Abstract
Problem
Regulatory T cells (Treg) are a vital immune cellular population at the maternal-fetal interface. They are likely to aid in immune tolerance by dampening the harmful effects of other immune cellular populations through cell-cell mediated interactions as well as by producing IL-10 and TGF-β. In addition to the anti-inflammatory properties, IL-10 has emerged as an important vascular cytokine choreographing endovascular interactions, angiogenesis and regulates hypertension.
Method of study
Review of innovative concepts to understand the temporal role of Tregs in both mouse and human pregnancy, particularly whether uterine Treg play a potential role in regulating vascular homeostasis and blood flow during pregnancy.
Results
Treg guard immune tolerance, getting cytotoxically activated under certain conditions, leading to adverse pregnancy outcome.
Conclusions
Despite increasing evidence of Treg tissue-specific expansion and functional plasticity, their role in vascular activity, preeclampsia or gestational diabetes is obscure and needs closer investigation to delineate its role later during pregnancy.
Keywords: Pregnancy, preeclampsia, regulatory T cells, interleukin-10, vascular activity
Introduction
Pregnancy is a unique immune phenomenon because the fetus and the placenta can develop in the womb without being attacked by the maternal immunesystem and despite admixing of maternal and fetal cells 1. One key reason may be the presence of uterine regulatory T cells (Tregs ) that play an important role in protecting the fetus by inhibiting harmful immune responses that would otherwise be detrimental to the fetus 2. In addition to their immuno-suppressive role during pregnancy, accumulating body of evidence now positions Tregs as modulators of vascular homeostasis and blood flow 3.
Regulatory T cells in pregnancy
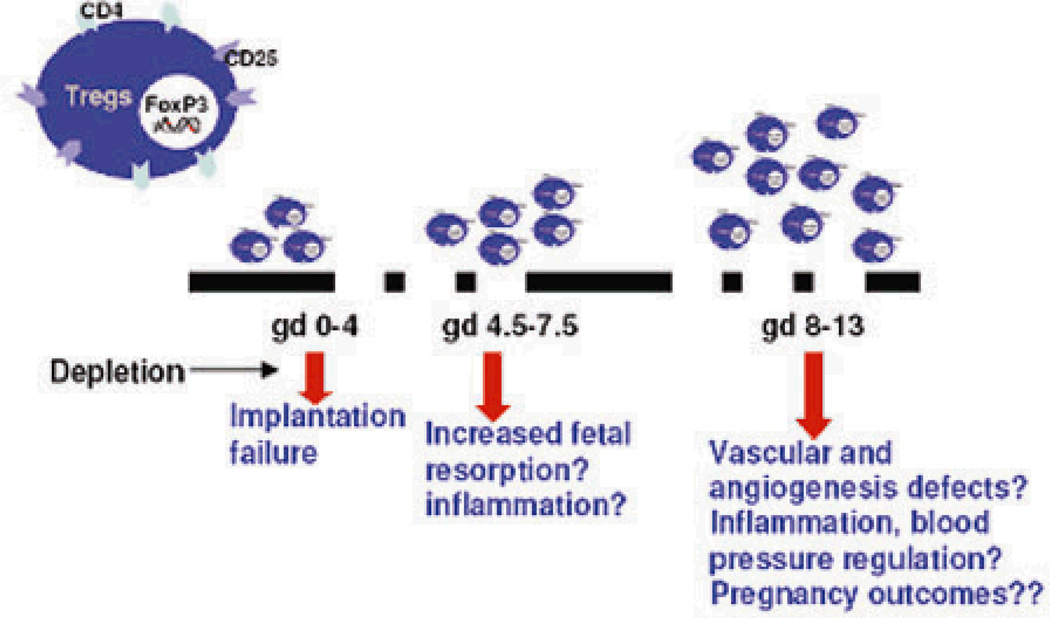
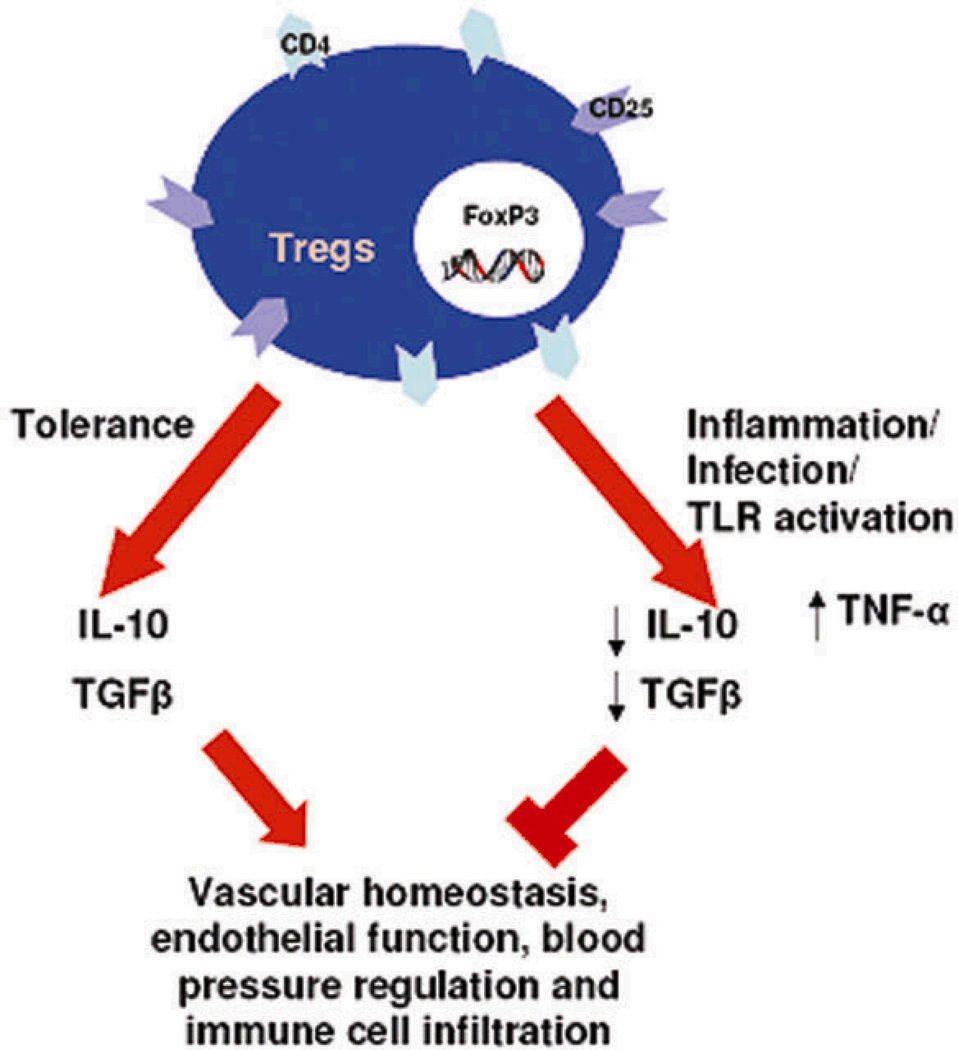
Regulatory T cells (Tregs ) are a specialized population of T lymphocytes known for their properties as potent suppressors of inflammatory immune responses and their ability to mediate immune homeostasis. Their unique properties, particularly their ability to suppress cytotoxically activated T cells and NK cells make them an integral part of immune tolerance during pregnancy. These cells are characterized by the surface expression of CD4, CD25, and the intracellular forkhead box transcription factor, Foxp3 4,5. In humans, regulatory T cells increase very early in pregnancy, peak during the second trimester and then begin to decrease to pre-pregnancy levels 3, 6. Mice exhibit a similar trend where Tregs begin to increase as early as day 2.5, peak during mid-gestation, and reach non-pregnant levels around gestational day (GD)17 7. Incidentally, the peak of Tregs coincides with the phase of pregnancy that entails intense vascular activity, trophoblast invasion, spiral artery remodeling and peak populace of uterine NK cells at the maternal-fetal interface. The importance of Treg cells for pregnancy was elegantly demonstrated by Aluvihare et al, where they adoptively transferred T lymphocytes depleted of CD4+CD25+ into pregnant T cell deficient mice 6. Here, the allogeneic fetal units were rejected in the absence of Tregs. Importantly, depletion of Tregs between GD2.5 and GD4.5 in mice resulted in implantation failure and significantly increased fetal resorption rates, whereas depletion of Tregs on GD10 did not induce discernable adverse pregnancies 8. These findings suggest that regulatory T cells are more important in the implantation phase and the early stages of pregnancies (Fig 1). The importance of Treg cells for successful pregnancy was further verified in another study where depletion of Tregs in pregnant mice resulted in fewer fetuses surviving at term 9. Furthermore, reduced levels of CD4+CD25+ cells were found in the decidual tissues of abortion prone DBA/J-mated CBA/J female mice. Adoptive transfer of Tregs isolated from normal pregnant mice but not non-pregnant mice prevented fetal demise 10. This implies that the hormonal changes associated with pregnancy uniquely influence Treg cell functions. Infertility has been proposed to be associated with reduced Treg cells in endometrial tissue 11. Spontaneous abortion cases and patients with recurrent miscarriage are also associated with lower systemic Tregs compared to that seen in normal pregnancies 12,13. It is now well appreciated that apart from cell-cell mediated immunoregulation, Tregs produce two immunosuppressive cytokines TGF-β and IL-10 that contribute to their anti-inflammatory effects. Since these cytokines also influence vascular activity, it is tempting to speculate that regulatory T cells may play a part in regulation of blood pressure. Conversely, dysregulated Tregs may contribute to the onset of preeclampsia (Fig 2).
Figure 1. Kinetics of possible functional implications of uterine Tregs.
Tregs begin to increase as early as gestational day (gd) 2.5, peak during mid-gestation (gd 12), then reach non-pregnant levels at term in mice. The importance of Tregs during implantation and early stages of pregnancies are supported by depletion studies.
Figure 2. Uterine Tregs and Vascular Homeostasis.
Tregs produce two immunosuppressive cytokines TGF-β and IL-10 that can influence vascular activity and may play a part in regulation of blood pressure. Conversely, dysregulated Tregs either due to infection, inflammation or TLR activation may perturb vascular homeostasis by producing TNFα.
Regulation of hypertension and vascular remodeling by IL-10
Interleukin-10 (IL-10), a key immunosuppressive cytokine, produced by Tregs, is increased early in pregnancy and remains elevated until the onset of labour 14. Various cellular populations are involved in its production at the maternal–fetal interface. Notable, villous cytotrophoblast produce IL-10 whereas extravillous trophoblasts (EVT) are poor producers of IL-10 15. IL-10 and its receptor (IL-10R) are expressed on a variety of cell types found at the maternal-fetal interface in both mice and humans namely, placental trophoblasts, decidual stromal cells, macrophages, and uNK cells 16,17. Mice genetically deficient in IL-10 have been very useful in the investigation into the precise functions of IL-10 during pregnancy. IL-10−/− mice are highly sensitive to pro-inflammatory stimuli such as lipopolysaccharide, polyinosinic:polycytidylic acid (poly I:C) and CpG, which are known ligands for toll-like receptors,18–21 as well as environmental toxicants like polychloride biphenyls22. Fetal resorption could be observed as early as GD9 in response to early treatment with polyI:C, a ligand for TLR-3 (unpublished data). Although it is known that Tregs host a repertoire of toll-like receptors and respond differentially in response to specific TLR ligation21, what is not clear is if the density and variety of TLR expressed by uterine Tregs is different from those cells in circulation or in spleen and if so what are their tissue-specific implications at different stages of gestations. In this context, it has been shown that viral infections sensed by TLR3 are associated with hypertensive disorders of pregnancy. Treatment of pregnant rats with the viral mimic poly I:C on gd 10 resulted in elevated systolic blood pressures, decreased aortic vasodilation, increased urinary protein concentrations, and had more malformed pups 23. This suggests that perturbations of Tregs during different phases of pregnancy may precipitate a diverse spectrum of pregnancy outcomes. In addition to its known anti-inflammatory functions, recent observations suggest that IL-10 can regulate vascular activity and endovascular interactions at the maternal-fetal interface23,24. Administration of recombinant IL-10 reversed hypoxia-induced hypertension, proteinuria and intrauterine growth restriction in IL-10−/− mice 24.Overall, these studies imply that IL-10 is likely to play key protective roles against preeclampsia.
Do Tregs play a role in vascular homeostasis during pregnancy?
Many developmental and immune-regulatory changes occur in pregnancy. Changes that involve the maternal adaptation to blood flow and vascular homeostasis include reduced pressor response to angiotensin II, increase in plasma volume resulting in a decrease in the proportion of red blood cells, peripheral vasodilation associated with significant increase in cardiac output, and transient low blood pressure25–28. Thus, understanding the blood flow in the context of changes that occur during normal pregnancy is important because failure to adjust to these changes may result in hypertensive disorders of pregnancies such as preeclampsia. Although not well understood, immune regulation may play a central role in the pathogenesis of hypertensive disorders of pregnancy. The focus has been on uterine NK cells. However, recent studies by Croy and colleagues demonstrate that uterine NK cells do not affect blood pressure regulation during pregnancy 29. This raises a question whether Tregs are involved in regulating blood flow and pressure during pregnancy.
Various reports have demonstrated that Tregs can modulate heart fibrosis in hypertension, control coronary arteriolar endothelial dysfunction in hypertensive mice and suppress angiotensin II-induced hypertension and vascular injury3,30,31. Angiotensin II is known for its role in the development of hypertension by mechanisms mediated in part by innate and adaptive immunity. Studies in which Tregs were adoptively transferred into Ang II-infused hypertensive mice prevented cardiac hypertrophy and reduced blood pressure, vascular damage and prevented Ang II-induced hypertension30,32. The concept that Tregs can control inflammation in a mouse model of chronic Ang II infusion demonstrated that Ang II does not directly influence Tregs. However, Treg-treatment maintained other T cell populations, signifying the mechanistic importance of these cells32.
The soluble fms-like tyrosine kinase receptor (sFlt1) and soluble endoglin have been implicated in the pathogenesis of preeclampsia33–36. Interestingly, soluble endoglin has been shown to precipitate hypertension and endothelial dysfunction by inhibiting TGFβ, 36 which incidentally is a vital cytokine essential to the tolerogenic actions of Tregs. Patients with preeclampsia produce high levels of Th1 cytokines (IL-2, IL-6, TNFα and IFN-γ), and reduced secretion of Th 2 cytokines (IL-10 and IL-15). Interestingly, IL-6 and TNF-α have been shown to induce hypertension and proteinuria, hallmark features of preeclampsia37. Several studies have reported a reduction in the numbers of Tregs in peripheral blood and also the placental bed sections in pre-eclamptic women compared to healthy pregnant controls38–41. Recently, our lab has shown that injection of preeclampsia serum in pregnant IL-10−/− mice causes hypertension and proteinuria42. Interestingly, injection of human preeclampsia serum resulted in full spectrum of PE-like symptoms and spiral artery remodeling defects and reduced Tregs population (unpublished results) in IL-10−/− mice43,44. The significance of persistence and peak amplification of Tregs around gd 12 in mice and by the end of first trimester of human pregnancy on placental angiogenesis and blood pressure regulation is not clear. Since Tregs reach peak on GD 12 in mice, it is tempting to speculate that besides immune reactions, Tregs may also play a role in these processes as well which become consequential at around this period during gestation.
Conclusions
Hypertension during pregnancy, particularly as related to preeclampsia, predominantly leads to preterm birth, intrauterine growth restriction (IUGR) and placental dysfunction45. Additionally, women with pre-existing and gestational diabetes are at an even higher risk for acquiring hypertensive disorders of pregnancies, which in turn, significantly increase the risk for future cardiovascular complications46. Thus clinicians and/or health care providers are faced with the challenge of controlling maternal blood pressure and glucose levels so as to improve maternal and fetal outcome. Tregs are guardians of immune tolerance, yet are flexible to get cytotoxically activated under certain conditions which may lead to adverse pregnancy outcomes. Increasing evidence is emerging regarding their tissue-specific expansion and functional plasticity; however, their role in vascular activity, preeclampsia or gestational diabetes is obscure and needs closer investigation to delineate its role later during pregnancy.
Acknowledgements
This research is supported by the National Institutes of Health grant P20RR018728 and by the Rhode Island Research Alliance Collaborative Research Award 2009-28.
References
- 1.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 2.Guerin LR, Prins JR, Robertson SA. Regulatory T cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update. 2009;15:517–535. doi: 10.1093/humupd/dmp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matrougui K, Zakaria AE, Kassan M, Choi S, Nair D, Gonzalez-Villalobos RA, Chentoufi AA, Kadowitz P, Belmadani S, Partyka M. Natural Regulatory T cells control coronary arteriolar endothelial dysfunction in hypertensive mice. Am J Pathol. 2011;178:434–441. doi: 10.1016/j.ajpath.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 5.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 6.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 7.Thuere C, Zenclussen ML, Schumacher A, Langwisch S, Schulte-Wrede U, Teles A, Paeschke S, Volk HD, Zenclussen AC. Kinetics of regulatory T cells during murine pregnancy. Am J Reprod Immunol. 2007;58:514–523. doi: 10.1111/j.1600-0897.2007.00538.x. [DOI] [PubMed] [Google Scholar]
- 8.Shima T, Sasaki Y, Itoh M, Nakashima A, Ishii N, Sugamura K, Saito S. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol. 2010;85:121–129. doi: 10.1016/j.jri.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Darrasse-Jeze G, Klatzmann D, Charlotte F, Salomon BL, Cohen JL. CD4 +CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol Lett. 2006;102:106–109. doi: 10.1016/j.imlet.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T, Kotsch K, Leber J, Volk HD. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005;166:811–822. doi: 10.1016/S0002-9440(10)62302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jasper MJ, Tremellen KP, Robertson SA. Primary unexplained infertility is associated with reduced expression of the regulatory T cell transcription factor Foxp3 in endometrial tissue. Mol Hum Reprod. 2006;12:301–308. doi: 10.1093/molehr/gal032. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10:347–353. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- 13.Arruvito L, Billordo A, Capucchio M, Prada ME, Fainboim L. IL-6 trans-signaling and the frequency of CD4+FOXP3+ cells in women with reproductive failure. J Reprod Immunol. 2009;82:158–165. doi: 10.1016/j.jri.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Hanna N, Hanna I, Hleb M, Wagner E, Dougherty J, Balkundi D, Padbury J, Sharma S. Gestational age-dependent expression of IL-10 and its receptor in human placental tissues and isolated cytotrophoblasts. J Immunol. 2000;164:5721–5728. doi: 10.4049/jimmunol.164.11.5721. [DOI] [PubMed] [Google Scholar]
- 15.Kalkunte S, Lai Z, Tewari N, Chichester C, Romero R, Padbury J, Sharma S. In vitro and in vivo evidence for lack of endovascular remodeling by third trimester trophoblasts. Placenta. 2008;29:871–878. doi: 10.1016/j.placenta.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin 10 and the interleukin 10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 17.Bennet WA, Lagoo-Deenadayalan S, Whitworth NS, Brackin MN, Hale E, Cowan BD. Expression and production of interleukin-10 by human trophoblast: relationship to pregnancy immunotolerance. Early Pregnancy. 1997;3:190–198. [PubMed] [Google Scholar]
- 18.Murphy SP, Fast LD, Hanna NN, Sharma S. Uterine NK cells mediate inflammation-induced fetal demise in IL-10 null mice. J Immunol. 2005;175:4048–4090. doi: 10.4049/jimmunol.175.6.4084. [DOI] [PubMed] [Google Scholar]
- 19.Murphy SP, Hanna NN, Fast LD, Shaw S, Berg G, Padbury JF, Romero R, Sharma S. Evidence for Participation of uterine natural killer cells in the mechanism responsible for spontaneous preterm labour and delivery. Am J Obstet Gyneocol. 2009;200:e1–e9. doi: 10.1016/j.ajog.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thaxton JE, Romero R, Sharma S. TLR9 activation coupled to IL-10 deficiency induces adverse pregnancy outcomes. J Immunol. 2009;183:1144–1154. doi: 10.4049/jimmunol.0900788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thaxton JE, Sharma S. Interleukin 10: a multi-faceted agent of pregnancy. Am J Reprod Immunol. 2010;63:482–491. doi: 10.1111/j.1600-0897.2010.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tewari N, Kalkunte S, Murray DW, Sharma S. The water channel aquaporin is a novel molecular target of polychlorinated biphenyls for in utero abnormalities. J Biol Chem. 2009;284:15224–15232. doi: 10.1074/jbc.M808892200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tinsley JH, Chiasson VL, Mahajan A, Young KJ, Mitchell BM. Toll-like receptor 3 activation during pregnancy elicits preeclampsia-like symptoms in rats. Am J Hypertens. 2009;22(12):1314–1319. doi: 10.1038/ajh.2009.185. [DOI] [PubMed] [Google Scholar]
- 24.Lai Z, Kalkunte S, Sharma S. A critical role of IL-10 in modulating hypoxia-induced preeclampsia-like disease in mice. Hypertension. 2011;57:505–514. doi: 10.1161/HYPERTENSIONAHA.110.163329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong AYH, Shathiyah K, Whiteley KJ, Qu D, Langille BL, Adamson SL. Maternal cardiovascular changes during pregnancy and postpartum. Am J Physiol Heart Circ Physiol. 2002;282:H918–H925. doi: 10.1152/ajpheart.00641.2001. [DOI] [PubMed] [Google Scholar]
- 26.Ahokas RA, Sibai BM, Anderson GD. Lack of evidence of a vasodepressor role for relaxin in spontaneously hypertensive and normotensive pregnant rats. Am J Obstet Gynecol. 1989;161:618–622. doi: 10.1016/0002-9378(89)90365-7. [DOI] [PubMed] [Google Scholar]
- 27.Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy: J Clin Invest. 1973;52:2682–2689. doi: 10.1172/JCI107462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novak K, Kaufman S. Effects of pregnancy, estradiol, and progesterone on pressor responsiveness to angiotensin II. Am J Physiol Regulatory Integrative Comp Physiol. 1991;261:R1164–R1170. doi: 10.1152/ajpregu.1991.261.5.R1164. [DOI] [PubMed] [Google Scholar]
- 29.Burke SD, Barrette VF, Bianco J, Thorne JG, Yamada AT, Pang SC, Adams MA, Croy BA. Spiral arterial remodeling is not essential for normal blood pressure regulation in pregnant mice. Hypertens. 2010;55:729–737. doi: 10.1161/HYPERTENSIONAHA.109.144253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schriffin T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertens. 2011;57:469–476. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- 31.Valencia X, Lipsky PE. CD4+CD25+Foxp3+ regulatory T cells in autoimmune diseases. Nat Clin Pract Rheumatol. 2007;3:619–626. doi: 10.1038/ncprheum0624. [DOI] [PubMed] [Google Scholar]
- 32.Burke SD, Barrette VF, Gravel J, Carter ALI, Hatta K, Zhang J, Chen Z, Leno-Duran Ester, Bianco J, Leonard S, Murrant C, Adams MA, Croy A. Uterine NK cells, spiral artery modification and the regulation of blood pressure during mouse pregnancy. Am J Reprod Immunol. 2010;63:472–481. doi: 10.1111/j.1600-0897.2010.00818.x. [DOI] [PubMed] [Google Scholar]
- 33.Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, Ecker J, Karumanchi SA. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J. Clin. Endocrinol Metab. 2004;89:770–775. doi: 10.1210/jc.2003-031244. [DOI] [PubMed] [Google Scholar]
- 34.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 35.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, Gomez R, Edwin S, Chaiworapongsa T, Levine RJ, Karumanchi SA. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J. Matern. Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D'Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 37.Zhou CC, Irani RA, Dai Y, Blackwell SC, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Autoantibody-Mediated IL6-Dependent Endothelin-1 Elevation Underlies Pathogenesis in a Mouse Model of Preeclampsia. J Immunol. 2011 doi: 10.4049/jimmunol.1004026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, Van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1994;101:669–674. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki Y, Darmochwal-Kolarz D, Suzuki D, Sakai M, Ito M, Shima T. Proportion of Peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in preeclampsia. Clin Exp Immunol. 2007;149:139–145. doi: 10.1111/j.1365-2249.2007.03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santer-Nanan B, Peek MJ, Khanam R, Richards L, Zhu E, Fazekas de St Groth B. Systemic in crease in the ratio between foxp3+ and IL-17 producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009;183:7023–7030. doi: 10.4049/jimmunol.0901154. [DOI] [PubMed] [Google Scholar]
- 41.Prin JR, Boelens HM, Heimweg J, Van der Heide S, Dubois AE, Van Oosterhout AJ. Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertens Pregnancy. 2009;61:398. doi: 10.1080/10641950802601237. [DOI] [PubMed] [Google Scholar]
- 42.Kalkunte S, Boij R, Norris W, Friedman J, Lai Z, Kurtis J. Sera from preeclampsia patients elicit symptoms of human disease in mice and provide a basis for an in vitro predictive assay. Am J Pathol. 2010;177:2387–2398. doi: 10.2353/ajpath.2010.100475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalkunte S, Nevers T, Norris WE, Sharma S. Vascular IL-10: a protective role in preeclampsia. J Reprod Immunol. 2011;88:165–169. doi: 10.1016/j.jri.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norris W, Nevers T, Sharma S, Kalkunte S. Review: hCG preeclampsia and regulatory T cells. Placenta. 2011;32:S182–S185. doi: 10.1016/j.placenta.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ness R, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am J Obstet Gynecol. 2006;195:1–1. doi: 10.1016/j.ajog.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan SD, Umans JG, Ratner R. Hypertension complicating diabetic pregnancies: pathphysiology, management, and controversies. J Clin Hypertens. 2011;13:275–284. doi: 10.1111/j.1751-7176.2011.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]