against cdk1, cdk2, cdk5, cdk7 and cdk9 (22–24). Downstream targets of cdks include proteins involved in the control of cell proliferation and cellular transcription. For example, Roscovitine-mediated cdk inhibition prevents phosphorylation of Rb and induces cell cycle arrest in several model systems (25–27). Depending on dose and cell type, Roscovitine can also induce apoptosis (28, 29). In addition, Roscovitine inhibits phosphorylation of the carboxy-terminal domain of the large subunit of RNA polymerase II, which is required for transcriptional activity (28). Some studies have found that the mechanism of action of Roscovitine involved the activity of the tumor suppressor gene, p53 (30–32). Finally, Roscovitine has been shown to induce cell cycle arrest or apoptosis in combination with other treatments such as doxorubicin, ErbB targeting agents, TRAIL induced apoptosis and inhibition of farnesyl protein transferase (33–36). Thus it appears that Roscovitine affects multiple targets within cell cycle and transcription activation pathways and that the best clinical utilization of Roscovitine may be in combination with other agents.
In this paper we show that either cdk inhibition or PP1 activation alone can induce apoptosis in cancer cells. However, when utilized in combination, the effect on cell number and apoptosis in breast and colon cancer cells is enhanced by approximately two-fold. The mechanisms involved in each of these processes is similar in that both appear to require the activity of E2F1, however, each process targets a different subset of Rb phosphorylation sites and each leads to a different pattern of expression of the bcl-2 protein family members which carry out apoptosis. Finally, the ability of PNUTS knockdown to potentiate the effect of Roscovitine appears to occur independent of p53 status in the cells utilized.
Results
PNUTS knockdown enhances Roscovitine-induced reduction in cell number
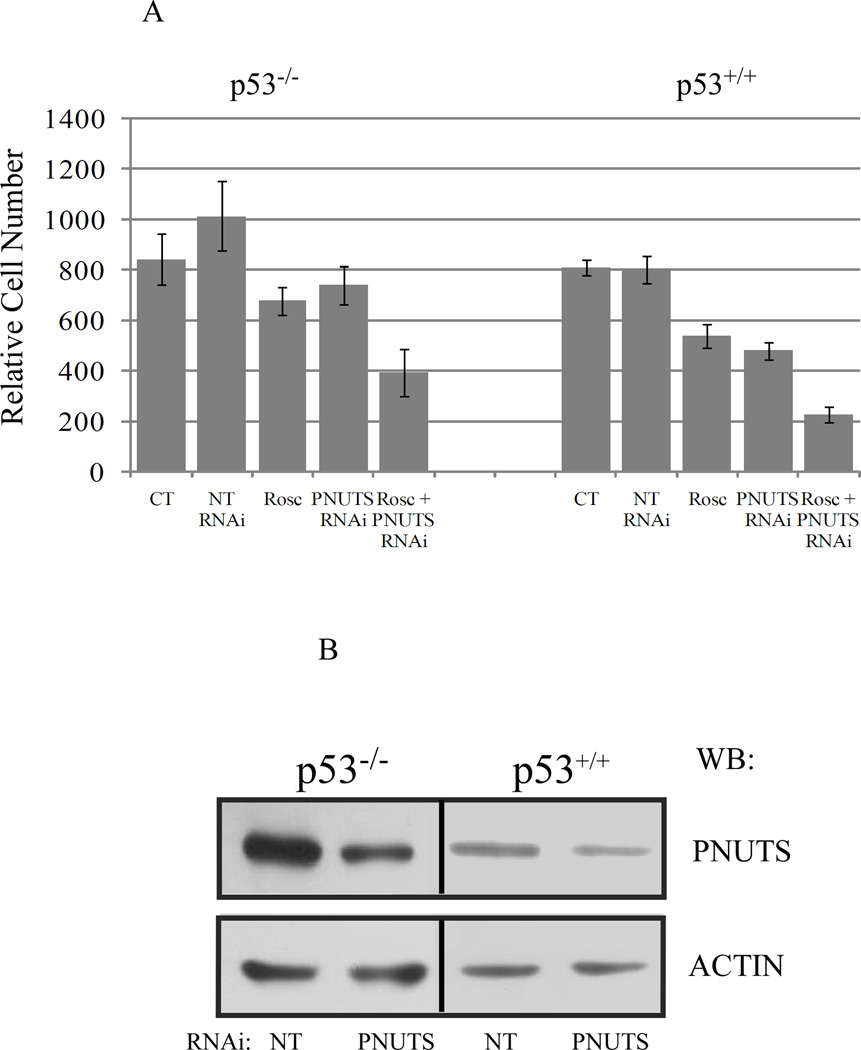
It has been previously shown that PNUTS knockdown or Roscovitine alone can cause a reduction in cell number (19,26). To determine whether combined depletion of cdk activity by Roscovitine and activation of PP1 activity by PNUTS siRNA would exert a combinatorial effect on the cells, we treated p53+/+ and p53−/− HCT116 colon cancer cells for eight hours with Roscovitine (25uM) followed by knockdown of PNUTS as described in the Materials and Methods. Viable cell number was measured 24 hours following transfection of PNUTS siRNA. We observed that knockdown of PNUTS alone or roscovitine treatment alone reduced cell number by approximately 30% in p53+/+ cells, with less of an effect in p53−/− cells (Figure 1A). However, when cells were subjected to both treatments, cell number in p53+/+ cells was reduced by 80% and cell number in p53−/− cells was reduced by 50%. The efficacy of silencing PNUTS gene expression in these experiments is shown (Figure 1B).
Figure 1. PNUTS knockdown increases the effect of Roscovitine on cell number in human cancer cells.
A. For combined treatments of Roscovitine (Rosc) and PNUTS RNAi, HCT116 cells (p53+/+ or p53−/−) were treated with 25uM Rosc and eight hours later cells were transfected with PNUTS RNAi as described in the Materials and Methods. Twenty four hours after transfection cells were counted. For Rosc treatment alone, eight hours after addition of drug, media was replaced and cells were incubated for 24 hours before counting was performed. For PNUTS RNAi alone, cells were treated with control nontargeting RNAi (NT) or PNUTS RNAi twenty four hours before cell counts were performed. Error bars represent standard deviation of the mean of triplicate samples. Data shown is representative of three independent experiments. B. Cell lysates obtained from cell counting experiments were subjected to immunoblotting. RNAi using a nontargeting control sequence (NT) or PNUTS RNAi was utilized. PNUTS knockdown and equal loading were confirmed in p53−/− and p53+/+ HCT116 cells by examining expression of PNUTS and β-actin, respectively. The antibodies utilized are indicated to the right of the figure.
PNUTS knockdown enhances cell death induced by Roscovitine
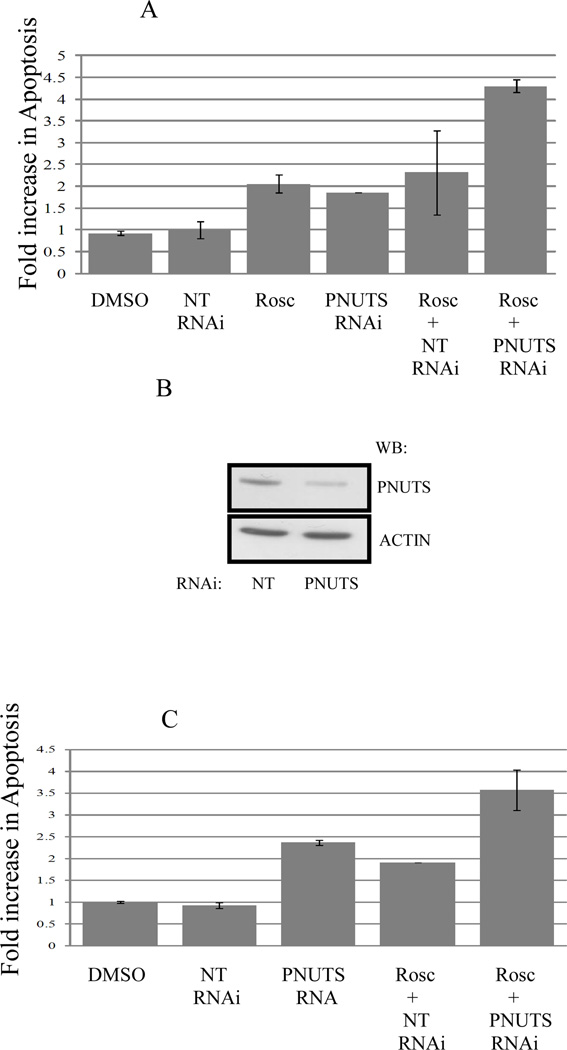
Previous studies utilizing siRNA to the PP1 targeting subunit PNUTS showed that dephosphorylation of Rb led to apoptosis in breast and colon cancer cells (19). Also, it has been shown that Roscovitine can cause cell death (28,29). Therefore in these experiments we measured whether the reduction in cell number observed when PNUTS siRNA was combined with Roscovitine led to an increase in cell death. We utilized MCF7 breast cancer cells which are p53+/+ and measured apoptosis by TUNEL assay. As shown in figure 2A, the apoptosis measured in controls (DMSO or NT-nontargeting RNAi) was normalized to 1. Roscovitine and PNUTS RNAi separately increased the induction of apoptosis by 2 fold, which was similar to results published previously (19). However, when MCF7 cells were treated first with Roscovitine (25uM, 8 hours) followed by PNUTS siRNA (24 hours) there was a 4 fold induction of apoptosis. The knockdown of PNUTS in MCF7 cells is shown (Figure 2B). We next utilized p53−/− HCT116 colon cancer cells and observed similar results using a cell death ELISA (Roche) to measure apoptosis (Figure 2C). Thus the reduction in cell number observed when cells are treated with both Roscovitine and PNUTS siRNA is due to activation of apoptosis.
Figure 2. PNUTS knockdown increases the apoptotic effect of Roscovitine in cancer cells.
A. MCF7 cells were treated as control (DMSO or nontargeting RNA) or transfected with PNUTS RNAi or treated with Rosc or these treatments were combined as described in figure 1. Analysis was performed by TUNEL assay as described in the Materials and Methods. The amount of apoptosis detected in control cells (DMSO) was normalized to one. The graph depicts the fold increase in degraded DNA observed in the cells under each condition. Error bars represent standard deviation of the mean of triplicate samples and data shown is representative of two independent experiments. B. PNUTS knockdown and equal loading were confirmed in MCF7 cells by examining expression of PNUTS and β-actin, respectively. The antibodies utilized are indicated to the right of the figure. C. Following treatment of p53−/− HCT116 cells as described in figure 1, apoptosis was measured by a Cell Death Detection ELISA (Roche Diagnostics) which detects degraded DNA released from the nucleus into the cytoplasm. The amount of apoptosis (degraded DNA) detected in control DMSO treated cells was normalized to one. The graph depicts the fold increase in degraded DNA observed due to PNUTS RNAi. Error bars represent standard deviation of the mean of triplicate samples and data shown is representative of two independent experiments.
Apoptosis induced by PNUTS knockdown is dependent on E2F1
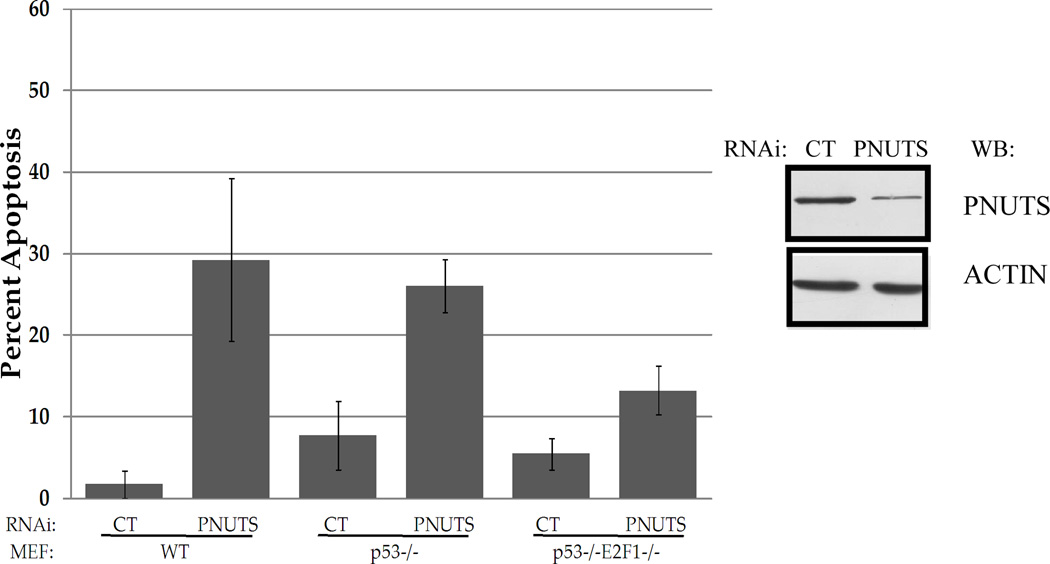
We next attempted to compare the mechanisms involved in apoptosis resulting from Roscovitine and/or PNUTS knockdown. Because Roscovitine and PNUTS siRNA each alter the phosphorylation state of Rb (19,25), and Rb phosphorylation regulates the association of Rb with E2F1 (37,38), we investigated the role of E2F1 in this series of experiments. Because it has clearly been shown that E2F1 is required for Roscovitine to induce apoptosis (36) we focused on the role of E2F1 in apoptosis induced by PNUTS knockdown. We utilized immortalized mouse embryonic fibroblasts that were either wild type, p53−/−, or DKO p53−/−E2F1−/−. Knockdown of PNUTS was performed as described in the Materials and Methods and reduced expression of PNUTS in these experiments is shown (Figure 3). Whereas PNUTS siRNA caused a 30% increase in apoptosis in WT cells, and a 25% increase in p53−/− MEFs, in p53−/−E2F1−/− DKO MEFs PNUTS knockdown caused only a 13% increase in apoptosis. Thus the loss of E2F1 reduced the induction of apoptosis induced by PNUTS siRNA by 50%. This data that E2F1 is involved in the mechanism by which PNUTS knockdown leads to apoptosis. In addition, it supports our earlier work that showed that p53 is not involved in apoptosis induced by PNUTS knockdown (19).
Figure 3. Apoptotic effect of PNUTS knockdown is dependent on E2F1.
Wild type (WT), p53−/− and p53−/−E2F1−/− mouse embryonic fibroblasts (MEFS) were transfected with nontargeting (control-CT) or PNUTS RNAi and 24 hours later apoptosis was measured by TUNEL assay as described in the Materials and Methods. Error bars represent standard deviation of the mean of triplicate samples and data shown is representative of two independent experiments. Knockdown of PNUTS was verified by immunoblotting cells treated with either control (CT) or PNUTS RNAi.
Rb Phosphorylation profiles in response to Roscovitine and PNUTS knockdown
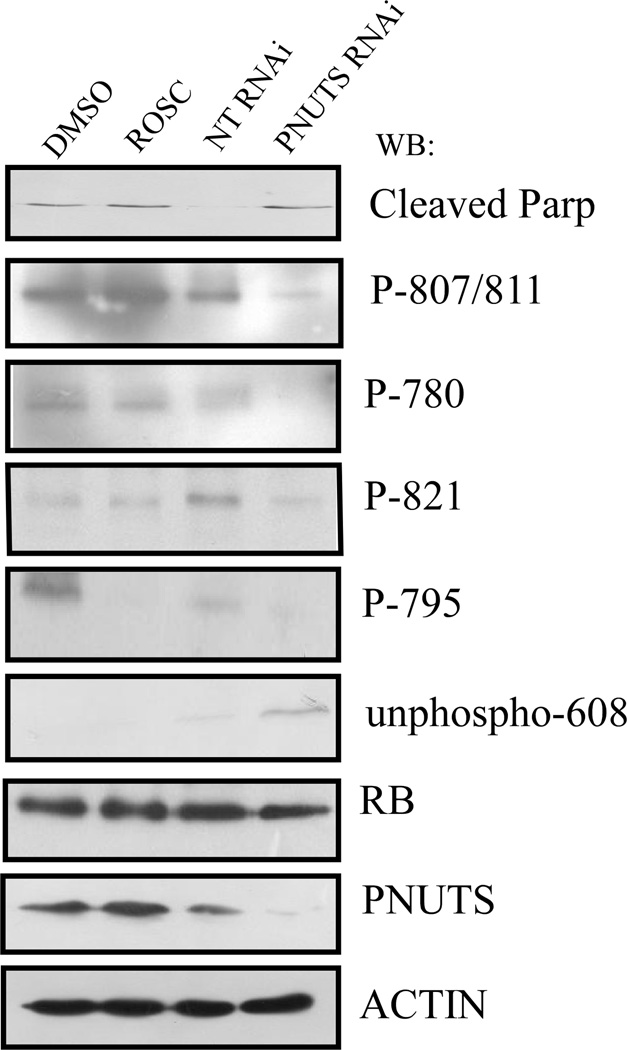
To compare the effects of cdk inhibition versus PP1 activation on Rb phosphorylation, we determined which sites of Rb were dephosphorylated in response to Roscovitine or PNUTS knockdown using p53−/− HCT116 cells. Roscovitine inhibits kinase activity toward Rb, while PNUTS siRNA activates phosphatase activity toward Rb. As shown in Figure 4, these two strategies that cause Rb dephosphorylation display differences in specificity toward Rb phosphorylation sites. For example, Roscovitine treatment causes dephosphorylation of amino acid Ser-795 but not of Thr-821, Ser-780 or Ser-807/811 whereas PNUTS knockdown causes the dephosphorylation of Ser-795 as well as Thr-821, Ser-780, Ser-807/811, and Ser-608. Thus inhibition of cdk activity effects specific sites of Rb phosphorylation, and activation of PP1 toward Rb (via PNUTS knockdown) causes the dephosphorylation of a distinct set of amino acid sites.
Figure 4. Effect of PNUTS knockdown versus Roscovitine treatment on Rb phosphorylation state.
p53−/− HCT116 cells were treated with DMSO or Rosc for eight hours or with nontargeting (NT) or PNUTS RNAi for twenty four hours followed by immunoblotting as described in the Materials and Methods. The antibodies utilized are indicated to the right of the figure. The antibodies to Rb amino acids 807/811, 780, 821, and 795 recognize the phosphorylated sites of Rb. However, the antibody to Rb-608 recognizes the unphosphorylated form of 608. Cleaved Parp is a marker of apoptosis (19). Total Rb expression is shown and PNUTS knockdown and equal loading were confirmed by examining expression of PNUTS and β-actin, respectively. Data shown is representative of two separate experiments.
Bcl-2 family protein expression profiles in response to Roscovitine and PNUTS knockdown
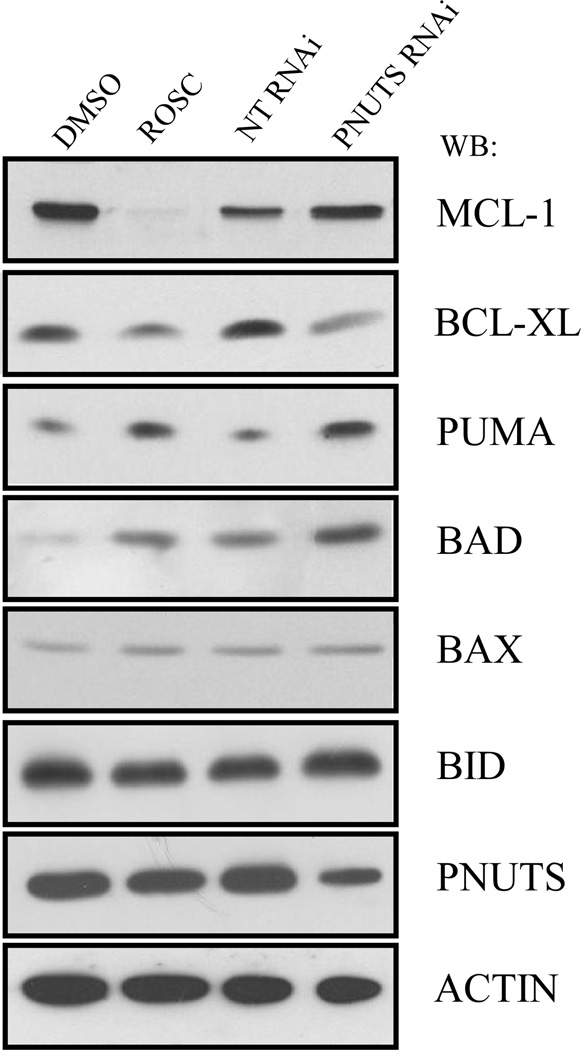
Because apoptosis is carried out by the bcl-2 family of proteins (39), we determined the effect of Roscovitine and PNUTS knockdown on the expression of several members of this family using p53−/− HCT116 cells. Although neither Roscovitine or PNUTS knockdown effected the BAX or BID proteins as shown in Figure 5, there were observable effects on other members of the group. Interestingly, Roscovitine dramatically reduced the expression of MCL-1, a finding which has previously been reported (28). However, PNUTS knockdown had no effect on the expression of this apoptosis mediator. Similarities in the effect produced by Roscovitine treatment and PNUTS knockdown include the decrease of the anti-apoptotic protein BCL-XL, and an increase in the pro-apoptotic proteins PUMA and BAD. Thus the effects of Roscovitine and PNUTS siRNA are similar on most of the bcl-2 family proteins with the notable exception of the effect on the expression of MCL-1.
Figure 5. Effect of PNUTS knockdown versus Roscovitine treatment on apoptosis regulators.
p53−/− HCT116 cells were treated with DMSO or Rosc for eight hours or with nontargeting (NT) or PNUTS RNAi for twenty four hours followed by immunoblotting as described in the Materials and Methods. PNUTS knockdown and equal loading were confirmed by examining expression of PNUTS and β-actin, respectively. The antibodies utilized are indicated to the right of the figure. Data shown is representative of two separate experiments.
Discussion
Alterations in the Rb pathway that lead to excessive phosphorylation of Rb have been observed in almost all cancer types (3, 40). These modifications include overexpression of cyclin proteins which activate cdks and/or the loss of endogenous cdk inhibitors (41). Here in this work we have examined the effect of targeting Rb phosphorylation in cancer cells. In previous studies we have shown that when Rb phosphatase is activated in cells using PNUTS siRNA, the Rb dephosphorylation that results leads to apoptosis (19). To further effect Rb phosphorylation, we utilized the cdk inhibitor Roscovitine. We found that utilized alone, Roscovitine or PNUTS siRNA caused a two-fold increase in apoptosis in breast and colon cancer cells. However, when cells are exposed to both of these treatments, apoptosis is stimulated four-fold. This increase in apoptosis induction observed when cells are treated with Roscovitine plus PNUTS siRNA suggests that the mechanism by which these two treatments act to induce apoptosis may be distinct.
Therefore we analyzed the phosphorylation of Rb at several amino acids in order to determine whether activation of phosphatase activity (PNUTS siRNA) or inhibition of cdk activity (Roscovitine) led to similar or different phosphorylation profiles of Rb. From this analysis of Rb phosphorylation, in these experiments, it appears that activation of phosphatase activity toward Rb (PNUTS knockdown) exerts a greater effect on Rb dephosphorylation than does cdk inhibition, evidenced by the increase in dephosphorylated sites on Rb. This result suggests that in these experiments the primary effect of Roscovitine may not be the dephosphorylation of Rb but of other targets of the cdks as discussed below.
Because most of the tumor suppressor functions of Rb are thought to be mediated via the E2F family of transcription factors, we next determined whether E2F1 was involved in the apoptosis observed in these studies. E2F1 has been implicated in apoptosis that occurs through modulation of Rb activity (42). It is known that Roscovitine increases the expression of E2F1 (36). In addition, E2F1 siRNA reduces Roscovitine induced apoptosis in cells that lack p53 and in TRAIL induced apoptosis (36,43). Finally inhibition of cdks by the cdk inhibitor flavopiridol causes dissociation of E2F from Rb and E2F1 activation (44). Thus it seems clear that Roscovitine does require the activity of E2F1 to cause apoptosis. In this study we determined that E2F1 is also required for apoptosis stimulated by activation of Rb phosphatase activity. This result further extends and strengthens our previous findings that E2F1 is involved in the mechanism by which PNUTS knockdown leads to apoptosis (19).
To understand the roles of the bcl-2 family of proteins in the mechanisms by which PNUTS knockdown and Roscovitine induce apoptosis, we evaluated the expression of this class of proteins after each treatment. The effects of both treatments on three apoptosis regulators were similar for both PNUTS knockdown and Roscovitine treatment. BCL-XL is an inhibitor of apoptosis (39) and was diminished in expression by both treatments. PUMA is an activator of apoptosis (45) and its expression was increased by the two treatments. Finally the expression of pro-apoptotic BAD (39) was also elevated by both the treatments. However, a striking difference occurred in MCL-1 expression between cdk inhibition and phosphatase activation. A reduction in MCL-1 levels alone can induce apoptosis (28). Thus it was striking to observe that Roscovitine abolished the expression of MCL-1 whereas PNUTS knockdown had no effect on MCL-1 expression. It has been shown that the effects of Roscovitine on cells can be mediated through the inhibition of phosphorylation of cdk substrates such as RNA polymerase II. Reduced phosphorylation of RNA polymerase II leads to inhibition of transcription, which results in a loss of MCL-1 expression (28). Thus in this study, it is likely that Roscovitine is acting via the inhibition of RNA polymerase activity, and not only through dephosphorylation of Rb.
Although the involvement of p53 in the apoptosis observed in this study was not directly assessed, it is interesting to note that loss of p53 in MEFs did not diminish the apoptosis observed when PNUTS expression was reduced (figure 3), suggesting that p53 is not required for PNUTS knockdown to cause apoptosis. This result is in agreement with our previous studies that showed that PNUTS knockdown was equally proficient in causing apoptosis in p53+/+ or p53−/− HCT116 colon cancer cells (19). However, p53 likely plays a role in Roscovitine induced apoptosis. Recent studies have shown that Roscovitine up-regulates p53 and induces apoptosis (46). However, here we show that even in the absence of p53, Roscovitine clearly blocks the expression of MCL-1, suggesting that p53 may not be required for Roscovitine to cause cell death. Further studies should elucidate the role of p53 in Roscovitine-mediated cell death.
Methods
Cell Culture
MCF7 cells were obtained from ATCC and grown in high-glucose Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS). HCT116 p53+/+ and HCT116 p53−/− isogenic human colorectal cancer cells were kindly provided by Bert Vogelstein (Johns Hopkins University, Baltimore, MD) and were grown in McCoy’s 5A media containing 10% FBS. Wild Type mouse embryonic fibroblasts (MEFs) were kindly provided by Jean Wang (University of California at San Diego, San Diego, CA). p53−/− and p53−/−E2F1−/− MEFs were a kind gift of Dr Olenski Petrenko, SUNY Stony Brook, NY. MEFs were grown in high-glucose DMEM containing 10% FBS. All cell types were grown in a 37°C humidified incubator containing 5% CO2 and maintained at or below 80–90% confluence.
RNAi Transfection/Drug treatment
Cells were plated at the appropriate density to reach 40–50% confluence in 24 hours and transfections were performed using the X-tremeGENE reagent (Roche) according to the protocols supplied by the manufacturer. The RNA oligonucleotides used in MCF7 and HCT116 cells were generated by Dharmacon based on the human mRNA for PNUTS: PNUTS RNAi: CAGCUAAACUGGUGAAGCA or Nontargeting RNAi: siCONTROL non-targeting siRNA #1 (Dharmacon) which has at least 4 mismatches with all known human, mouse or rat genes which is sufficient to eliminate nonspecific silencing of genes with similar sequences. Mouse specific interfering RNA was obtained from Santa Cruz Biotechnology for the homologous mouse mRNA PNUTS sequence (siRNA-SC61378) which was used in MEF experiments. Transfection mixtures (containing final concentration 100nM of RNA) were added to the cells. Twenty four hours later cells were counted or underwent apoptosis assays or were lysed for protein analysis. For drug treated cells, Roscovitine (Calbiochem) was diluted in DMSO and cells were exposed to 25uM Roscovitine for 8 hours. Control cells received DMSO alone.
Cell proliferation/Apoptosis assays
Cell viability was determined using the Quick Cell proliferation assay kit (Biovision) based on the cleavage of the tetrazolium salt WST-1 to formazan. Percentage of viability was determined by comparing the number of viable cells in treated cultures to the number of viable cells in controls after subtraction of background values from each. The Cell Death Detection ELISA (Roche) was performed as directed by the manufacturer. Briefly, 104 cells from each condition were lysed and subjected to a slow spin centrifugation to pellet nuclei. Extracts from the cytoplasmic fraction were used to detect fragmented DNA. For TUNEL analysis cells were plated on chamber slides at a density of 5,000 cells/uL. Cells were fixed in 4% para-formaldehyde for one hour at room temperature and the TUNEL assay was carried out as per manufacturer’s instructions (Chemicon, ApopTag® Plus Fluorescein In Situ Apoptosis Detection Kit). Results were analyzed by fluorescence microscopy using an Olympus BX60 fluorescence microscope equipped with a monochrome digital acquisition camera. Counts were obtained by counting on random fields and by scoring at least 500 cells per experimental group.
Immunoblotting
SDS-PAGE and Western blotting was performed as previously described(13). In this study we utilized the following primary antibodies: PNUTS (Transduction Laboratories); Rb-phospho-780, Rb-phospho-795, Rb-phospho-807/811, cleaved PARP, BAD, MCL-1, BAK, BID, Bcl-xL, Bcl-2(Cell Signaling Technology); Rb (G3-245), unphosphorylated Rb (608) (Pharmingen), Rb-phospho-821 (Biosource), and β-Actin (Sigma).
Acknowledgements
This work was supported by NIH CA120019 awarded to N. Krucher
References
- 1.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nature Reviews Mol Cell Bio. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan Weinberg: Hallmarks of Cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 3.Mittnacht S. The retinoblastoma protein-from bench to bedside. Eur. J. Cell Biol. 2005;84:97–107. doi: 10.1016/j.ejcb.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Clarke AR, Maandag ER, van Roon M, van der Lugt NM, van der Valk M, Hooper ML, Berns A. Requirement for a functional Rb-1 gene in murine development. Nature. 1992;359:328–330. doi: 10.1038/359328a0. [DOI] [PubMed] [Google Scholar]
- 5.Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA. Effects of an Rb mutation in the mouse. Nature. 1992;359:295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- 6.Lee EYHP, Chang CY, Hu N, Wang YCJ, Lai CC, Herrup K, Lee WH, Bradley A. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature. 1994;359:288–294. doi: 10.1038/359288a0. [DOI] [PubMed] [Google Scholar]
- 7.Haas-Kogan D, Kogan SC, Levi D, Dazin P, T’Ang A, Fung YK, Israel MA. Inhibition of apoptosis by retinoblastoma gene product. EMBO J. 1995;14:461–472. doi: 10.1002/j.1460-2075.1995.tb07022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knudsen KE, Booth D, Naderi S, Sever-Chroneos Z, Fribourg AF, Hunton IC, Feramisco JR, Wang JY, Knudsen ES. RB-dependent S-phase response to DNA damage. Mol Cell Bio. 2000;20:7751–7763. doi: 10.1128/mcb.20.20.7751-7763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherr CJ. Cancer Cell Cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 10.Nelson DA, Krucher NA, Ludlow JW. High molecular weight protein phosphatase type 1 dephosphorylates the retinoblastoma protein. J. Biol. Chem. 1997;272:4528–4535. doi: 10.1074/jbc.272.7.4528. [DOI] [PubMed] [Google Scholar]
- 11.Ianquinta PJ, Lees JA. Life and death decisions by the E2F transcription factors. 2007;19:649–657. doi: 10.1016/j.ceb.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dou QP, An B, Will PL. Induction of a retinoblastoma phosphatase activity by anticancer drugs accompanies p53-independent G1 arrest and apoptosis. Proc Natl. Acad. Sci. USA. 1995;92:9019–9023. doi: 10.1073/pnas.92.20.9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krucher NA, Rubin E, Tedesco VC, Roberts MH, Sherry TC, De Leon G. Dephosphorylation of Rb (Thr-821) in response to cell stress. Exp. Cell Res. 2006;312:2757–2763. doi: 10.1016/j.yexcr.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Popowski M, Ferguson HA, Sion AM, Koller E, Knudsen E, Van Den Berg CL. Stress and IGF-1 differentially control cell fate through mammalian target of rapamycin (mTOR) and Retinoblastoma Protein (pRB) J. Biol. Chem. 2008;283:28265–28273. doi: 10.1074/jbc.M805724200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen PT. Protein Phosphatase 1- targeted in many directions. J. Cell Sci. 2000;115:241–256. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- 16.Allen PB, Kwon Y-G, Nairn AC, Greengard P. Isolation and characterization of PNUTS, a putative protein phosphatase 1 nuclear targeting subunit. J. Biol. Chem. 1998;273:4089–4095. doi: 10.1074/jbc.273.7.4089. [DOI] [PubMed] [Google Scholar]
- 17.Kim YM, Watanabe T, Allen PB, Kim YM, Lee SJ, Greengard P, Nairn AC, Kwon YG. PNUTS, a protein phosphatase 1 (PP1) Targeting Subunit. Characterization of its PP1 and RNA binding domains and regulation by phosphorylation. J. Biol. Chem. 2003;278:13819–13828. doi: 10.1074/jbc.M209621200. [DOI] [PubMed] [Google Scholar]
- 18.Udho E, Tedesco V, Zygmunt A, Krucher NA. PNUTS (PP1 Nuclear Targeting Subunit) inhibits retinoblastoma-directed PP1 activity. Biochem. Biophys. Res. Commun. 2002;297:463–468. doi: 10.1016/s0006-291x(02)02236-2. [DOI] [PubMed] [Google Scholar]
- 19.De Leon G, Sherry TC, Krucher NA. Reduced expression of PNUTS leads to activation of Rb-phosphatase and caspase-mediated apoptosis. Cancer Biol. Ther. 2008;7:833–841. doi: 10.4161/cbt.7.6.5839. [DOI] [PubMed] [Google Scholar]
- 20.Lee SJ, Lim CJ, Min JK, Lee JK, Kim YM, Won MH, Lee JY, Kwon YG. Protein phosphatase 1 nuclear targeting subunit is a hypoxia inducible gene:its role in post-translational modification of p53 and MDM2. Cell Death Diff. 2007;14:1106–16. doi: 10.1038/sj.cdd.4402111. [DOI] [PubMed] [Google Scholar]
- 21.Malumbres M, Pevarello P, Barbacid M, Bischoff JR. CDK inhibitors in cancer therapy: What is next? Trends Pharmacol Sci. 2008;29:16–21. doi: 10.1016/j.tips.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, Inagaki M, Delcros JG, Moulinoux JP. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 23.McClue SJ, Blake D, Clarke R, Cowan A, Cummings L, Fischer PM, Mackenzie M, Melville J, Stewart K, Wang S, Zhelev N, Zheleva D, Lane DP. In vitro and in vivo antitumor properties of the cyclin dependent kinase inhibitor CYC202 (Roscovitine) Int. J. Cancer. 2002;102:463–468. doi: 10.1002/ijc.10738. [DOI] [PubMed] [Google Scholar]
- 24.Fischer PM, Gianella-Borradori A. CDK inhibitors in clinical development for the treatment of cancer. Expert. Opin. Investig. Drugs. 2003;12:955–970. doi: 10.1517/13543784.12.6.955. [DOI] [PubMed] [Google Scholar]
- 25.Whittaker SR, Walton MI, Garrett MD, Workman P. The cyclin-dependent kinase inhibitor CYC202 (R-Roscovitine) inhibits retinoblastoma protein phosphorylation, causes loss of cyclin D1, and activates the mitogen-activated protein kinase pathway. Cancer Res. 2004;64:262–272. doi: 10.1158/0008-5472.can-03-0110. [DOI] [PubMed] [Google Scholar]
- 26.Whittaker SR, te Poele RH, Chan F, Linardopoulos S, Walton MI, Garrett MD, Workman P. The cyclin-dependent kinase inhibitor seliciclib (R-roscovitine; CYC202) decreases the expression of mitotic control genes and prevents entry into mitosis. Cell Cycle. 2007;6:3114–3131. doi: 10.4161/cc.6.24.5142. [DOI] [PubMed] [Google Scholar]
- 27.Payton M, Chung G, Yakowec P, Wong A, Powers D, Xiong L, Zhang N, Leal J, Bush TL, Santora V, Askew B, Tasker A, Radinsky R, Kendall R, Coats S. Discovery and Evaluation of Dual cdk1 and cdk2 inhibitors. Cancer Res. 2006;66:4299–4308. doi: 10.1158/0008-5472.CAN-05-2507. [DOI] [PubMed] [Google Scholar]
- 28.MacCullem DE, Melville J, Frame S, Watt K, Anderson S, Gianella-Borradori A. Seliciclib (CYC202, R-Roscovitine) induces cell death in multiple myeloma cells by inhibition of RNA polymerase II-dependent transcription and down regulation of mcl-1. Cancer Res. 2005;65:5399–5407. doi: 10.1158/0008-5472.CAN-05-0233. [DOI] [PubMed] [Google Scholar]
- 29.Wesierska-Gadek J, Borza A, Walzi E, Krystof V, Maurer M, Komina O, Wandl S. Outcome of treatment of human HeLa Cervical Cancer cells with Roscovitine strongly depends on the dosage and cell cycle status prior to treatment. J. Cell Biochemistry. 2009;106:937–955. doi: 10.1002/jcb.22074. [DOI] [PubMed] [Google Scholar]
- 30.Dey A, Wong ET, Cheok CF, Tergainkar V, Lane DP. R-Roscovitine simultaneously targets both the p53 and NF-kB pathways and causes potentiation of apoptosis:implications in cancer therapy. Cell Death Diff. 2008;15:263–273. doi: 10.1038/sj.cdd.4402257. [DOI] [PubMed] [Google Scholar]
- 31.Paprskarova M, Krystof V, Jorda R, Dzubak P, Hajduch M, Wesierska-Gadek J, Strnad M. Functional p53 in cells contributes to the anticancer effect of the cyclin-dependent kinase inhibitor roscovitine. J. Cell Biochem. 2009;107:428–437. doi: 10.1002/jcb.22139. [DOI] [PubMed] [Google Scholar]
- 32.Lu W, Chen L, Peng Y, Chen J. Activation of p53 by roscovitine-mediated suppression of mdm2 expression. Oncogene. 2001;20:3206–3216. doi: 10.1038/sj.onc.1204412. [DOI] [PubMed] [Google Scholar]
- 33.Fleming IN, Hogben M, Frame S, McClue SJ, Green SR. Synergistic inhibition of erbB signaling by combined treatment with seliciclib and ErbB-targeted agents. Cin Cancer Res. 2008;14:4326–4335. doi: 10.1158/1078-0432.CCR-07-4633. [DOI] [PubMed] [Google Scholar]
- 34.Lambert LA, Qiao N, Hunt KK, Lambert DH, Mills GB, Meijer L, Keyomarsi K. Autophagy: A novel mechanism of synergistic cytotoxicity between doxorubicin and Roscovitine in a sarcoma model. Cancer Res. 2008;68:7966–7974. doi: 10.1158/0008-5472.CAN-08-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wesierska-Gadek J, Maurer M, Schmid G. Inhibition of farnesyl protein transferase sensitizes human MCF7 breast cancer cells to Roscovitine-mediated cell cycle arrest. J. Cell Biochem. 2007;102:736–747. doi: 10.1002/jcb.21325. [DOI] [PubMed] [Google Scholar]
- 36.Ortiz-Ferron G, Yerbes R, Eramo A, Lopez-Perez AI, De Maria R, Lopez-Rivas A. Roscovitine sensitizes breast cancer cells to TRAIL-induced apoptosis through a pleiotropic mechanism. Cell Res. 2008;18:664–676. doi: 10.1038/cr.2008.54. [DOI] [PubMed] [Google Scholar]
- 37.Knudsen ES, Wang JYJ. Dual mechanisms for the inhibition of E2F binding to RB by cyclin-dependent kinase mediated RB phosphorylation. Mol. Cell. Bio. 1997;17:5771–5783. doi: 10.1128/mcb.17.10.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown V, Phillips RA, Gallie BL. Cumulative effect of phosphorylation of pRB on regulation of E2F activity. Mol Cell Bio. 1999;19:3246–3256. doi: 10.1128/mcb.19.5.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cory S, Adams JM. The BCL2 family:regulators of the cellular life or death switch. Nature Reviews Cancer. 2002;2:647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 40.Sherr CJ, McCormick F. The RB p53 pathways in cancer. Cancer Cell. 2002;2:103–112. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 41.Johansson M, Persson JL. Cancer Therapy: Targeting Cell Cycle Regulators Anti-Cancer Agents in Medicinal Chemistry. 2008;8:723–731. doi: 10.2174/187152008785914833. [DOI] [PubMed] [Google Scholar]
- 42.Tsai KY, Hu Y, Macleod KF, Crowley D, Yamasaki L, Jacks T. Mutation of E2F1 suppresses apoptosis and inappropriate S phase entry and extends survival of Rb-deficient mouse embryos. Mol. Cell. 1998;2:293–304. doi: 10.1016/s1097-2765(00)80274-9. [DOI] [PubMed] [Google Scholar]
- 43.Ma Y, Freeman SN, Cress WD. E2F4 deficiency promotes drug-induced apoptosis. Cancer Biology and Therapy. 2004;3:1262–1269. doi: 10.4161/cbt.3.12.1239. [DOI] [PubMed] [Google Scholar]
- 44.Jiang J, Matranga CB, Cai D, Latham VM, Zhang X, Lowel AM, Martelli F, Shapiro GI. Flavopiridol-induced apoptosis during S phase requires E2F1 and inhibition of cyclin A dependent kinase activity. Cancer Res. 2003;63:7410–7422. [PubMed] [Google Scholar]
- 45.Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cells. Molecular Cell. 2001;7:673–682. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- 46.Wesierska-Gadek J, Wandl S, Kramer MP, Pickem C, Krystof V, Hajek SB. Roscovitine upregulates p53 protein and induces apoptosis in human HeLaS (3) cervix carcinoma cells. J. Cell Biochem. 2008;105:1161–1171. doi: 10.1002/jcb.21903. [DOI] [PubMed] [Google Scholar]