Abstract
Objectives
To determine long-term effects on substance use and substance use disorder (SUD), up to 8 years after childhood enrollment, of the randomly assigned 14-month treatments in the multisite Multimodal Treatment Study of Children with Attention-Deficit/Hyperactivity Disorder (MTA; n=436); to test whether (a) medication at follow-up, (b) cumulative psychostimulant treatment over time, or (c) both relate to substance use/SUD; to compare substance use/SUD in the ADHD sample to the non-ADHD childhood classmate comparison group (n=261).
Method
Mixed-effects regression models with planned contrasts were used for all tests except the important cumulative stimulant treatment question, for which propensity score matching analysis was used.
Results
The originally randomized treatment groups did not differ significantly on substance use/SUD by the 8 year follow-up or earlier (M age = 17 years). Neither medication at follow-up (mostly stimulants) nor cumulative stimulant treatment was associated with adolescent substance use/SUD. Substance use at all time points, including use of two or more substances and SUD, were each greater in the ADHD than non-ADHD samples, regardless of sex.
Conclusions
Medication for ADHD did not protect from, nor contribute to, visible risk of substance use or SUD by adolescence, whether analyzed as randomized treatment assignment in childhood, as medication at follow-up, or as cumulative stimulant treatment over an 8 year follow-up from childhood. These results suggest the need to identify alternative or adjunctive adolescent-focused approaches to substance abuse prevention and treatment for boys and girls with ADHD, especially given their increased risk for use and abuse of multiple substances that is not improved with stimulant medication. Clinical trial registration information—Multimodal Treatment Study of Children with Attention Deficit and Hyperactivity Disorder (MTA); http://clinical trials.gov/; NCT00000388.
Keywords: attention-deficit/hyperactivity disorder (ADHD), substance uses
Introduction
Risk of substance use or substance abuse or dependence disorders for children with attention-deficit/hyperactivity disorder (ADHD) is a topic of substantial interest for researchers, practitioners, and parents. Recent meta-analytic reviews of pertinent longitudinal studies have reported moderately higher rates of substance abuse and dependence by early adulthood for children with the disorder in relation to non-ADHD comparison groups1,2. For example, although results across studies are heterogeneous, children with ADHD are more than twice as likely as comparison peers to meet diagnostic criteria for marijuana abuse or dependence by early adulthood2. The included studies do not, however, provide data about the developmental course of substance use, and they tend to emphasize diagnosis of substance abuse or substance dependence even for adolescents whose addiction risk is often better measured by more developmentally sensitive, continuous variables (e.g., frequency of alcohol use)3,4. Nevertheless, the results raise important questions about factors, such as treatment history, that might affect ADHD-related addiction vulnerability.
Although not the only evidence-based treatment for ADHD5, stimulants (e.g., methylphenidate, amphetamine) are the most commonly used and widely accessible treatment, and prescription rates have increased markedly in recent years6. These medications reliably decrease ADHD symptoms for over 80% of diagnosed children, sometimes dramatically7,8. To the extent that symptoms of ADHD contribute to addiction vulnerability, treatment with stimulant medication should theoretically decrease risk. Indeed, a growing number of studies have shown that ADHD symptom persistence correlates with substance use9–12. In other accounts, however, risk of substance abuse has been hypothesized to increase for children medicated with stimulants13. This possibility, potentially linked to the overlap in brain-based mechanisms for both addictive behaviors and stimulant medication efficacy14, has been supported, with mixed results, in animal studies showing an enhanced response to drugs of abuse following methylphenidate exposure15. However, route of administration, relative dosages, developmental timing of medication receipt, and limits of cross-species parallels (or lack thereof) complicate interpretation.
An early, small meta-analytic review of stimulant treatment and risk of substance abuse reported a protective effect of medication use in adolescence and, to a lesser extent, in early adulthood16. However, considering recently published studies, the accumulated data appear mixed. Some studies have found protective associations17–20; one reported predisposing associations (i.e., more medication treatment, more substance use)13,21–22; and three reported no association23–25. Barkley et al.25 reported one positive association for adulthood cocaine use, which fell to non-significance after controlling for conduct disorder. A retrospective chart review of a birth cohort in Rochester, MN, reported protection from substance abuse by age 1826. Two other prospective longitudinal studies recently reported (1) no association between years of stimulant treatment and substance use severity or disorder for 16 to 22 year olds27 and (2) no association between childhood treatment with stimulant medication (including course of medication) and SUD or tobacco use to age 2428.
Substantial and important variability in the methodologic features of these studies makes it difficult to synthesize the findings. Samples vary considerably in age both within and across studies, and age is a potentially confounding variable that is strongly related to both treatment and substance use (W. Pelham, unpublished conference presentation, 2004 October). Compounding this problem is that most relevant research is based on sample sizes of about 100, which can be insufficient for examining the association between stimulant treatment and a variable that has a low to moderate frequency occurrence even in high risk samples (i.e., substance abuse). For example, marijuana abuse and dependence peak at 5.6% of 18–25 year olds nationally and are even less frequent at younger ages29. A few of the relevant studies measured actual substance use21,24,25,27 whereas others relied on diagnostic assessments of abuse or dependence (which, as mentioned earlier, may not identify teens on a developmental trajectory toward abuse/dependence in adulthood). Studies also vary in their operationalization of psychoactive treatment history, which has resulted in fundamentally different questions being addressed across studies comprising a relatively small literature.
The longitudinal follow-up of the children in the Multimodal Treatment Study of ADHD (MTA) provides an opportunity to examine the association between ADHD treatment and substance abuse with a large, diverse, narrow age-band sample recruited in childhood, treated for 14 months in a randomized clinical trial, and followed prospectively with substance use assessments through adolescence14. The randomized trial design of the MTA in childhood (see below) provided partial protection from the influences of self-selection into treatment at an age when medication treatment is at its peak30,31. In the first published MTA report of substance use24 at three years after study baseline, no protective or predisposing associations were found between substance use and early medication exposure, measured as either (a) randomly assigned treatment or (b) proportion of days medicated in the year leading up to the substance use assessment. However, substance use was nascent in the first follow-up report, with limited opportunity to detect treatment effects because ages ranged from 10 to 13 years. We now turn to the eight year follow-up (M age 16.8), when substance use is more prevalent32.
Hence, one aim of the current study was to test the association between medication treatment (both as randomly assigned during childhood and naturalistically used following the clinical trial) and adolescent substance use/SUD by mean age 17. We opted for a three-pronged approach to examine substance use/SUD as a function of (a) random assignment to medication in childhood (at a mean age of 8.5); (b) concurrent medication use across the multiple follow-up assessments; and most importantly, (c) duration of medication (specifically psychostimulant treatment) from study entry to the eight year follow-up. Thus, we expanded our analyses to consider putative long-term exposure effects of stimulant medication that have been hypothesized to either protect children with ADHD from or increase risk for substance use/SUD14. Although the initial randomization affected subsequent parental choices about medication and therefore provided only partial protection against the effects of confounding variables underlying natural selection into medication over time, initial group differences in medication use were lost by the three year follow-up33. To control for these and other individual differences in propensity to medicate over time, we selected an analytic approach—propensity score matching—favored for managing the effects of confounding variables34. A similar propensity analysis was conducted at 3-year follow-up for effects of medication on ADHD symptoms, without significant results35.
A second aim was to compare substance use/SUD at the 8 year follow-up between the children with ADHD and an MTA-recruited and followed comparison group without ADHD. This analysis tested an extension of our prior findings24 of ADHD-related risk for substance use experimentation to heavier levels of use/SUD for the high school age range. Polysubstance use is highly prevalent among teen substance users, but questions have arisen about ADHD-related drug specialization, especially for tobacco because of its distinct pharmacologic (stimulant) properties36; we examined use of other drugs by tobacco users. We also tested whether our failure to find sex differences in early adolescent substance use24 would extend to this older age range. Because of the larger research literature on ADHD for boys than for girls, it is not surprising that little research has been conducted on use/SUD in girls with ADHD histories (for exceptions, see Babinski et al.37; Biederman et al.38; Hinshaw et al.39,40).
Method
Participants
The participants with ADHD in the MTA were 579 children with DSM-IV ADHD combined type. Each of the six participating sites randomized 95 to 98 children to one of four treatment groups: Medication Management (MedMgt), Behavior Therapy (Beh), Combined MedMgt plus Beh (Comb), and Community Comparison (CC). At baseline (pretreatment), participants were 7.0 to 9.9 years of age (M=8.5 years, SD=0.8 years). The MTA recruitment strategy, procedures for diagnosing ADHD, treatment specifics, and sample demographics have been described elsewhere41–46.
The children were reassessed at 3 and 9 months, at completion of the 14-month treatment phase, at 24 and 36 months following randomization, and again at 6 and 8 years after randomization. Participation rates dating from 14 months were 97%, 93%, 84%, 78%, and 75% of the original 579 enrolled, respectively. There were no significant differences in any baseline characteristics between participants and non-participants for the 36-month assessment33. However, children lost to the 8-year follow-up assessment, compared with those retained, were more often male, had younger mothers, less-educated parents, lower parent income, and parents more likely to have antisocial personality disorder at baseline (p<.025 corrected for experimentwise-error). There were no significant differences on the remaining sociodemographic or adversity variables (e.g., age, grade, ethnicity/race, parent marital status, stable residency, on welfare, parent job loss, child health, birthweight) or on baseline measures of intellect and achievement, parent and teacher report of ADHD and oppositional defiant disorder (ODD) symptoms, parent-reported aggression and conduct problems, parental diagnoses of mood, anxiety, alcohol, or other drug use disorders, or randomized treatment group assignment. Mean ages at the 6- and 8-year follow-up assessments were 14.9 (SD=1.0) and 16.8 (SD=1.0) years, respectively.
A local normative comparison group (LNCG, N=289) was recruited at 24 months to reflect the local populations from which the ADHD sample was drawn. The LNCG children were randomly selected from the same schools and grades and in the same sex proportions as the children with ADHD. LNCG children were not excluded from recruitment because of symptoms of ADHD, but those found to have diagnosable ADHD (n=31) were excluded from the present analyses as we have done previously47. The assessment battery included the Diagnostic Interview Schedule for Children Version-IV (DISC-IV48) and teacher-reported ratings of ADHD symptoms, which afforded examination of DSM-IV diagnoses and ADHD symptom severity. The LNCG had the same entry criteria as the children with ADHD except for ADHD diagnosis and age; they were matched to the age of the ADHD sample at 24 months after randomization. Thus, data for the LNCG are available starting at the 24-month assessment. At that time, average age of the LNCG (M=10.4 yrs, SD=1.08 yrs) did not differ significantly from that of the ADHD sample (t811=1.04, p=.36) at that time point. The percentage of female subjects was similar in the LNCG (18.7%, n=54/289) and the ADHD samples (19.7%, n=114/579, χ21=0.13, ns). The percentage of retained LNCG participants by 6 and 8 years was 87% (252/289) and 90% (261/289), respectively. The LNCG participants lost to the 8-year assessment had less stable residency and younger mothers than those retained, but all other baseline variables were non-discriminating. LNCG mean ages at the 6- and 8-year assessments were 14.5 (SD=1.2) and 16.6 years (SD=1.2), respectively.
Measures
The substance use outcomes were measured at all interviews beginning with the 24 month assessment. Use (e.g., consumption) of substances was measured separately from DSM symptoms of abuse or dependence49. The primary analyses made use of two variables: a single substance use variable and a single abuse/dependence variable, each composited across substances. Secondary analyses explored substance-specific associations.
Substance Use
Substance use was assessed with a child/adolescent-reported questionnaire50 adapted for the MTA. The measure included items for lifetime and current (past 6 months) use of alcohol, cigarettes, chewing tobacco, marijuana, and other street drugs. Also included were items for non-prescribed use or other misuse of psychoactive medications, including stimulants. The measure was modeled after similar substance use measures in longitudinal or national survey studies of alcohol and other drug use51–53 that also rely on confidential youth self-report as the best source of data54. A National Institutes of Health (NIH) Certificate of Confidentiality further strengthened the assurance of privacy.
Our primary substance use variable was a binary composite which allowed consideration of multiple substance use classes simultaneously. Analysis of substance use as an ordinal variable is a common approach—particularly for studying adolescents55. Substance use was coded positive if any of the following behaviors, selected after examining distributions, were endorsed as occurring in the participant’s lifetime: (1) drank alcohol (more than just a sip) more than five times or drunk at least once; (2) smoked cigarettes or tried chewing tobacco more than a few times; (3) used marijuana more than once; or (4) used inhalants, hallucinogens, cocaine, or any of amphetamines/stimulants, barbiturates/sedatives, opioids/narcotics without a prescription or misused a prescription (used more in quantity or more often than prescribed). Each of the four types of substances, as well as daily use of tobacco and the number of substance use classes endorsed (0, 1, 2 or more), were explored in secondary analyses.
For the analysis of stimulant treatment duration in relation to substance use at the 8 year follow-up, the primary outcome was number of substances used in the past six months, in order to ensure that most stimulant treatment received would have preceded substance use. Component variables included (1) “drunk” once or more or drank alcohol 3–4 times or more; (2) one or more cigarettes/day in the past month (time frame exception specific to tobacco); (3) marijuana two or more times; and (4) any other illicit drug use or prescription medication misuse. Secondary analyses explored each class of substances separately.
Substance abuse or dependence (SUD)
DSM-IV abuse or dependence was based on a positive parent or child report with the Diagnostic Interview Schedule for Children Ver. 2.3/3.0 (DISC48) at the 6 and 8 year follow-up assessments. The DISC includes both lifetime and past year diagnoses. The Diagnostic Interview Schedule-IV56 was used at the 8 year follow-up for 18+ year olds (n=111). SUD was defined as presence or absence of any abuse or dependence in the lifetime (excluding tobacco dependence, due to differences in the meaning of abuse/dependence for tobacco versus other substances). Additional analyses explored SUD for alcohol, tobacco, and marijuana/other drugs (illicit or misuse of prescription medications) separately.
For the analysis of stimulant treatment exposure over time in relation to substance use at the 8 year follow-up, the primary outcome variable was SUD in the past year for any substance (excluding tobacco). Secondary analyses explored alcohol and marijuana/other drug use disorders separately.
Psychoactive medication treatment
In addition to original randomized assignment to treatment, two medication treatment variables were calculated from responses to the Services for Children and Adolescents–Parent Interview,57,58 administered at all assessments to collect a cumulative history of medication treatment since enrollment into the study. Detailed information about the children’s psychoactive medication over the follow-up period has been previously reported47. One variable was the proportion of days medicated with psychoactive medications in the past year at each follow-up assessment. These medications were predominantly stimulants but occasionally included others (atomoxetine, guanfacine, and typical alternative medications such as clonidine and amitriptyline). A second medication variable was calculated for the current study to reflect the cumulative number of days stimulant-treated since enrollment into the study.
Statistical Approach
The analyses addressed (1) ADHD vs. LNCG group differences in substance use and SUD; (2) prediction of substance use and SUD from original randomized treatment assignment, including covariation with concurrent (past year) medication at each assessment; and 3) prediction of substance use and SUD from cumulative days stimulant-treated since study entry (using propensity score matching analysis).
The main analytic approach for (1) and (2) was generalized estimating equations (GEE) analyses. As one method for mixed-effects regression with repeated measures, the GEE analysis is an extension of the general linear model (for a relevant overview, see Hedeker and Gibbons59, especially pp. 131–146). These analyses tested whether groups (subject group; assigned treatment group) differed as a function of time. In contrast to traditional repeated measures analysis of variance, mixed-effects regression allows inclusion of subjects with complete data on covariates but partial data on the outcome variable. Thus, all subjects with substance use data from at least one follow-up assessment between 24 months and the 8 year follow-up were included in tests of group × time and treatment × time effects (n=520 ADHD; n=258 LNCG). For question (2), the ADHD sample size was reduced to n=515 because of missing data for medication use. We tested individual point-in-time contrasts, treating group and time as fixed effects and the intercept as a random effect, to test the significance of group differences overall and at each follow-up assessment. Because of some variability in age at each assessment, age was controlled as a time-varying covariate (as expected, it was always a statistically significant correlate). Power was sufficient (.80 or higher) to detect small treatment group differences (effect size of .28 or larger) at p<.05 or less. We used binary logit models for dichotomous outcomes and multinomial cumulative logit models for secondary analyses with ordinal count variable outcomes. We explored whether participant sex moderated results.
For the ADHD group only, effects of treatment were tested with three orthogonal contrasts following statistically significant treatment × time interactions (or for endpoint-only analyses, following statistically significant main effects of treatment): Comb+MedMgt vs. Beh+CC, termed the MTA Medication Algorithm effect; Comb vs. MedMgt, the Multimodality effect; and Beh vs. CC, the Behavioral Substitution effect60. Consistent with prior analyses24,47, we also tested an alternate set of planned contrasts in which behavioral treatment rather than medication algorithm was the primary divider: Comb+Beh vs. MedMgt+CC (Intensive Behavioral effect); Comb vs. Beh (Medication Addition effect); and MedMgt vs. CC (Intensity of Medication effect). Covariates were site, age at each assessment and proportion of days medicated in the past year (time-varying for repeated measures analyses); and p-values > .025 are not reported as significant to adjust for alpha inflation.
For (3), to test prediction of substance use and SUD from the number of days stimulant treated since study entry, we used propensity score matching analyses for ordinal treatment variables34. Propensity scores are calculated to represent each subject’s propensity (e.g., probability) to be stimulant-medicated over time as a function of baseline characteristics (e.g., original randomly assigned treatment, ADHD symptom severity, parent education, and family income). Participants with higher vs. lower stimulant medication exposure are matched on the basis of these scores to statistically control for observed characteristics that may influence the likelihood of natural selection into treatment over time. We used matched pair analyses to compare the substance use and SUD variables between subjects with higher vs. lower stimulant medication exposure.
Results
Medication Use Over Time
To explicate the medication variable, we first examined medication use over time. As previously reported, proportion of days medicated in the past year declined over time to M=.31(SD=.42) by the 8-year follow-up. In contrast, at 14 months when study-delivered treatment ended, M (SD) was; .71 (.22) for Comb; .71 (.24) for MedMgt; .54 (.41) for CC; .16 (.28) for Beh. At the 8 year follow-up, only 32.5% (132/406 with complete medication data) were medicated over 50% of days in the past year (vs. 63.3% or 257/406 at 14 months). Treatment was still predominantly with stimulants (83%) or stimulants plus non-stimulant treatment (8%). Cumulative number of days stimulant-treated since enrollment into the study—with M (SD) =1,402 (985) days (M [SD] = 3.84 [2.70] yrs)—was correlated .91 with cumulative dose, p<.01.
ADHD vs. LNCG
Substance use
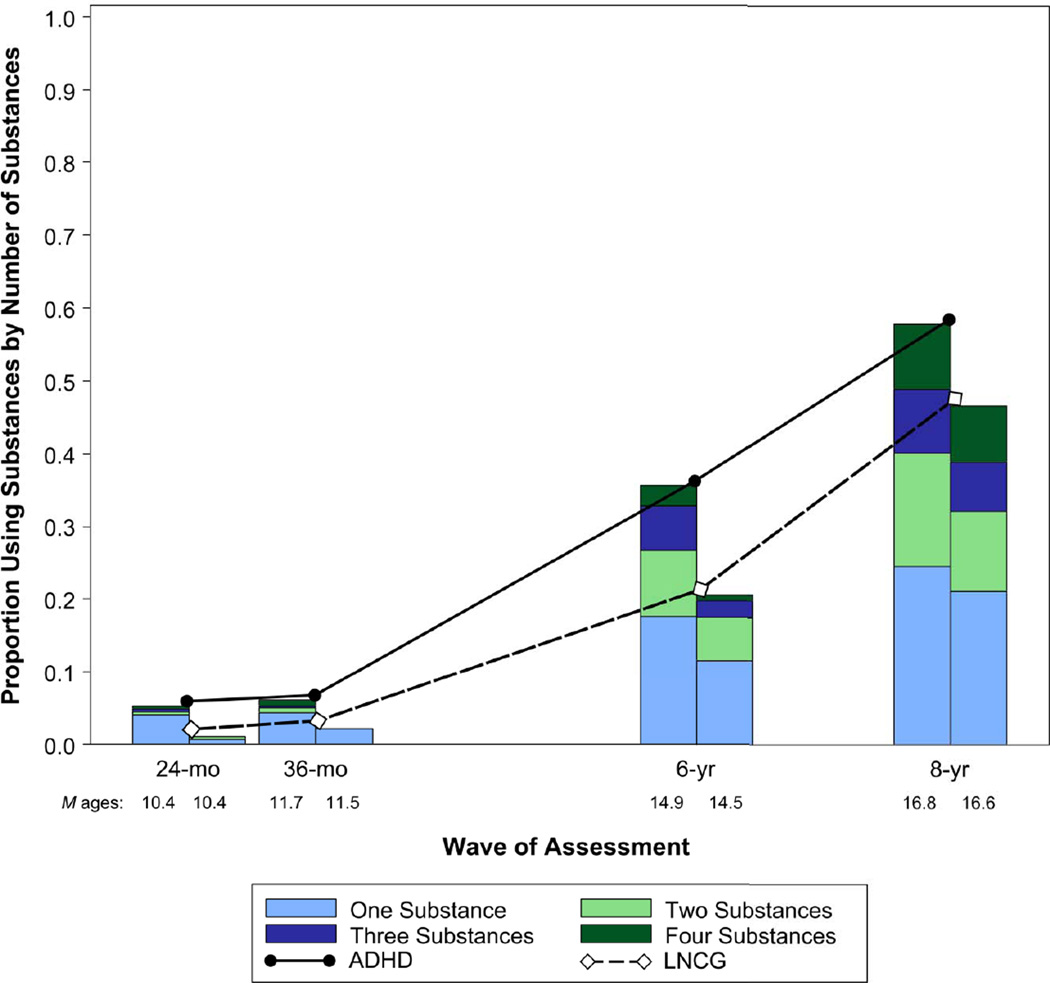
There was a main effect of ADHD vs. LNCG group between the 24 month and 8 year follow-ups, χ2=20.59, p<.0001. Figure 1 depicts this finding as well as the expected increase in substance use with time. There was a barely significant group × time interaction, p=.05, indicating variable group effects with time. Group differences were significant at each follow-up: 24 months, χ2(1)=12.54, p<.01; 36 months, χ2(1)=6.65, p=.01; 6 years, χ2(1)=11.11; p<.01, 8 years, χ2=4.24, p=.04. However, the largest group difference was at the 6 year follow-up, when 35% (n = 155/443) of ADHD and 19.5% (n = 44/226) of LNCG participants reported substance use. Results were similar for number of substances used, with the ADHD vs. LNCG group difference greatest at the 6 year follow-up, with 17.8% (n=79/443) of ADHD and 8% (n=18/226) of LNCG participants reporting use of 2 or more substances (see Fig.1).
Figure 1.
Proportion of Participants Using Substances (by Number of Substances) from the 24-month through 8-year Follow-up Assessments. ADHD = Attention-Deficit/Hyperactivity Disorder; LNCG = Local Normative Comparison Group.
Type of substance
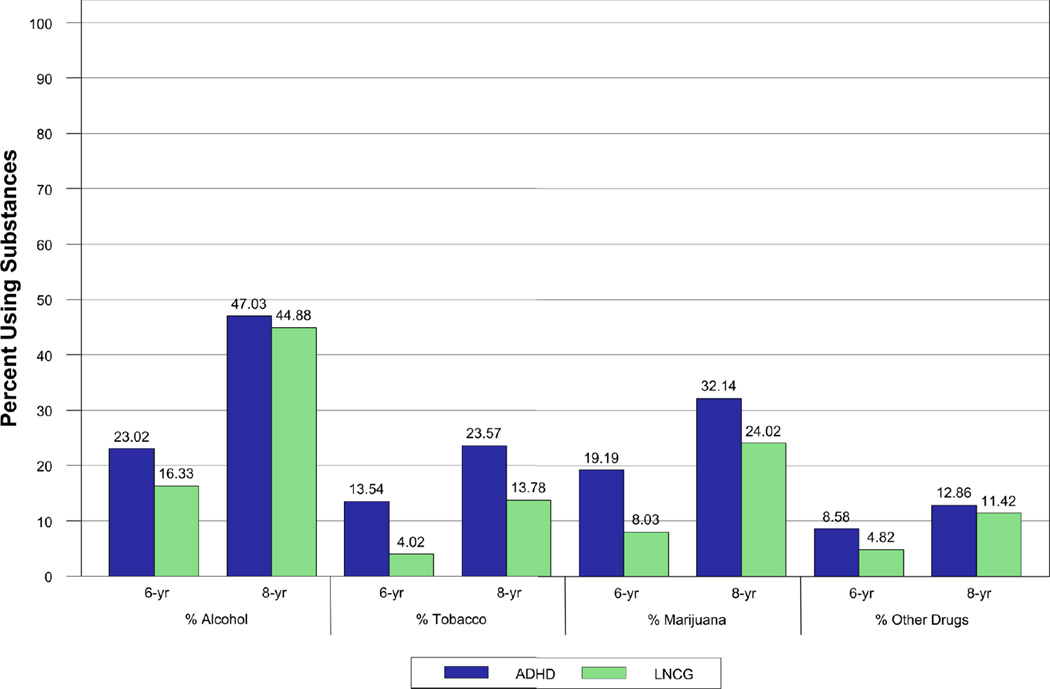
ADHD/LNCG group differences were apparent for all substances. There was a main effect of ADHD vs. LNCG group for alcohol use between the 24 month and 8 year follow-ups, χ2(1)=5.99, p=.01, but no group × time interaction. There was a main effect of ADHD vs. LNCG group for tobacco use, χ2(1)=23.98, p<.01 (group × time interaction not tested because no LNCG subjects reported smoking at 24 or 36 months). Few participants were sole tobacco users (i.e., did not also report other substance use), 3.6% of ADHD (n=15/420) vs. 0% of LNCG. There were more daily smokers at 8 years for ADHD, 16.7%, than for LNCG, 7.9%, χ2(1)=10.58, p<.01. There was a main effect of ADHD vs. LNCG group for marijuana and other drug use, χ2(1)=16.47, p<.01, but no group × time interaction. Figure 2 shows the expected age-related increases in substance use and the high prevalence of alcohol and marijuana use for both groups at mean age 17. The bar graphs also illustrate the relatively stronger contribution of tobacco and marijuana use to the ADHD vs. LNCG group differences.
Figure 2.
Percent of Participants Reporting Alcohol, Tobacco, Marijuana, and Other Drug Use at Each of the 6- and 8-year Follow-up Assessments. ADHD = Attention-Deficit/Hyperactivity Disorder; LNCG = Local Normative Comparison Group.
Rates of illicit drug use other than marijuana, including misuse of prescription medications, were low and not significantly different by group. At the 8 year follow-up, the following categories of substance use had base rates below 5%: amphetamines/stimulants, barbiturates/sedatives, opioids/narcotics, inhalants, hallucinogens/psychedelics, and cocaine. These rates included misuse (more use than prescribed or use without a prescription) of amphetamine/stimulant medications for the ADHD (8/422, 1.9%) and LNCG (4/230, 1.7%) groups.
SUD
There was a main effect of group (ADHD vs. LNCG) on SUD, but no main effect of time or group × time interaction. The group effect was statistically significant at 6 years, χ2(1)=11.01, p<.01, but not at 8 years, χ2(1)=1.45, p=.23 (for rates, see Table 1).
Table 1.
Percent (n) of Participants Reporting Substance Use Disorders at Each of the 6- and 8-year Follow-Up Assessments.
| 6 year | 8 year | GEE Model Results, χ2 (df), p | |||||
|---|---|---|---|---|---|---|---|
| ADHD | LNCG | ADHD | LNCG | Group (ADHD vs. LNCG) | Time | Group by Time | |
| Any SUD | 9.93 (44) | 3.14 (7) | 15.06 (64) | 10.76 (24) | 8.66 (1), p<.01 | .04 (1), p=.84 | 3.79 (1), p=.05 |
| Alcohol | 5.19 (23) | 2.69 (6) | 8.24 (35) | 7.17 (16) | 1.05 (1), p=.31 | .12 (1), p=.73 | 1.16 (1), p=.28 |
| Marijuana/other drug | 7.22 (32) | 1.79 (4) | 12.50 (53) | 6.73 (15) | 12.66 (1), p<.01 | .07 (1), p=.80 | 1.69 (1), p=.19 |
| Nicotine dependence | 3.39 (15) | 0 (0) | 4.47 (19) | 1.79 (4) | 11.55 (1), p<.01 | 1.33 (1), p=.25 | — |
Note: Nicotine dependence diagnoses are not included in the proportions reported for diagnosis with any Substance Use Disorder (SUD). Attention-deficit/hyperactivity disorder (ADHD) sample sizes are n=443 (6 years) and n=425 (8 years). Local normative comparison group (LNCG) sample size is n=223 (6 and 8 years). GEE = Generalized Estimating Equations.
Type of SUD
For alcohol abuse or dependence, there was no main effect of group, no main effect of time, and no group × time interaction (for all rates, see Table 1). For marijuana/other drug abuse or dependence, there was a main effect of group, but no main effect of time and no group × time interaction. For nicotine dependence, there was a main effect of group, but no main effect of time. In each case, the ADHD group had greater use. There were too few nicotine dependence diagnoses among LNCG subjects to evaluate a group by time interaction.
Sex
There was no main effect of sex, χ2(1)=.49, p=.49, and no sex by ADHD group interaction, χ2(1)=.30, p=.59 for substance use. Results were parallel for number of substances used, χ2(1)=.82, p=.37, and χ2(1)=.19, p=.66, respectively. There was a main effect of sex for SUD, χ2(1)=4.21, p=.04, with more boys (17.9%) than girls (11.5%) meeting diagnostic criteria. There was no group × sex interaction, χ2(1)=.02, p=.89.
Associations with Treatment: Randomly Assigned in Childhood and Naturalistic Medication Use at each Follow-Up Assessment
Substance use
Neither treatment assignment at baseline nor proportion of days medicated in the past year was significantly associated with substance use at any follow-up assessment. For the orthogonal treatment contrasts, ps ranged from .03 to .96 (with an average p=.52). The overall effect of the proportion of days medicated was not significant, p=.68, and there were no statistically significant medication × time interactions. Removal of the treatment group assignment variables (because of their correlation with medication treatment at the earlier assessments) did not change the pattern of results (p=.59). Results were similar for number of substances used.
Type of substance
There were no statistically significant associations between original randomized treatment assignment and alcohol use, average p=.60, tobacco use, average p=.26, or marijuana/drug use, p=.58 (ps ranged from .07 to .99). There were also no associations between these variables and ADHD medication use at each assessment for alcohol use, average p=.55, for tobacco use, average p=.25, or for marijuana/drug use, average p=.46 (ps ranged from .22 to .82).
SUD
Neither randomized treatment assignment nor proportion of days medicated in the past year was a statistically significant predictor (correlate) of SUD at the 6 and 8 year assessments. Across all orthogonal treatment contrasts and across assessments, the average p-value for the treatment group comparisons was p=.51 (range = .03 to .89). The association between proportion of days medicated and SUD was also not statistically significant, p=.37, and did not interact with assessment point, p=.48. There were no statistically significant associations for any specific type of SUD (alcohol, marijuana/drug) or for nicotine dependence.
Cumulative Psychostimulant Treatment and Substance Use/SUD at 8 years
For the propensity score matching analyses, and following Lu et al.,34 the distribution of days that participants had been stimulant treated since baseline was divided into five ordinal groups using quintile cut points. Using an ordinal logit model, quintile group membership was regressed on 36 variables selected for their potential to predict stimulant treatment. These included demographics (e.g., parent education and income; marital status; child sex), receipt of medication or school assistance prior to study enrollment, ADHD symptom severity (parent and teacher report), parent-reported impairment, other behavior problems (e.g., parent and teacher ratings of oppositionality and aggression), social skills ratings by parent and teacher, achievement test scores and IQ estimate, parental psychopathology (mood, anxiety, alcohol, or drug use disorder), and original randomly assigned treatment group (references for these variables are reported elsewhere45). A propensity score was produced for each participant, estimated from baseline characteristics, as an index of the likelihood of receiving more or fewer days of stimulant treatment between baseline and the 8 year follow-up. Participants were subsequently matched in pairs to minimize their differences in propensity scores and maximize their differences in days of stimulant treatment. These matched pairs produced two groups distinguished by more versus fewer days treated. As planned, the groups differed significantly in days stimulant treated, M = 2071.10, SD = 728.87, versus M = 763.08, SD = 765.00, p<.01, but not in any of the 36 baseline variables, all ps > .10; average p-value =.70.
Matched pair analyses were performed for each of the primary (composited number of substances used; any SUD excluding nicotine dependence) and secondary (alcohol; marijuana; cigarettes; SUD for alcohol and SUD for marijuana/other drugs) variables. There were no statistically significant associations for any of these variables. For the primary outcomes, the Wilcoxon signed-rank test for number of substances used was z = −.34, p=.73; McNemar’s chi-square test for any SUD was χ2(1)=.44, p=.62. For the exploratory analyses: alcohol use, z=.42, p=.68; cigarettes, z=−.54, p=.59; marijuana, z=.45, p=.65, alcohol abuse or dependence, χ2(1)=.00, p=1.00; marijuana or other drug abuse or dependence, χ2(1)=1.29, p=.35.
Discussion
The current study first tested the hypothesis that children with combined type ADHD have increased risk of substance use and SUD in adolescence. We found a significantly higher prevalence of substance use by adolescents with, versus former classmates without, ADHD histories. Overall group differences for any SUD (DSM-IV abuse or dependence) were also observed, but these occurred at comparatively lower rates and only at certain ages—for marijuana and nicotine only—highlighting the importance of measuring levels of certain types of substance use in adolescence that may foreshadow later SUD in adulthood. The multi-site nature of the MTA sample, including demographic diversity (e.g., 20% female) and other unique sample features (see below), underscores for the first time the significance of the substance abuse risk for both boys and girls with childhood ADHD. This finding renders all the more important our examination of treatment effects on substance use/SUD risk. Across all analyses, we found no associations to suggest either harm or benefit from medication (mostly stimulant) treatment in regard to rates of adolescent substance use or SUD. As this is the first study to include a period of randomized assignment to treatment and the first to consider a large number of potential confounding variables, these findings are the strongest test to date of the association between medication for ADHD and adolescent substance abuse.
About one-third of the MTA sample fell into a high risk group for adult substance abuse based on their repeated use of substances as adolescents. At the six year follow-up (mean age of 15), group differences were greatest with 35% of the ADHD versus 20% of the LNCG (non-ADHD) groups reporting use of one or more substances. We also detected group differences in SUD at the six year follow-up, but for fewer participants, which may explain other smaller studies’ failure to find statistically significant group differences in adolescent SUD50,61. A large body of research has shown that substance use at a young age is prognostic of later abuse/dependence in adulthood62,63.
Marijuana and tobacco use most clearly distinguished the ADHD from LNCG groups. Only a few longitudinal studies of children diagnosed with ADHD have examined marijuana use, as opposed to abuse/dependence, and the results have been mixed50,64–65. In the current study, a third of the children with ADHD (32%) had used marijuana more than once by the 8 year follow-up (mean age of 17) versus 24% of the LNCG. These high rates for both groups reflect the increasing use of marijuana by teens in the United States66 but they also indicate a relatively higher risk of marijuana use for teens with ADHD histories. This risk was barely visible in early adolescence but picked up speed by the 6 year follow-up assessment (mean age of 15). At this age, nearly one-fifth of our participants with ADHD histories reported marijuana use more than once, indicating that this outcome variable should be directly targeted for future research. Marijuana use at a young age has been associated with a host of negative cognitive sequelae such as neurocognitive decrements, decreased academic achievement, and poor physical health67–72. Given the long-term impact of childhood ADHD on educational, occupational, and psychosocial outcomes of adulthood9, early and chronic marijuana use has the potential to significantly undermine later adult accomplishments for this population.
Tobacco use (mostly cigarette smoking) was strongly associated with childhood ADHD. Nearly a quarter (24%) of the ADHD sample had smoked cigarettes or used other forms of tobacco more than a few times by the 8 year follow-up, and 17% were already daily smokers. These are high rates compared to both the LNCG (8% were daily cigarette users) and to daily smoking rates for high school students nationally, 11.2%73. Combined with other reports of high rates of daily smoking from single site samples9,74, these findings suggest that the MTA children are headed toward a previously projected4 doubling of the 19.3% adulthood prevalence of smoking currently found in the USA75. Cigarette use that begins in adolescence is associated with an increased likelihood of nicotine addiction into adulthood and with a lower likelihood of successful quitting76,77. Adolescent cigarette use is also associated with use and abuse of other drugs78—a finding strongly supported by the current data for both the ADHD and non-ADHD groups. In fact, we found extremely few adolescents who reported using only cigarettes.
Assignment to study treatments in childhood did not predict any high school-aged substance-related outcomes. This result parallels our previously reported failure to find treatment group differences for any of the other 24 symptom, behavioral, and functioning (e.g., arrests, psychiatric hospitalizations, etc.) variables examined at the same age48. Many of these variables (e.g., impulsivity, conduct problems, academic achievement) are long-established predictors of substance use for teens in the general population79–81 and in other studies they correlate with substance use for adolescents with ADHD histories12,82,83. To the extent that these variables were not differentially affected by assigned treatment group in childhood47, it follows that substance use/SUD in adolescence should not be differentially affected. Collectively, these findings are in stark contrast to the widely held belief that starting treatment early (here, mean age of 8) will, in and of itself, change long-term outcomes that also include SUD vulnerability. Instead, treatments found to be efficacious for adolescent substance abuse84,85 may hold some promise for this population due to their targeting of the same behavioral, academic, and familial/peer vulnerabilities known to accompany adolescent ADHD.
Our findings did not provide any evidence that ADHD medication protects from, or increases risk for, adolescent substance use or SUD. This finding held for recent medication and for days cumulatively treated with stimulants. Unmeasured confounders may have been operating due to the naturalistic follow-up study design, and we did not statistically control for psychopathology and functioning at the follow-up assessments. However, we conducted the most carefully controlled analysis to date using a statistical method (propensity score matching analysis) that allowed consideration of multiple baseline factors that might contribute to long-term medication treatment or termination. Moreover, our prior report that medication at high school age does not relate to multiple indicators of symptoms and daily functioning47 revealed that addition of these covariates was not indicated. Our findings conflict with anecdotal clinical experience with long-term patients who appear to derive benefit from continued medication. We speculate that these experiences reflect a subset of well-monitored teens who, unlike the majority of our sample, continue to be accepting of medication recommendations and other concurrent protective strategies and/or may have a low risk profile for developing substance use.
The observed lack of associations between stimulant exposure over time and adolescent substance use/SUD do not discount the possibility that brain-based changes in neural mechanisms underlying addiction vulnerability are occurring as a function of prolonged stimulant treatment. Volkow et al. recently demonstrated, for stimulant-naïve adults with ADHD, an up-regulation of the dopamine transporter and decreased D2 receptor availability in the striatum following one year of methylphenidate treatment86,87. Although demonstrated with adults, these findings heighten concern about potential stimulant-related adjustments in dopaminergic mechanisms pertinent to drug abuse vulnerability. On the other hand, Castellanos et al. (2002) suggested neuroprotection from stimulant use in childhood and adolescence for youth with ADHD88. Studies of differential responsivity to drug challenges in adulthood as a function of prior stimulant treatment would be useful. In adolescence, as in the present study, other factors that increase substance abuse risk, such as parental and peer influences, may outweigh developing biological vulnerability.
The substance use/SUD outcomes for the MTA should be considered in the context of several unique study features and limitations. All of the children in the MTA were diagnosed with the combined type of DSM-IV ADHD, and generalization of study results should generally not extend beyond this subtype. It is interesting that conduct disorder, a common comorbidity for the combined subtype that is also a strong proximal mediator of ADHD-related substance use/SUD, was infrequent. Compared to other clinic-based longitudinal studies of ADHD, for which CD rates range from 25% to 30% for boys64,83 and 18% to 23% for girls38,39, the MTA rate of 8% at the 8 year assessment47, and even the rate of 14% at baseline44, is low. The sample recruitment that included, in addition to psychiatric clinics, pediatrician offices and school settings41 may have contributed to this finding89). Our follow-up assessments, which relied on self-report and often with two-year windows, may have missed episodes of substance use and rates may be underestimated. Similarly, the precision of our treatment data relies on the accuracy of parent recall at each assessment which no doubt includes some degree of error.
Thus, children with ADHD have increased risk of substance use, in particular tobacco and marijuana, against a backdrop of high rates of alcohol use for all teens, and our standard treatments (short-term psychosocial, medication) for ADHD have not, to date, countered this risk. Given the ubiquity of medicinal treatment for ADHD, and our failure to find relations between ADHD medication treatment and adolescent substance use/SUD, the findings argue for additional research on neurobiological and psychosocial factors that fuel the vulnerability and can inform the types of treatment so urgently needed.
Acknowledgments
The work reported was supported by cooperative agreement grants and contracts from NIMH and the National Institute on Drug Abuse (NIDA) to the following: University of California–Berkeley: U01 MH50461, N01MH12009, and HHSN271200800005-C; DA-8-5550; Duke University: U01 MH50477, N01MH12012, and HHSN271200800009-C; DA-8-5554; University of California–Irvine: U01 MH50440, N01MH 12011, and HHSN271200800006-C; DA-8-5551; Research Foundation for Mental Hygiene (New York State Psychiatric Institute/Columbia University): U01 MH50467, N01 MH12007, and HHSN271200800007-C; DA-8-5552; Long Island–Jewish Medical Center U01 MH50453; New York University: N01MH 12004, and HHSN271200800004-C; DA-8-5549; University of Pittsburgh: U01 MH50467, N01 MH 12010, and HHSN 271200800008C; DA-8-5553; and McGill University N01MH12008, and HHSN271200800003-C; DA-8-5548. The Office of Special Education Programs of the U.S. Department of Education, the Office of Juvenile Justice and Delinquency Prevention of the Justice Department, and NIDA also participated in funding.
Dr. Hinshaw has received an honorarium from the American Psychological Association for his editorship of Psychological Bulletin. Dr. Arnold has received research funding from Curemark, Eli Lilly and Co., and Shire; advisory board honoraria from Biomarin, Noven, Seaside Therapeutics, and Shire; and travel support from Noven. Dr. Swanson has served on the advisory board of Noven Pharmaceuticals, and has received travel support from Shire and Jannsen to attend separate, professional meetings. Dr. Pelham has received a research grant from and has served on the advisory board of Noven Pharmaceuticals. Dr. Hechtman has received research funds from and has served on the advisory boards and speakers’ bureaus for Eli Lilly and Co., Janssen, Ortho, Purdue, and Shire. Dr. Wigal has received research support and consulting honoraria from, and has served on the speakers’ bureau for Eli Lilly and Co., Noven, Rhodes, Otsuka, and Shire. Dr. Abikoff has received royalties from Multi-Health Systems regarding the Children’s Organizational Skills Scale. Dr. Greenhill has received grant support from Shire and Rhodes, and has served as a member of the Scientific Advisory Board of BioBDX LLC. Dr. Jensen has received honoraria for three keynote addresses given at European conferences on attention-deficit/hyperactivity disorder treatment outcomes (two from Shire and one from Janssen-Cilag), and has received a charitable donation from Shire. Dr. Wells has received royalties from Multi-Health Systems, the publisher of Conners’ Rating Scales.
Appendix
Drs. Gibbons, Howard, Hur, Lu, and Marcus served as the statistical experts for this research.
The Multimodal Treatment Study of Children with Attention-Deficit/Hyperactivity Disorder (ADHD) (MTA) was an NIMH cooperative agreement randomized clinical trial involving six clinical sites. Collaborators from NIMH: Peter S. Jensen, M.D., of the Mayo Clinic; L. Eugene Arnold, M.D., M.Ed., of Ohio State University; Joanne B. Severe, M.S., of the Clinical Trials Operations and Biostatistics Unit, Division of Services and Intervention Research; Benedetto Vitiello, M.D., of the Child and Adolescent Treatment and Preventive Interventions Research Branch; Kimberly Hoagwood, Ph.D., of Columbia University; previous contributors from NIMH to the early phase: John Richters, Ph.D., of the National Institute of Nursing Research; and Donald Vereen, M.D., of NIDA. Principal investigators and co-investigators from the clinical sites are: University of California–Berkeley/San Francisco: Stephen P. Hinshaw, Ph.D., of Berkeley; and Glen R. Elliott, Ph.D., M.D., of San Francisco; Duke University: C. Keith Conners, Ph.D.; Karen C. Wells, Ph.D.; John March, M.D., M.P.H.; and Jeffery Epstein, Ph.D.; University of California–Irvine/Los Angeles: James Swanson, Ph.D., of Irvine; Dennis P. Cantwell, M.D., deceased, of Los Angeles; and Timothy Wigal, Ph.D., of Irvine; Long Island Jewish Medical Center/Montreal Children's Hospital: Howard B. Abikoff, Ph.D., of New York University School of Medicine; and Lily Hechtman, M.D., McGill University; New York State Psychiatric Institute/Columbia University/Mount Sinai Medical Center: Laurence L. Greenhill, M.D., of Columbia University; and Jeffrey H. Newcorn, M.D., of Mount Sinai School of Medicine; University of Pittsburgh: William E. Pelham, Ph.D., of Florida International University; Betsy Hoza, Ph.D., of the University of Vermont; and Brooke Molina, Ph.D. Original statistical and trial design consultant: Helena C. Kraemer, Ph.D., of Stanford University. Follow-up phase statistical collaborators: Robert D. Gibbons, Ph.D., of the University of Illinois–Chicago; Sue Marcus, Ph.D., of Mt. Sinai College of Medicine; and Kwan Hur, Ph.D., of the University of Illinois–Chicago. Collaborator from the Office of Special Education Programs/US Department of Education: Thomas Hanley, Ed.D. Collaborator from Office of Juvenile Justice and Delinquency Prevention/Department of Justice: Karen Stern, Ph.D.
The opinions and assertions contained in this report are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of Health and Human Services, the National Institutes of Health (NIH), or NIMH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Drs. Molina, Hoza, Epstein, Vitiello, Gibbons, Howard, Hur, Lu, and Marcus, and Ms. Houck report no biomedical financial interests or potential conflicts of interest.
References
- 1.Charach A, Yeung E, Climans T, Lillie E. Childhood Attention-Deficit/Hyperactivity Disorder and future substance use disorders: Comparative meta-analyses. J Am Acad Child Adolesc Psychiatry. 2011;50(1):9–21. doi: 10.1016/j.jaac.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: A meta-analytic review. Clin Psychol Rev. 2011;31(3):328–341. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derefinko KD, Pelham WE. ADHD and substance use. In: Sher KJ, editor. Oxford Handbook of Substance Use Disorders. New York, NY: Oxford University Press; 2013. [Google Scholar]
- 4.Molina BSG. Delinquency and substance use in ADHD: Adolescent and young adult outcomes in developmental context. In: Evans SW, Hoza B, editors. Attention Deficit Hyperactivity Disorder: State of the Science and Best Practices. Vol. 2. Kingston, NJ: Civic Research Institute; 2011. [Google Scholar]
- 5.Pelham WE, Fabiano GA. Evidence-based psychosocial treatment for attention deficit/hyperactivity disorder: An update. J Clin Child Adolesc Psychol. 2008;37(1):185–214. doi: 10.1080/15374410701818681. [DOI] [PubMed] [Google Scholar]
- 6.Swanson JM, Wigal TL, Volkow ND. Contrast of medical and nonmedical use of stimulant drugs, basis for the distinction, and risk of addiction: Comment on Smith and Farah (2011) Psychol Bull. 2011;137(5):742–748. doi: 10.1037/a0024898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold LE. Methylphenidate vs. amphetamine: Comparative review. J Atten Disord. 2000;3(4):200–211. [Google Scholar]
- 8.Faraone SV, Buitelaar J. Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. Eur Child Adolesc Psychiatry. 2010;19:353–364. doi: 10.1007/s00787-009-0054-3. [DOI] [PubMed] [Google Scholar]
- 9.Barkley RA, Murphy KR, Fischer M. ADHD in adults: What the science says. New York, NY: Guilford Press; 2008. [Google Scholar]
- 10.Chang Z, Lichtenstein P, Larsson H. The effects of childhood ADHD symptoms on early-onset substance use: A Swedish twin study. J Abnorm Child Psychol. 2012;40:425–435. doi: 10.1007/s10802-011-9575-6. [DOI] [PubMed] [Google Scholar]
- 11.Knop J, Penick EC, Nickel EJ, et al. Childhood ADHD and conduct disorder as independent predictors of male alcohol dependence at age 40. J Stud Alcohol Drugs. 2009;70:169–177. doi: 10.15288/jsad.2009.70.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molina BSG, Pelham WE, Cheong J, Marshal MP, Gnagy EM, Curran PJ. Childhood ADHD and growth in adolescent alcohol use: The roles of functional impairments, ADHD symptom persistence, and parental knowledge. J Abnorm Psychol. 2012;121(4):922–935. doi: 10.1037/a0028260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambert NM, McLeod M, Schenk S. Subjective responses to initial experience with cocaine: an exploration of the incentive–sensitization theory of drug abuse. Addiction. 2006;101:713–725. doi: 10.1111/j.1360-0443.2006.01408.x. [DOI] [PubMed] [Google Scholar]
- 14.Volkow ND, Swanson JM. Does childhood treatment of ADHD with stimulant medication affect substance abuse in adulthood? Am J Psychiatry. 2008;165(5):553–555. doi: 10.1176/appi.ajp.2008.08020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valvassori SS, Frey BN, Martins MR, et al. Sensitization and cross-sensitization after chronic treatment with methylphenidate in adolescent Wistar rats. Behav Pharmacol. 2007;18:205–212. doi: 10.1097/FBP.0b013e328153daf5. [DOI] [PubMed] [Google Scholar]
- 16.Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of Attention-Deficit/Hyperactivity Disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111(1):179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- 17.Biederman J, Wilens T, Mick E, Spencer TJ, Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104(2):e20. doi: 10.1542/peds.104.2.e20. [DOI] [PubMed] [Google Scholar]
- 18.Loney J, Kramer JR, Salisbury H. Medicated vs. unmedicated ADHD children: Adult involvement with legal and illegal drugs. In: Jensen PS, Cooper JR, editors. Attention Deficit Hyperactivity Disorder. State of the Science Best Practices. Kingston, NJ: Civic Research Institute; 2002. [Google Scholar]
- 19.Mannuzza S, Klein RG, Truong NL, et al. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: Prospective follow-up into adulthood. Am J Psychiatry. 2008;165:604–609. doi: 10.1176/appi.ajp.2008.07091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilens TE, Adamson J, Monuteaux MC, et al. Effect of prior stimulant treatment for attention-deficit/hyperactivity disorder on subsequent risk for cigarette smoking and alcohol and drug use disorders in adolescents. Arch Pediatr Adolesc Med. 2008;162(10):916–921. doi: 10.1001/archpedi.162.10.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambert NM. The contribution of childhood ADHD, conduct problems, and stimulant treatment to adolescent and adult tobacco and psychoactive substance abuse. Ethical Human Psychol Psychiatry. 2005;7(3):197–221. [Google Scholar]
- 22.Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31(6):533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- 23.Biederman J, Monuteaux MC, Spencer T, et al. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: A naturalistic controlled 10-year follow-up study. Am J Psychiatry. 2008;165(5):597–603. doi: 10.1176/appi.ajp.2007.07091486. [DOI] [PubMed] [Google Scholar]
- 24.Molina BSG, Flory K, Hinshaw SP, et al. Delinquent behavior and emerging substance use in the MTA at 36-months: Prevalence, course, and treatment effects. J Am Acad Child Adolesc Psychiatry. 2007;46(8):1027–1039. doi: 10.1097/chi.0b013e3180686d96. [DOI] [PubMed] [Google Scholar]
- 25.Barkley RA, Fischer M, Smallish L, Fletcher KE. Does the treatment of Attention-Deficit/Hyperactivity Disorder with stimulants contribute to drug use/abuse? A 13-year prospective study. Pediatrics. 2003;111(1):97–109. doi: 10.1542/peds.111.1.97. [DOI] [PubMed] [Google Scholar]
- 26.Katusic SK, Barbaresi WJ, Colligan RC, Weaver AL, Leibson CL, Jacobson SJ. Psychostimulant treatment and risk for substance abuse among young adults with a history of Attention-Deficit/Hyperactivity Disorder: A population-based, birth cohort study. J Child Adolesc Psychopharmacol. 2005;15(5):764–776. doi: 10.1089/cap.2005.15.764. [DOI] [PubMed] [Google Scholar]
- 27.Harty SC, Ivanov I, Newcorn JH, Halperin JM. The impact of conduct disorder and stimulant medication on later substance use in an ethnically diverse sample of individuals with Attention-Deficit/Hyperactivity Disorder in childhood. J Child Adolesc Psychopharmacol. 2011;21(4):331–339. doi: 10.1089/cap.2010.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winters KC, Lee S, Botzet A, Fahnhorst T, Realmuto GM, August GJ. A prospective examination of the association of stimulant medication history and drug use outcomes among community samples of ADHD youths. Journal of Child and Adolescent Substance Abuse. 2011;20(4):314–329. doi: 10.1080/1067828X.2011.598834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Substance Abuse and Mental Health Services Administration. Rockville, MD: Office of Applied Studies; 2009. Results from the 2008 National Survey on Drug Use and Health: National Findings. NSDUH Series H-36, HHS Publication No. (SMA) 09-4434. [Google Scholar]
- 30.Swanson JM, Arnold LE, Kraemer H, et al. Evidence, interpretation, and qualification from multiple reports of long-term outcomes in the Multimodal Treatment Study of Children with ADHD (MTA) Part I: Executive Summary. J Atten Disord. 2008;12(1):4–14. doi: 10.1177/1087054708319345. [DOI] [PubMed] [Google Scholar]
- 31.Swanson JM, Arnold LE, Kraemer H, et al. Evidence, interpretation, and qualification from multiple reports of long-term outcomes in the Multimodal Treatment Study of Children with ADHD (MTA) Part II: Supporting details. J Atten Disord. 2008;12(1):15–43. doi: 10.1177/1087054708319525. [DOI] [PubMed] [Google Scholar]
- 32.Swendsen J, Burstein M, Case B, et al. Use and abuse of alcohol and illicit drugs in US adolescents. Arch Gen Psychiatry. 2012;69(4):390–398. doi: 10.1001/archgenpsychiatry.2011.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen PS, Arnold LE, Swanson JM, et al. 3-Year Follow-up of the NIMH MTA Study. J Am Acad Child Adolesc Psychiatry. 2007;46(8):988–1001. doi: 10.1097/CHI.0b013e3180686d48. [DOI] [PubMed] [Google Scholar]
- 34.Lu B, Zanutto E, Hornik R, Rosenbaum PR. Matching with doses in an observational study of a media campaign against drug abuse. J Am Stat Assoc. 2001;96(456):1245–1253. doi: 10.1198/016214501753381896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swanson JM, Hinshaw SP, Arnold LE, et al. Secondary evaluations of MTA 36-month outcomes: Propensity score and growth mixture model analyses. J Am Acad Child Adolesc Psychiatry. 2007;46(8):1002–1013. doi: 10.1097/CHI.0b013e3180686d63. [DOI] [PubMed] [Google Scholar]
- 36.Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry. 2001;49(3):258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- 37.Babinski DE, Pelham WE, Molina BSG, et al. Late adolescent and young adult outcomes of girls diagnosed with ADHD in childhood: An exploratory investigation. J Atten Disord. 2011;15(3):204–214. doi: 10.1177/1087054710361586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biederman J, Monuteaux MC, Mick E, et al. Psychopathology in females with attention-deficit/hyperactivity disorder: a controlled five-year prospective study. Biol Psychol. 2006;60:1098–1105. doi: 10.1016/j.biopsych.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 39.Hinshaw SP, Owens EB, Sami N, Fargeon S. Prospective follow-up of girls with Attention-Deficit/Hyperactivity Disorder into adolescence: Evidence for continuing cross-domain impairment. J Consult Clin Psychol. 2006;74(3):489–499. doi: 10.1037/0022-006X.74.3.489. [DOI] [PubMed] [Google Scholar]
- 40.Hinshaw SP, Owens EB, Zalecki C, et al. Prospective follow-up of girls with attention-deficit hyperactivity disorder into early adulthood: Continuing impairment includes elevated risk for suicide attempts and self-injury. J Consult Clinical Psychol. 2012;80(6):1041–1051. doi: 10.1037/a0029451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arnold LE, Abikoff HB, Cantwell DP, et al. NIMH collaborative multimodal treatment study of children with ADHD (MTA): Design, methodology, and protocol evolution. J Atten Disord. 1997;2:141–158. [Google Scholar]
- 42.Greenhill LL, Abikoff HB, Arnold LE, et al. Medication treatment strategies in the MTA: Relevance to clinicians and researchers. J Am Acad Child Adoelsc Psychiatry. 1996;35(10):1304–1313. doi: 10.1097/00004583-199610000-00017. [DOI] [PubMed] [Google Scholar]
- 43.Hinshaw SP, March JS, Abikoff HB, et al. Comprehensive assessment of childhood attention-deficit hyperactivity disorder in the context of a multisite, multimodal clinical trial. J Atten Disord. 1997;1:217–234. [Google Scholar]
- 44.The MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 1999;56(12):1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- 45.The MTA Cooperative Group. Moderators and mediators of treatment response for children with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 1999;56(12):1088–1096. doi: 10.1001/archpsyc.56.12.1088. [DOI] [PubMed] [Google Scholar]
- 46.Wells KC, Pelham WE, Kotkin RA, et al. Psychosocial treatment strategies in the MTA study: Rationale, methods, and critical issues in design and implementation. J Abnorm Child Psychol. 2000;28:483–505. doi: 10.1023/a:1005174913412. [DOI] [PubMed] [Google Scholar]
- 47.Molina BSG, Hinshaw SP, Swanson JM, et al. The MTA at 8 years: Prospective follow-up of children treated for combined type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48(5):484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH diagnostic interview schedule for children version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 49.American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: DSM-IV; 1994. [Google Scholar]
- 50.Molina BSG, Pelham WE. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. J Abnorm Psychol. 2003;112(3):497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- 51.Donovan JE. The teen drinking questionnaire. Pittsburgh (PA): Pittsburgh Adolescent Alcohol Research Center, University of Pittsburgh; 1994. [Google Scholar]
- 52.Jessor R, Donovan JE, Costa FM. Health Behavior Questionnaire: High School Version. Boulder, CO: Institute of Behavioral Science; 1989. [Google Scholar]
- 53.NHSDA. National Household Survey on Drug Abuse. National Institute on Drug Abuse, U.S. Dept of Health and Human Services. 1992 OMB No: 0930-0110.
- 54.Winters KC, Fahnhorst T. Assessment issues in adolescent drug abuse treatment research. In: Galanter M, editor. Recent Developments in Alcoholism: Vol XVII: Research on Alcohol Problems in Adolescents and Young Adults. Washington, D.C.: American Psychiatric Press; 2005. pp. 407–425. [DOI] [PubMed] [Google Scholar]
- 55.Hedeker D, Mermelstein RJ. Analysis of longitudinal substance use outcomes using ordinal random-effects regression models. Addiction. 2000;95(suppl. 3):S381–S394. doi: 10.1080/09652140020004296. [DOI] [PubMed] [Google Scholar]
- 56.Robins LN, et al. The Diagnostic Interview Schedule for the DSM-IV (DIS-IV) St. Louis, MO: Washington University School of Medicine; 2000. [Google Scholar]
- 57.Hoagwood K, Jensen PS, Arnold LE, et al. Reliability of the services for children and adolescents parent interview (SCAPI) J Am Acad Child Adolesc Psychiatry. 2004;43:1345–1454. doi: 10.1097/01.chi.0000139558.54948.1f. [DOI] [PubMed] [Google Scholar]
- 58.Jensen P, Hoagwood K, Roper M, et al. The services for children and adolescents parent interview (SCAPI): Development and performance characteristics. J Am Acad Child Adolesc Psychiatry. 2004;43:1334–1344. doi: 10.1097/01.chi.0000139557.16830.4e. [DOI] [PubMed] [Google Scholar]
- 59.Hedeker D, Gibbons RD. Longitudinal Data Analysis. New York: John Wiley and Sons; 2006. [Google Scholar]
- 60.Swanson JM, Kraemer HC, Hinshaw SP, et al. Clinical relevance of the primary findings of the MTA: Success rates based on severity of ADHD and ODD symptoms at the end of treatment. J Am Acad Child Adolesc Psychiatry. 2001;40:168–179. doi: 10.1097/00004583-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 61.Biederman J, Wilens T, Mick B, et al. Is ADHD a risk factor for psychoactive substance use disorders? Findings from a four-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1997;36:21–29. doi: 10.1097/00004583-199701000-00013. [DOI] [PubMed] [Google Scholar]
- 62.Chassin L, Pitts SC, Prost J. Binge drinking trajectories from adolescence to emerging adulthood in a high-risk sample: Predictors and substance use outcomes. J Clin Child Psychol. 2002;70(1):67–78. [PubMed] [Google Scholar]
- 63.Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: Results from the National Longitudinal Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- 64.Barkley RA, Fischer M, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1990;29:546–557. doi: 10.1097/00004583-199007000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Hartsough CS, Lambert NM. Pattern and progression of drug use among hyperactives and controls: A prospective short-term longitudinal study. J Child Psychol Psychiatry. 1987;28:543–553. doi: 10.1111/j.1469-7610.1987.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 66.Substance Abuse and Mental Health Services Administration. Rockville,MD: Substance Abuse and Mental Health Services Administration; 2011. Results from the 2010 National Survey on Drug Use and Health: National Findings. NSDUH Series H-41, HHS Publication No. (SMA) 11-4658. [Google Scholar]
- 67.Brook JS, Stimmel MA, Zhang C, et al. The association between earlier marijuana use and subsequent academic achievement and health problems: A longitudinal study. Am J Addict. 2008;17:155–160. doi: 10.1080/10550490701860930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crean RD, Crane NA, Mason BJ. An evidence-based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5(1):1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grant I, Gonzalez R, Carey CL, et al. Non-acute (residual) neurocognitive effects of cannabis use: A meta-analytic study. J Int Neuropsychol Soc. 2003;9:679–689. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- 70.Hanson KL, Medina KL, Padula CB, Tapert SF, Brown SA. Impact of adolescent alcohol and drug use on neuropsychological functioning in young adulthood: 10-year outcomes. J Child Adolesc Subst Abuse. 2011;20(2):135–154. doi: 10.1080/1067828X.2011.555272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pope HG, Gruber AJ, Hudson JI, et al. Early-onset cannabis use and cognitive deficits: What is the nature of the association? Drug Alcohol Depend. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- 72.Meier MH, Caspi A, Harrington H, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. P Natl Acad Sci USA. :E2657–E2664. doi: 10.1073/pnas.1206820109. [published online ahead of print August 27, 2012]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Centers for Disease Control and Prevention. Youth Risk Behavior Surveillance – United States, 2009. [Accessed March 27, 2012];MMWR CDC Surveill Summ. 2010 59(SS-5) [Google Scholar]
- 74.Milberger S, Biederman J, Faraone SV, Chen L, Jones J. ADHD is associated with early initiation of cigarette smoking in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1997;36:37–44. doi: 10.1097/00004583-199701000-00015. [DOI] [PubMed] [Google Scholar]
- 75.Centers for Disease Control and Prevention. Vital Signs: Current cigarette Smoking Among Adults Aged >18 Years—United States, 2005–2010. [Accessed January 24, 2012];MMWR CDC Surveill Summ. 2011 60(33):1207–1212. [Google Scholar]
- 76.Chen J, Millar W. Age of smoking initiation: Implications for quitting. Health Rep. 1998;9(4):39–46. [PubMed] [Google Scholar]
- 77.Riggs N, Chou C-P, Li C, Pentz MA. Adolescent to emerging adulthood smoking trajectories: When do smoking trajectories diverge, and do they predict early adulthood nicotine dependence? Nicotine Tob Res. 2007;9(11):1147–1154. doi: 10.1080/14622200701648359. [DOI] [PubMed] [Google Scholar]
- 78.Chen X, Unger JB, Palmer P, et al. Prior cigarette smoking initiation predicting current alcohol use: Evidence for a gateway drug effect among California adolescents from eleven ethnic groups. Addict Behav. 2002;27(5):799–817. doi: 10.1016/s0306-4603(01)00211-8. [DOI] [PubMed] [Google Scholar]
- 79.Petraitis J, Flay BR, Miller TQ. Reviewing theories of adolescent substance use: Organizing pieces in the puzzle. Psychol Bull. 1995;117(1):67–86. doi: 10.1037/0033-2909.117.1.67. [DOI] [PubMed] [Google Scholar]
- 80.Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: Common and specific influences. Annu Rev Clin Psychol. 2008;4(1):325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- 81.Zucker RA, Donovan JE, Masten AS, Mattson ME, Moss HB. Early developmental processes and the continuity of risk for underage drinking and problem drinking. Pediatrics. 2008;121(suppl. 4):S252–S272. doi: 10.1542/peds.2007-2243B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brook DW, Brook JS, Zhang C, Koppel J. Association between Attention-Deficit/Hyperactivity Disorder in adolescence and substance use disorders in adulthood. Arch Pediatr Adolesc Med. 2010;164(10):930–934. doi: 10.1001/archpediatrics.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gittelman R, Mannuzza S, Shenker R, Bonagura N. Hyperactive boys almost grown up: I. Psychiatric status. Arch Gen Psychiatry. 1985;42:937–947. doi: 10.1001/archpsyc.1985.01790330017002. [DOI] [PubMed] [Google Scholar]
- 84.Waldron HB, Turner CW. Evidence-based psychosocial treatments for adolescent substance abuse. J Clin Child Adolesc Psychol. 2008;37(1):238–261. doi: 10.1080/15374410701820133. [DOI] [PubMed] [Google Scholar]
- 85.Spas J, Ramsey S, Paiva AL, Stein LAR. All might have won, but not all have the prize: Optimal treatment for substance abuse among adolescents with conduct problems. Subst Abuse. 2012;6:1–16. doi: 10.4137/SART.S10389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Volkow ND, Wang GJ, Tomasi D, et al. Methylphenidate-elicited dopamine increases in ventral striatum are associated with long-term symptom improvement in adults with attention deficit hyperactivity disorder. J Neurosci. 2012;32(3):841–849. doi: 10.1523/JNEUROSCI.4461-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang GJ, Volkow ND, Wigal T, et al. Chronic treatment with methylphenidate increases dopamine transporter density in patients with attention deficit hyperactive disorder. J Nucl Med. 2009;50(suppl. 2):1283. [Google Scholar]
- 88.Castellanos FX, Lee PP, Sharp W, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with Attention-Deficit/Hyperactivity Disorder. JAMA. 2002;288(14):1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- 89.Brown RT, Freeman WS, Perrin JM, et al. Prevalence and Assessment of Attention-Deficit/Hyperactivity Disorder in Primary Care Settings. Pediatrics. 2001;107(3):e43. doi: 10.1542/peds.107.3.e43. [DOI] [PubMed] [Google Scholar]