Abstract
Eosinophilic esophagitis (EoE) is a chronic immune-mediated condition where infiltration of eosinophils into the esophageal mucosa leads to symptoms of esophageal dysfunction. It has rapidly emerged as an important cause of upper GI morbidity in patients of all ages and is encountered in a substantial proportion of patients undergoing diagnostic upper endoscopy. This review discusses the clinical, endoscopic, and histologic features of EoE and presents the most recent guidelines for diagnosis of EoE. It describes selected diagnostic dilemmas including distinguishing EoE from gastroesophageal reflux disease and addressing the newly recognized clinical entity of proton pump inhibitor responsive esophageal eosinophilia. It also highlights evidence to support both pharmacologic and non-pharmacologic treatments, including topical corticosteroids, dietary elimination therapy, and endoscopic dilation.
Keywords: eosinophilic esophagitis, diagnosis, treatment, epidemiology, endoscopy, allergy
Introduction
Eosinophilic esophagitis (EoE) is currently defined as a chronic immune-mediated condition where infiltration of eosinophils into the esophageal mucosa leads to symptoms of esophageal dysfunction.1 While the first case was described in the late 1970s,2 the disease as it is now recognized was reported in children and adults in the early 1990s.3-5 EoE was initially felt to be rare, but data from multiple centers now show that the incidence and prevalence are increasing rapidly and have outpaced the increased recognition of the disease.6-12 In fact, over the past ten years EoE has become an important and frequent cause of upper gastrointestinal symptoms in both children and adults.13,14 More than 6% of patients undergoing upper endoscopy for any reason, and more than 15% having the procedure for an indication of dysphagia will be diagnosed with EoE.15-17 The prevalence of EoE has been estimated to range between 43-52/100,000 in the general population,10,18,19 a level that is beginning to approach the population prevalence of inflammatory bowel disease.20
The increasing recognition and evolving epidemiology of EoE has led to an explosion of research interest. While many questions related to EoE are unanswered, there has been substantial progress towards understanding the pathogenesis and genetic basis of the disease,21-23 the clinical presentation, and effective treatment strategies. This review will discuss clinical, endoscopic, and histologic features of EoE, present the most recent guidelines for diagnosis of EoE and selected diagnostic dilemmas, and highlight evidence to support both pharmacologic and non-pharmacologic treatment.
Patient history
EoE has been described throughout the world including North America, Europe, South America, Australia, and Asia, but the prevalence appears to be highest in the U.S. and Western Europe as compared with Japan and China.9,10,19,24-26 It also occurs in patients of all ages,1,27,28 ut it is more frequent in children and adults under the age of 40.27-29 For reasons that are not understood, EoE is seen three to four times more frequently in males than in females, and is also more common in whites.1,8,9,16,17,28,30 However, as centers accrue more experience and report data from larger populations of subjects from more diverse areas, racial minorities have been found to have EoE.31-34
The clinical presentation of EoE varies by patient age.1,6,8,35,36 In infants and toddlers, symptoms are non-specific, and can include failure-to-thrive, fussiness, poor growth, feeding intolerance or food aversion, abdominal pain, nausea, vomiting, and regurgitation.29,35,37 In contrast, dysphagia is the most characteristic symptom in adolescents and adults, and in some studies this symptom is nearly universal.8,15,17,28,29 For patients who present to an emergency department with a food impaction, EoE is the cause at least 50% of the time.38-40 It is important to note that patients can minimize symptoms of dysphagia by avoiding solid foods, lubricating foods, drinking copious liquids during meals, and chewing carefully, so asking about these dietary modifications on history is necessary. Heartburn can affect patients with EoE of any age, and in 1-8% of those with proton-pump inhibitor (PPI) refractory reflux symptoms, EoE is the cause.15,17,29,41-44 Because of the many potential symptoms and because no single symptom is specific for EoE, there is often a delay in making the diagnosis.45
EoE is also strongly associated with atopic diseases such as asthma, allergic rhinitis and sinusitis, atopic dermatitis, and food allergies. This relationship was first reported in children where up to 80% can have atopy, and helped to support the allergic etiology of EoE.29,37,46,47 While fewer adults with EoE have atopy, it is still a prominent feature in this population.8,48,49 Interestingly, there have been several reports of seasonal variation in the diagnosis of EoE as well as variation based on climate zone.8,9,50,51
Endoscopic features
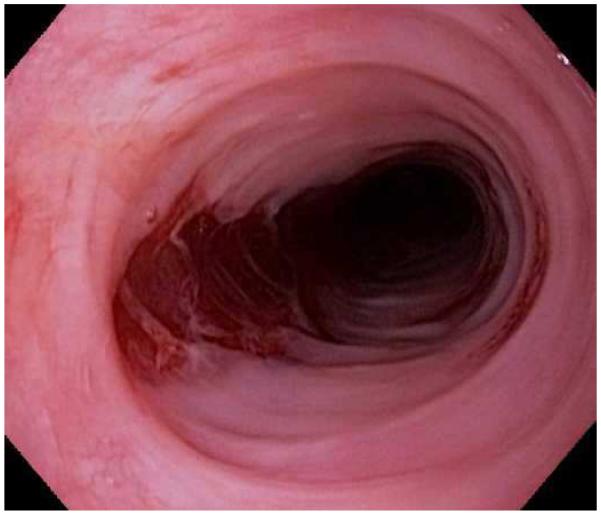
Upper endoscopy is required to evaluate the clinical symptoms of EoE, assess for other possible causes, and perform esophageal biopsies. Multiple characteristic endoscopic findings of EoE have been reported,1,52-54 but in up to 10% of cases the esophageal mucosa can appear normal and biopsies are required or the diagnosis will be missed.55 These findings have a fair to good inter- and intra-observer reliability,56,57 and efforts are underway to standardize reporting and scoring of endoscopic findings in EoE.58
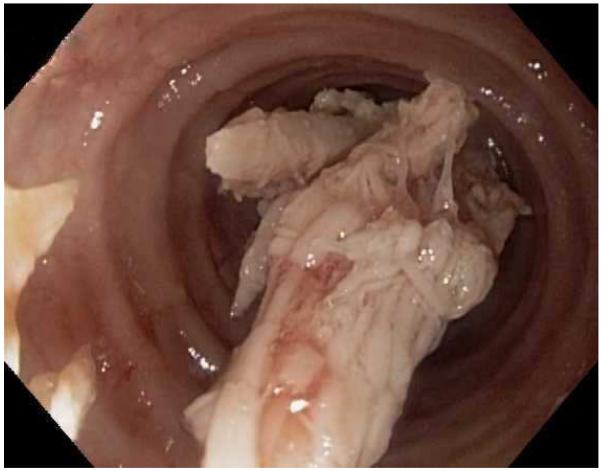
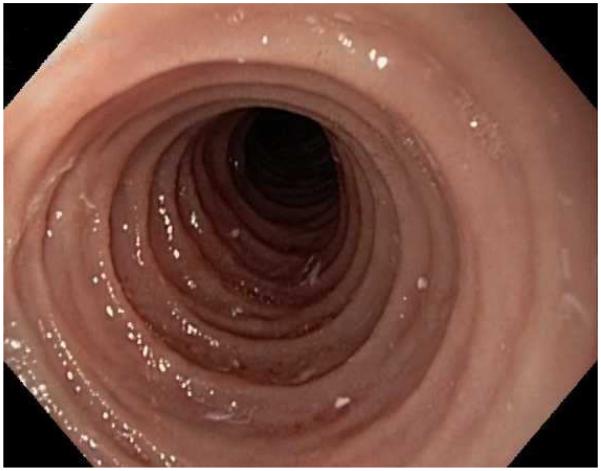
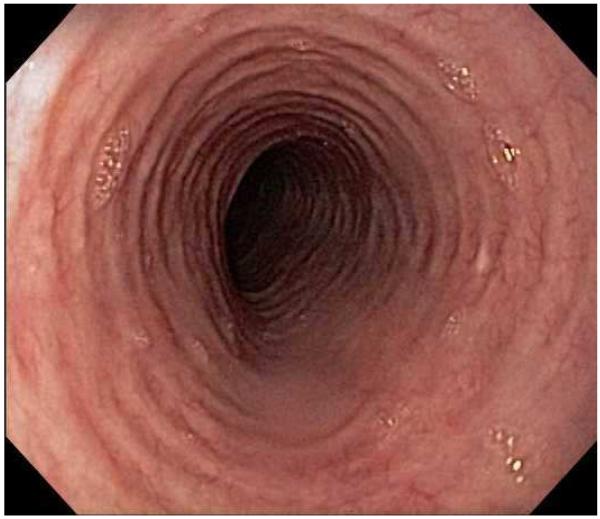
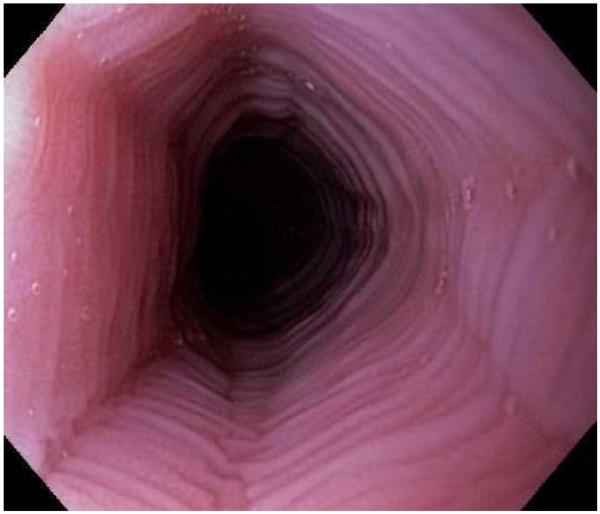
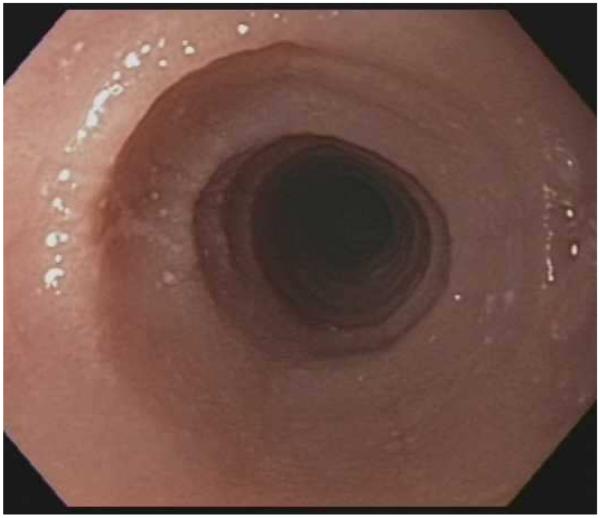
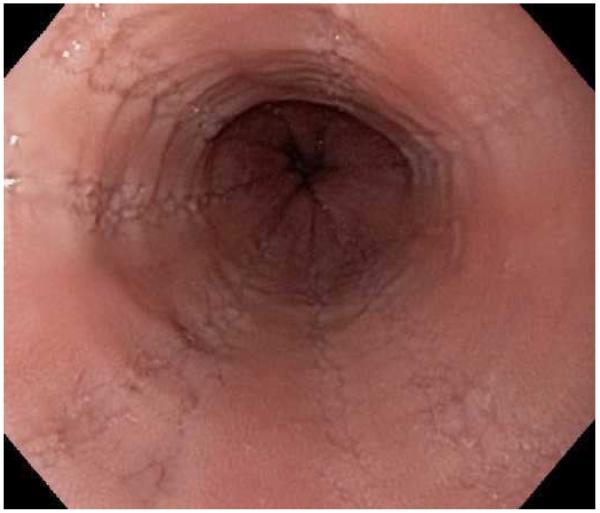
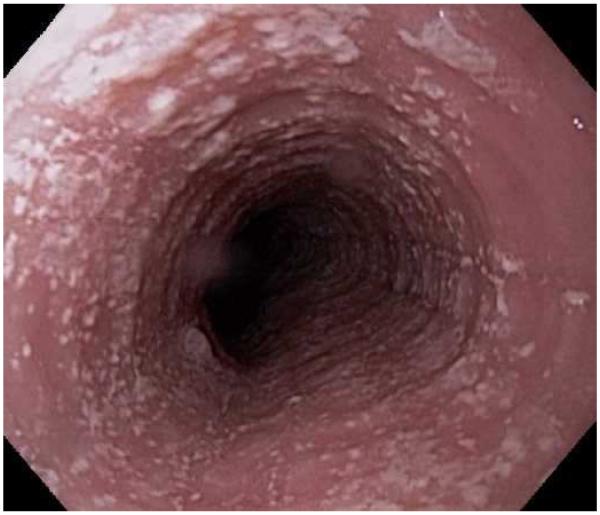
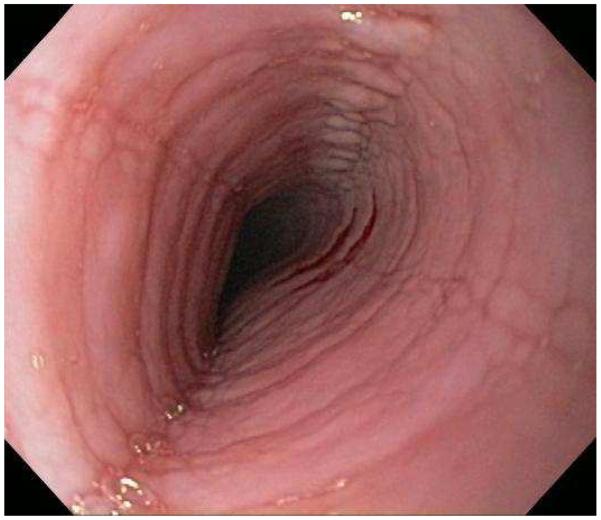
Typical endoscopic findings of EoE are presented in Figure 1, and include:
Esophageal rings. These can be fixed (previously referred to as esophageal trachealization or corrugation) or transient (sometimes termed felinization).
Narrow caliber esophagus. This can be difficult to appreciate on visual inspection alone, but there can be resistance to the scope passage without seeing a clear stricture.
Focal esophageal strictures.
Linear furrows. These are grooves in the esophageal mucosa that run parallel to the axis of the esophagus.
White plaques or exudates. These are punctate white spots on the esophageal mucosa that can be confused for esophageal candidiasis.
Decreased vascularity. Here, the normal mucosal vascular pattern is lost and the esophagus appears pale, congested, or edematous.
Crêpe-paper mucosa. This is a manifestation of mucosal fragility in EoE where the mucosa tears with passage of the endoscope, but a focal stricture or resistance is not appreciated.
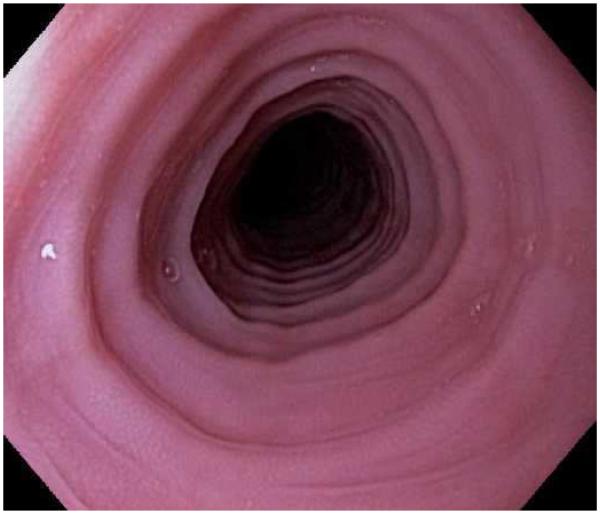
Figure 1.
The range of endoscopic findings in EoE. (A) A patient with an acute food bolus impaction. (B) After the bolus is cleared, fixed esophageal rings are noted. (C) A patient with less prominent esophageal rings. (D) Felinization of the esophageal with rings that are transiently present. Congestion and loss of vascular marking area also noted. (E) A narrow caliber esophagus which prevented passage of an adult upper endoscope. Rings, congestion, and decreased vasculature are also present. (F) Linear furrows in the distal esophagus, associated subtle rings and decreased vasculature. (G) Prominent white plaques and exudates, as well as subtle rings and furrows. In this patient, brushings were negative for candida and biopsies showed multiple eosinophilic microabscesses. (H) Crêpe-paper mucosa where this tear was noted after uneventful passage of the endoscope. The mucosa is also congested with decreased vascularity. (I) A patient with multiple findings of rings, furrows, decreased vascularity, narrow caliber esophagus, and crêpe-paper mucosa.
These features can occur in isolation, but more commonly occur together. There are some data to suggest that younger children tend to have more inflammatory features such as linear furrows, white plaques, and decreased vasculature, while adults (particularly those with a long symptom duration) tend to have more fibrotic features such as rings and strictures.8,59,60 It is important to note that the endoscopic findings of EoE are not pathognomonic, and a recent meta-analysis found that the sensitivity, specificity, and predictive values of endoscopic findings in EoE were not high enough to be the basis for diagnostic decisions. This emphasizes the importance obtaining esophageal biopsies when EoE is suspected clinically. This is reiterated in recent guidelines which recognize that the eosinophilic infiltrate in EoE is patchy and that increasing numbers of biopsies increase diagnostic sensitivity.61-64 Therefore, it is recommend for the endoscopist to obtain 2-4 biopsies from the distal esophagus and an additional 2-4 samples from the proximal esophagus.1
Histologic features
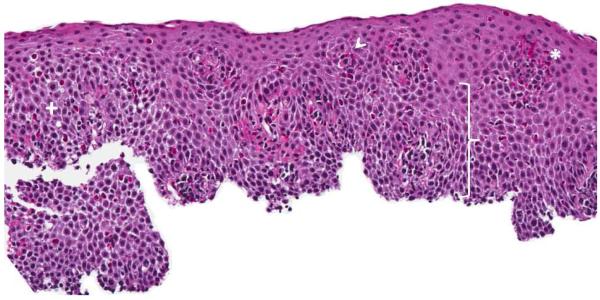
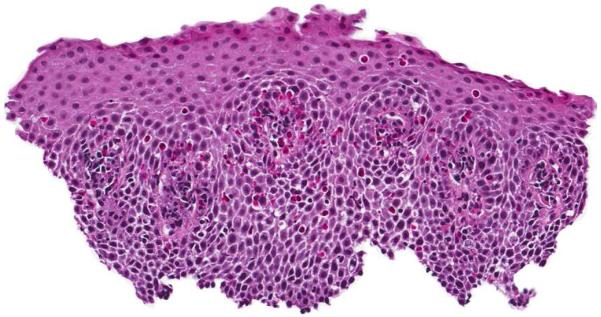
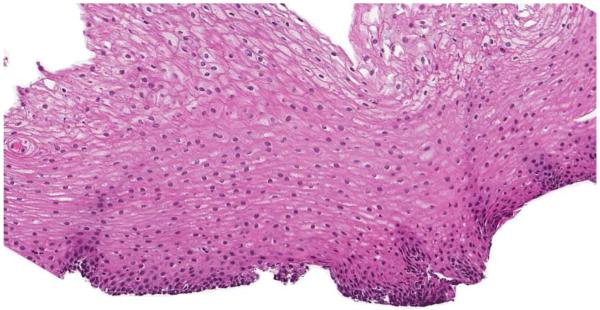
Infiltration of the esophageal mucosa with eosinophils is the histologic hallmark of EoE.65,66 Currently, finding at least 15 eosinophils per high-power microscopy field (eos/hpf) is suggestive of EoE.1 However, as discussed in more detail below, the finding of esophageal eosinophilia on biopsy is not specific for EoE. When biopsy samples are examined, in addition to the eosinophil count which by convention represents the peak value in the most highly inflamed area on the biopsy, other features are also present (Figure 2).1,65,66 Eosinophils are often found toward the apical aspect of the epithelium, and clusters of eosinophils can form eosinophilic microabscesses. Because eosinophils are activated in EoE, they degranulate and the granule proteins can be seen extracellularly. There is also basal layer hypertrophy, where the cells in the basal layer expand and the rete pegs elongate. Spongiosis, also termed dilated intracellular spaces, is frequently observed. Finally, if the biopsy sample is deep enough to contain lamina propria, fibrosis of this area can be noted.
Figure 2.
Esophageal biopsy specimen in EoE. This shows a brisk eosinophilic infiltration in the esophageal epithelium, as well as spongiosis (+), eosinophil degranulation (arrowhead), basal zone hyperplasia (bracket), and an eosinophilic microabscess (*).
Diagnostic criteria
Consensus guidelines
Because of heterogeneity in disease definition and reporting of data pertaining of EoE,28,67,68 an initial set of diagnostic guidelines were proposed in 2007 and represented a major step forward for the field.69 These guidelines have been recently updated after taking into account advances in understanding and complexities related to diagnosis.1 In this most recent document, EoE is defined conceptually as a “chronic immune/antigen-mediated esophageal disease characterized clinically by symptoms related to esophageal dysfunction and histologically by eosinophil-predominant inflammation”. Three specific criteria were required to diagnose EoE:
Symptoms related to esophageal dysfunction;
A peak eosinophil count of ≥ 15 eos/hpf on esophageal biopsy, with few exceptions;
Eosinophilia limited to the esophagus with other causes of esophageal eosinophilia excluded.
Taken together, the conceptual definition and the diagnostic criteria emphasize that EoE is a clinicopathologic condition. Specifically, the entire clinical and histologic picture must to be considered in order to make a diagnosis, and no single feature is diagnostic on its own.
Diagnostic dilemmas
Despite having a set of diagnostic guidelines, the ability to characterize patients clinically and endoscopically, and well defined histologic findings with a marker, the eosinophil count, that is reliable and reproducible if approached systematically,70 diagnosis of EoE can still be challenging.
Just as symptoms of dysphagia and findings of esophageal rings on endoscopy are not specific for EoE, there is a differential diagnosis for esophageal eosinophilia on biopsy.1,69 A number of conditions that have been reported to cause or be associated with esophageal eosinophilia, such as achalasia, infections (fungal, viral, parasitic), connective tissue disease, graft-versus host disease, Crohn’s disease, adrenal insufficiency, hypereosinophilic syndrome, and eosinophilic gastroenteritis, have their own distinct clinical presentations and can typically diagnosed with a focused history and physical exam and limited supplemental evaluation. However, two conditions, gastroesophageal reflux-disease (GERD) and PPI-responsive esophageal eosinophilia (PPI-REE) deserve special attention when attempting to diagnose EoE.
There is substantial overlap between GERD and EoE, including symptoms of heartburn, chest pain, and dysphagia, and biopsy findings of eosinophilia; even very high eosinophil counts do not distinguish the two conditions.44,71 In the 2007 EoE diagnostic guidelines, exclusion of GERD, either with a high-dose PPI trial or with pH monitoring, was required prior to definitively diagnosing EoE.69 As more clinical experience was gained, it was clear that there were some patients who had coexisting EoE and GERD and that the relation between the two conditions was complicated.71
In addition, there was the observation that some patients who appeared to have EoE would have a clinical and histologic response to PPI therapy. This was first reported in a case series by Ngo and colleagues, and since then a number of studies have reported that one third or more of patients with esophageal eosinophilia have a response to PPIs.72-77 This phenomenon has been termed PPI-REE.1 It is not currently known whether this is a distinct clinical entity, a sub-type of GERD, or phenotype of EoE, but it’s role must currently be addressed in the diagnostic algorithm for EoE. Specifically, when esophageal eosinophilia is found on biopsy in a clinical setting where there is suspicion for EoE, a high dose PPI trial (20-40 mg twice daily of any of the available agents for 8 weeks) is necessary. If a patient responds to this regimen, then additional clinical evaluation can be performed to determine if GERD was the cause of the esophageal eosinophilia or if the patient has PPI-REE. If a patient has persistent symptoms of EoE and there are still at least 15 eos/hpf on esophageal biopsy, than EoE can be diagnosed.
Because of these challenges and the somewhat rudimentary way in which EoE is diagnosed, there is active research aimed at identifying better ways to diagnose EoE. Some methods under investigation include clinical scoring systems,8,78,79 endoscopic imaging techniques,56,80,81 functional luminal assessment of the esophagus,82 biomarker measurements on esophageal biopsies,83-87 radio-labelling of esophageal biopsies,88 non-invasive assessment of esophageal cytokines,89 non-invasive serum biomarkers,90-92 and genetic testing.21,93 While these are promising, none of these techniques have been validated or are ready to be used in clinical practice.
Treatment
There are now a number of evidence-based treatment options for EoE. Pharmacologic agents are commonly used, but non-pharmacologic approaches such as dietary elimination therapy and endoscopy dilation are also effective. Of note, there are currently no Food and Drug Administration-approved medications for EoE, so all pharmacologic treatment options are off-label. The overall goal of treating patients with EoE is to improve symptoms and normalize the esophageal mucosa without adversely impacting quality of life, although specific endpoints have been difficult to define in clinical trials.94 Because EoE is chronic, for the majority of patients when medications or dietary therapy are stopped, symptoms will recur.95-97 For this reason, in patients with severe, frequent, or recurrent symptoms, or in patients who have had complications such as esophageal strictures, food impactions requiring emergency evaluation for endoscopy and bolus clearance, or esophageal perforations, long-term treatment may be required.
Pharmacologic treatment
Corticosteroids
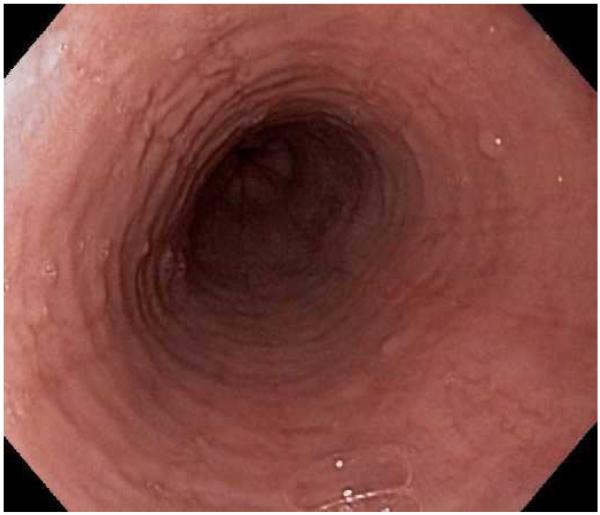
Corticosteroid medications are an effective and commonly used treatment for EoE (Figure 3). Initially, systemic steroids were administered with good effect,95,98 but because of concerns about side effects, this class was not a viable option for long-term used. Instead, a “topical” method of administration of steroids was developed.99 Patients were instructed to use asthma medication preparations, either in multi-dose inhaler (MDI) or aqueous solution formulations, and instead of inhaling the medications they swallowed them to coat the esophagus. With this technique, a number of studies showed that agents like fluticasone, budesonide, mometasone, beclomethasone, and ciclesonide all effectively decreased esophageal eosinophil counts and improved clinical symptoms.99-107 In addition, a randomized control trial (RCT) comparing topical fluticasone with prednisone by Schaefer and colleagues showed that the two agents were equivalent for improving symptoms and eosinophil counts.108
Figure 3.
(A) Endoscopic appearance of the esophagus prior to topical steroid therapy with rings, furrows, and degreased vascularity noted. (B) Appearance has completely normalized after topical steroid therapy. A similar improvement is noted on this patients biopsies before (C) and after (D) treatment.
The two most commonly used topical steroids are fluticasone and budesonide. The first placebo controlled RCT in EoE was conducted in children by Konikoff and colleagues using fluticasone.109 They showed that 50% of the 21 subjects receiving fluticasone had complete histologic remission (defined as ≤ 1 eos/hpf) compared with 9% of the 15 subjects receiving placebo. Recently, a similar study was conducted in adults by Alexander and colleagues.110 Here, 62% of the 21 adults treated with fluticasone had a > 90% decrease in eosinophil counts, compared with 0% of the 15 subjects in the placebo arm. When used clinically, the typical dose of fluticasone is 880-1760 mcg/d, administered twice daily, with the final dose determined by patient age or size.
Topical budesonide has also been studied. Here, the aqueous formulation of the medication is typically mixed with a sugar substitute such as sucralose, and the resultant slurry (which has been termed oral viscous budesonide, or OVB) is swallowed.104,111 In the first RCT in children, Dohil and colleagues reported that 87% of the 15 subjects who received OVB had a histologic response (≤ 6 eos/hpf) where none of the 9 patients in the placebo group had this response. The marked histologic response seen with budesonide was confirmed in a larger placebo-controlled dose-finding trial of 80 children, but in this study, symptoms significantly improved in both the active therapy and placebo arms, making the results somewhat difficult to interpret and highlighting a trend that has been seen in some other recent trials.112 There have also been two RCTs of budesonide in adults. Straumann and colleagues compared a nebulized and then swallowed protocol for budesonide to placebo, and found that budesonide was highly effective for improving symptoms and decreasing eosinophil counts.113 The second study compared OVB to the nebulized/swallowed budesonide administration protocol, and showed that OVB was more effective.114 When used clinically, the typical dose of budesonide is 1-2 mg/d, mixed with 5g of sucralose and administered twice daily, with the final dose determined by patient age or size.
Topical steroids are generally considered safe and well tolerated. Adrenal suppression has not been reported with an initial course of treatment,110,112,114,115 but longer term safety data are needed.1 The main adverse effect with topical steroids is local. The rate of candidal esophagitis ranges from 0-32% in prospective studies, though many of these cases were detected incidentally on follow-up endoscopy.108-110,112-115 There is also a case report of herpes esophagitis complicating topical steroid use.116
Biologic agents
There has been burgeoning interest in non-steroid treatment modalities for eosinophilic esophagitis, and biologic agents targeting key factors in the pathogenesis of EoE are intriguing options. To date, however, these are either still in the experimental phase or have not been shown to be effective.
Mepolizumab and reslizumab are monoclonal anti-IL-5 antibodies. A small series showed that mepolizumab had some efficacy for EoE, so these agents were subsequently studied in three RCTs, one in adults and two in children.117-119 While there was a mild to moderate improvement in eosinophils counts, the medications did not tend to improve symptoms in the active treatment arms compared to placebo, and primary endpoints of resolution of eosinophilia and symptoms were not met. These agents are not available clinically at this time.
Infliximab, an anti-tumor necrosis factor (TNF) antibody frequently used in inflammatory bowel disease, was examined in a small case series of steroid-refractory EoE patients, but did not improve symptoms or histology.120
Omalizumab, a monoclonal antibody against IgE and used for allergic asthma, was studied in a placebo-controlled RCT by Fang and colleagues in 30 adults with EoE.121 There was no difference in any of the outcomes between the groups, and this agent is not recommended for use in EoE.
Other agents
Given the strong association between EoE and allergic diseases, leukotriene antagonists have been studied, but the data are conflicting. In an initial report in adults by Atwood and colleagues, high doses of montelukast (20-40 mg/d) were effective, though associated with nausea and vomiting.122 Two additional studies, one in adults and one in children, were less encouraging,123,124 and this agent is not routinely used in EoE.
Mast cell stabilizers such as cromolyn have also been examined, but were not effective.29 There are currently no data in EoE for ketotifen, an anti-histamine with anti-mast cells properties.
Immunomodulators such as azathioprine and 6MP have been studied in a single series of three patients with steroid-dependent EoE.125 While the medication was effective for improving eosinophilic counts and maintaining symptom control, given the potential toxicity and paucity of data, this medication is not recommended for routine use in EoE.
A number of investigations into novel agents to treat EoE are ongoing. For example, anti-IL-13 and anti-eotaxin-3 antibodies are under development,1 and pilot data were recently presented on a Th2 cell prostaglandin D2 receptor antagonist, which represents a potentially new medication class for EoE.126 With the increasing understanding of pathways involved in the pathogenesis of EoE, it is likely that a wide variety of therapeutic modalities will be studied over the coming years.
Non-pharmacologic treatment
There are two major strategies for non-pharmacologic treatment of EoE. The first is dietary elimination, where specific foods or groups of foods are removed from the diet in order to identify potential food allergens that trigger EoE. Similar to pharmacologic therapy, dietary restriction reduces esophageal eosinophilia. The second is endoscopic dilation, where strictures, rings, or a narrow-caliber esophagus are stretched in order to relieve symptoms of dysphagia. This technique does not impact the underlying inflammation in the esophagus.
Dietary elimination
There are three approaches to dietary elimination for treatment of EoE. First is a completely allergen-free elemental formula, composed of only amino acids, medium chain triglycerides, and simple carbohydrates. This treatment was initially used in one of the early reports of EoE where ten children responded within weeks of starting the formula.5 In a study of 51 children, Markowitz and colleagues found that 96% of subjects had near-complete resolution of esophageal eosinophilia and symptoms within 1-2 weeks,127 and other studies have reported similar results.29,128 Until recently, all of the data on elemental diets were in children, but a study by Peterson and colleagues confirms the short-term utility of this approach in adults as well, if they can tolerate the formulation.129 Though elemental diets are very effective for EoE treatment, they are unpalatable, expensive, sometimes require gastrostomy tubes for administration, and may not be feasible for many patients with EoE.
The second approach, the so-called six food elimination diet (SFED), addresses the difficulty with compliance found with the elemental diet. In the SFED, the six most highly allergenic food groups, dairy, egg, wheat, soy, nuts, and seafood, are removed from the diet. This approach compares quite favorably with the elemental diet in children, with three-quarters of subjects having resolution of esophageal eosinophilia and almost all having improvement in symptoms.128,130,131 Gonsalves and colleagues have now shown that the SFED is equally effective in adult patients as well.132
Third is a targeted elimination diet where foods are removed based on reactivity on allergy testing. This is less restrictive than either elemental or SFED diets, but the efficacy is also somewhat less, likely due to the fact that allergy testing itself is not completely reliable for detecting food triggers.1,128,133 A number of studies have explored this approach, and the overall response rate ranges between 50-75% in children and lower in adults, depending on the center and the methods of allergy testing used.29,128,134-138
When selecting dietary modification as the treatment modality for a patient with EoE, it is important to have a multidisciplinary approach with collaboration between gastroenterologists, allergists, and nutritionists. This ensures not only that patients are having their nutrition requirements met, but that they have enough information about food choices and potential sources of contamination to maximize the likelihood of success of the elimination diet.
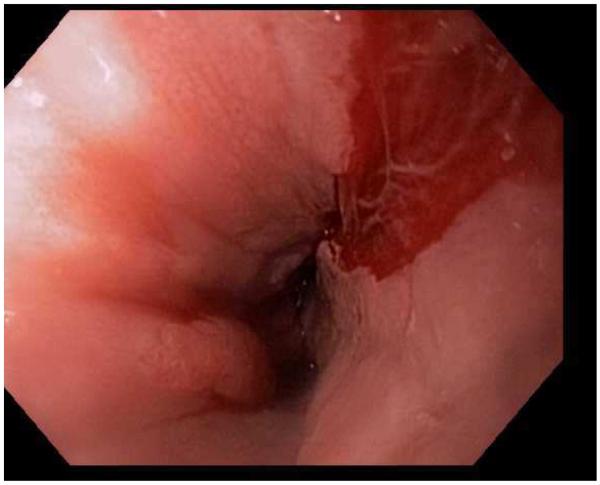
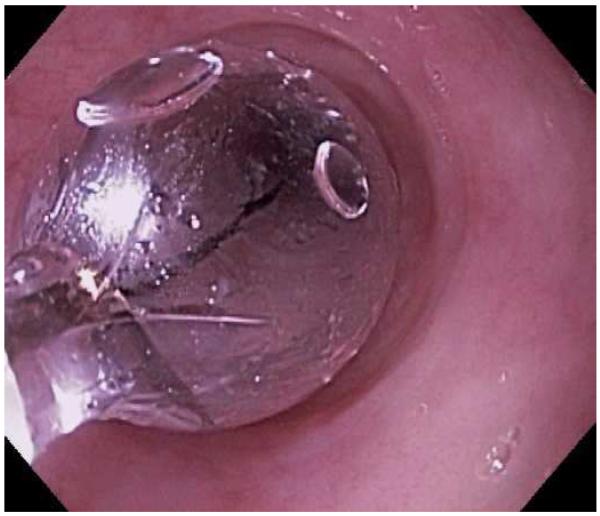
Endoscopic dilation
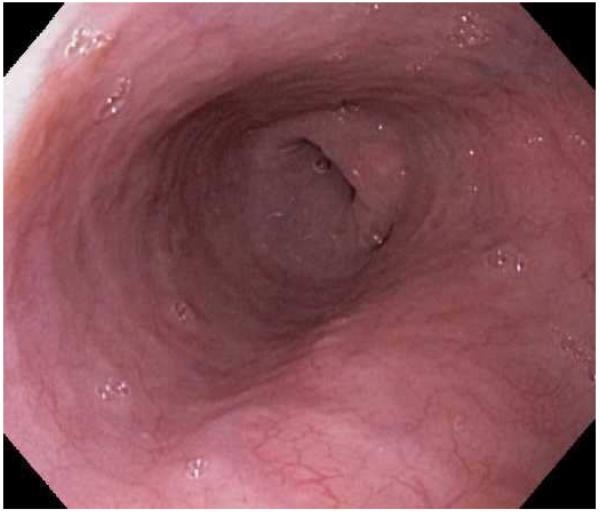
Endoscopic dilation is an effective way to treat dysphagia due to strictures or narrow caliber esophagus in patients with EoE (Figure 4). When the technique was first reported, however, there were high rates of complications such as esophageal perforation, mucosal rents, and hospitalization for post-procedural chest pain, and safety was a significant concern.52,53,139-142 As more experience with dilation in EoE patients has accumulated, however, the technique appears safer than originally believed and a cautious approach has been endorsed.1 In a number of recently published studies and two systematic reviews,143-151 the overall perforation rate is 0.3%, which is similar to the cited rate for upper endoscopy with dilation in patients who do not have EoE.152
Figure 4.
Dilation for treatment of EoE. (A) Endoscopic appearance of a esophageal narrowing with a tight series of rings, as well as mucosal pallor, congestion, and decreased vasculature. (B) Through-the-scope balloon deployed and inflated to 10 mm. (C) Desired post-dilation effect with mucosal disruption.
Dilation is also consistently reported to improve symptoms of dysphagia, even though the underlying eosinophilic inflammation is not affected.147,148,150,151 In one study by Schoepfer and colleagues, after one dilation session and in the absence of other medical or dietary treatments, the symptom response after dilation persisted for 1 year in 46% and for 2 years in 41%.147 This same study also observed that if patients are asked prospectively, approximately three-quarter will report post-dilation chest discomfort.
There are few data on the timing of dilation. Unless there is a critical stricture noted on the first endoscopy, dilation of a narrow-caliber esophagus is typically postponed until the follow-up endoscopy assessing the effect of medical or dietary therapy.1,69 There are also few data on the specific dilation technique, and both balloon and bougie dilation have been reported to be effective.148,153,154 Therefore regardless of the equipment choice, dilation should be approached with caution, care should be taken in choosing the initial dilator size, dilator size should not be increased after a mucosal rent is created, and patients should be informed about the risk, benefits, and likelihood of discomfort after the procedure.
Treatment non-response
While the majority of patients respond to medical or dietary therapy, there are a proportion who remain refractory to therapy. In these cases, a systematic assessment should be made to identify the reason for non-response, specifically determining whether ongoing esophageal eosinophilia is the cause of symptoms or if there are other contributing factors. Potential reasons for non-response include:
Non-adherence to the prescribed treatment
Inadequate dosing of a topical steroid
Candidal esophagitis complicating steroid therapy. In these cases, the eosinophilic inflammation has typically improved, but the infection causes dysphagia and odynophagia.
Persistent esophageal stricture or narrowing. Here, the eosinophilic inflammation has also resolved, but fibrotic changes in the esophagus remain and require dilation therapy for symptom improvement. Many times, subtle narrowing can be difficult to appreciate on endoscopic examination.
Superimposed motility disorders. While there is not a single characteristic pattern of esophageal dysmotility in EoE, esophageal longitudinal muscle dysfunction has been reported in a subset of patients with EoE,155-157 and the esophagus is also less compliant in EoE patients.82
Persistent esophageal eosinophilia that is truly refractory to therapy. This could be due to failure to respond to adequate doses of steroid medications, or due to persistent (or inadvertent) allergen exposure. In this situation, if a patient has failed topical steroid therapy, they should be tried on dietary elimination. Conversely, if they have failed dietary therapy, they should be placed on topical steroids.
Summary
Eosinophilic esophagitis is a chronic allergen-mediated disorder defined by symptoms of esophageal dysfunction and epithelial eosinophilia on biopsy. It has rapidly emerged as an important cause of upper GI morbidity in patients of all ages and is encountered in a substantial proportion of patients undergoing diagnostic upper endoscopy.
Diagnosis of EoE is based on consensus guidelines, but can be challenging because none of the symptoms, endoscopic findings, or histologic features are specific for EoE on their own. From a practical standpoint, EoE is suspected when a patient presents with symptoms of dysphagia, food impaction, or in children with feeding intolerance, abdominal pain, or vomiting. There are often concomitant atopic disorders present. On endoscopic evaluation, typical findings include rings, furrows, white plaques or exudates, decreased vascularity or congestion, esophageal narrowing or strictures, and mucosal fragility. When biopsies reveal at least 15 eos/hpf, EoE is a diagnostic consideration. However, other causes of eosinophilic must be considered and in particular, PPI-REE must be excluded with a PPI trial for 8 weeks and a repeat endoscopy. If symptoms persist and biopsies continue to show a peak of at least 15 eos/hpf after the PPI trial, then EoE is diagnosed.
For treatment, either swallowed topical corticosteroids or dietary elimination therapy are reasonable first line options. The choice will depend both on patient preference and local expertise. In cases where there are severe esophageal strictures, dilation is also performed. After an initial trial of treatment for 8 weeks, a follow-up endoscopy is useful to assess the mucosal response to the anti-eosinophil treatment and to determine if further endoscopic dilation is required. For patients who do not respond to either dietary or medical treatment, there are limited options but a number of new agents are under investigation and will likely change the treatment algorithm in the future.
Key Points.
Over the past ten years, EoE has become a major cause of GI symptoms, including dysphagia and food impaction in adolescents and adults, and feeding intolerance, failure-to-thrive, regurgitation, heartburn, and vomiting in children.
EoE is a clinicopathologic condition, so the entire clinical and histologic picture must to be considered in order to make a diagnosis; no single feature is diagnostic on its own.
EoE is now diagnosed based on consensus guidelines requiring symptoms of esophageal dysfunction, at least 15 eosinophils per high-power microscopy field on esophageal biopsy, and eosinophilia limited to the esophagus with other causes of esophageal eosinophilia (including proton pump inhibitor responsive esophageal eosinophilia) excluded.
Effective first line treatment strategies include topical steroids, such as swallowed fluticasone or budesonide, or dietary therapy with either an elemental formula, a six-food elimination diet, or a targeted elimination diet.
Acknowledgments
This work was supported in part by NIH award number 1K23 DK090073-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No conflicts of interested pertaining to this manuscript. Dr. Dellon has received research support from AstraZeneca, Meritage Pharma, Olympus, NIH, ACG, AGA, and CURED Foundation. Dr. Dellon has been a consultant for Oncoscope.
References
- 1.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20.e6. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 2.Landres RT, Kuster GG, Strum WB. Eosinophilic esophagitis in a patient with vigorous achalasia. Gastroenterology. 1978;74:1298–301. [PubMed] [Google Scholar]
- 3.Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci. 1993;38:109–16. doi: 10.1007/BF01296781. [DOI] [PubMed] [Google Scholar]
- 4.Straumann A, Spichtin HP, Bernoulli R, Loosli J, Vogtlin J. Idiopathic eosinophilic esophagitis: a frequently overlooked disease with typical clinical aspects and discrete endoscopic findings. Schweiz Med Wochenschr. 1994;124:1419–29. [PubMed] [Google Scholar]
- 5.Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology. 1995;109:1503–12. doi: 10.1016/0016-5085(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 6.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med. 2004;351:940–1. doi: 10.1056/NEJM200408263510924. [DOI] [PubMed] [Google Scholar]
- 7.Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol. 2005;115:418–9. doi: 10.1016/j.jaci.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Dellon ES, Gibbs WB, Fritchie KJ, et al. Clinical, endoscopic, and histologic findings distinguish eosinophilic esophagitis from gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2009;7:1305–13. doi: 10.1016/j.cgh.2009.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prasad GA, Alexander JA, Schleck CD, et al. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2009;7:1055–61. doi: 10.1016/j.cgh.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hruz P, Straumann A, Bussmann C, et al. Escalating incidence of eosinophilic esophagitis: A 20-year prospective, population-based study in Olten County, Switzerland. J Allergy Clin Immunol. 2011;128:1349–50 e5. doi: 10.1016/j.jaci.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Cherian S, Smith NM, Forbes DA. Rapidly increasing prevalence of eosinophilic oesophagitis in Western Australia. Arch Dis Child. 2006;91:1000–4. doi: 10.1136/adc.2006.100974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Rhijn BD, Verheij J, Smout AJ, Bredenoord AJ. Rapidly increasing incidence of eosinophilic esophagitis in a large cohort. Neurogastroenterol Motil. 2012 doi: 10.1111/nmo.12009. [DOI] [PubMed] [Google Scholar]
- 13.Katzka DA. Eosinophilic Esophagitis: From Rookie of the Year to Household Name. Clin Gastroenterol Hepatol. 2009;7:370–1. doi: 10.1016/j.cgh.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 14.Kidambi T, Toto E, Ho N, Taft T, Hirano I. Temporal trends in the relative prevalence of dysphagia etiologies from 1999-2009. World J Gastroenterol. 2012;18:4335–41. doi: 10.3748/wjg.v18.i32.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasad GA, Talley NJ, Romero Y, et al. Prevalence and Predictive Factors of Eosinophilic Esophagitis in Patients Presenting With Dysphagia: A Prospective Study. Am J Gastroenterol. 2007;102:2627–32. doi: 10.1111/j.1572-0241.2007.01512.x. [DOI] [PubMed] [Google Scholar]
- 16.Mackenzie SH, Go M, Chadwick B, et al. Clinical trial: eosinophilic esophagitis in patients presenting with dysphagia: a prospective analysis. Aliment Pharmacol Ther. 2008;28:1140–6. doi: 10.1111/j.1365-2036.2008.03795.x. [DOI] [PubMed] [Google Scholar]
- 17.Veerappan GR, Perry JL, Duncan TJ, et al. Prevalence of Eosinophilic Esophagitis in an Adult Population Undergoing Upper Endoscopy: A Prospective Study. Clin Gastroenterol Hepatol. 2009;7:420–6. doi: 10.1016/j.cgh.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Spergel JM, Book WM, Mays E, et al. Variation in prevalence, diagnostic criteria, and initial management options for eosinophilic gastrointestinal diseases in the United States. J Pediatr Gastroenterol Nutr. 2011;52:300–6. doi: 10.1097/MPG.0b013e3181eb5a9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sealock RJ, Rendon G, El-Serag HB. Systematic review: the epidemiology of eosinophilic oesophagitis in adults. Aliment Pharmacol Ther. 2010;32:712–9. doi: 10.1111/j.1365-2036.2010.04411.x. [DOI] [PubMed] [Google Scholar]
- 20.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–9. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116:536–47. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothenberg ME, Spergel JM, Sherrill JD, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet. 2010;42:289–91. doi: 10.1038/ng.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sherrill JD, Rothenberg ME. Genetic dissection of eosinophilic esophagitis provides insight into disease pathogenesis and treatment strategies. J Allergy Clin Immunol. 2011;128:23–32. doi: 10.1016/j.jaci.2011.03.046. quiz 3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujishiro H, Amano Y, Kushiyama Y, Ishihara S, Kinoshita Y. Eosinophilic esophagitis investigated by upper gastrointestinal endoscopy in Japanese patients. J Gastroenterol. 2011;46:1142–4. doi: 10.1007/s00535-011-0435-5. [DOI] [PubMed] [Google Scholar]
- 25.Kinoshita Y, Furuta K, Ishimaura N, et al. Clinical characteristics of Japanese patients with eosinophilic esophagitis and eosinophilic gastroenteritis. J Gastroenterol. 2012 doi: 10.1007/s00535-012-0640-x. [DOI] [PubMed] [Google Scholar]
- 26.Shi YN, Sun SJ, Xiong LS, Cao QH, Cui Y, Chen MH. Prevalence, clinical manifestations and endoscopic features of eosinophilic esophagitis: a pathological review in China. J Dig Dis. 2012;13:304–9. doi: 10.1111/j.1751-2980.2012.00593.x. [DOI] [PubMed] [Google Scholar]
- 27.Kapel RC, Miller JK, Torres C, Aksoy S, Lash R, Katzka DA. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology. 2008;134:1316–21. doi: 10.1053/j.gastro.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Dellon ES, Aderoju A, Woosley JT, Sandler RS, Shaheen NJ. Variability in diagnostic criteria for eosinophilic esophagitis: A systematic review. Am J Gastroenterol. 2007;102:2300–13. doi: 10.1111/j.1572-0241.2007.01396.x. [DOI] [PubMed] [Google Scholar]
- 29.Liacouras CA, Spergel JM, Ruchelli E, et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol. 2005;3:1198–206. doi: 10.1016/s1542-3565(05)00885-2. [DOI] [PubMed] [Google Scholar]
- 30.Franciosi JP, Tam V, Liacouras CA, Spergel JM. A case-control study of sociodemographic and geographic characteristics of 335 children with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2009;7:415–9. doi: 10.1016/j.cgh.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Sperry SLW, Woosley JT, Shaheen NJ, Dellon ES. Influence of race and gender on the presentation of eosinophilic esophagitis. Am J Gastroenterol. 2012;107:215–21. doi: 10.1038/ajg.2011.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bohm M, Malik Z, Sebastiano C, et al. Mucosal Eosinophilia: Prevalence and Racial/Ethnic Differences in Symptoms and Endoscopic Findings in Adults Over 10 Years in an Urban Hospital. J Clin Gastroenterol. 2012;46:567–74. doi: 10.1097/MCG.0b013e31823d3305. [DOI] [PubMed] [Google Scholar]
- 33.Sharma HP, Mansoor DK, Sprunger AC, et al. Racial disparities in the presentation of pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2011;127:AB110. [Google Scholar]
- 34.Moawad FJ, Veerappan GR, Dias JA, Maydonovitch CL, Wong R. Race may play a role in the clinical presentation of eosinophilic esophagitis. Am J Gastroenterol. 2012;107:1263. doi: 10.1038/ajg.2012.176. [DOI] [PubMed] [Google Scholar]
- 35.Putnam PE. Evaluation of the Child who has Eosinophilic Esophagitis. Immunol Allergy Clin North Am. 2009;29:1–10. doi: 10.1016/j.iac.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 36.Straumann A. Clinical Evaluation of the Adult who has Eosinophilic Esophagitis. Immunol Allergy Clin North Am. 2009;29:11–8. doi: 10.1016/j.iac.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Spergel JM, Brown-Whitehorn TF, Beausoleil JL, et al. 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr. 2009;48:30–6. doi: 10.1097/MPG.0b013e3181788282. [DOI] [PubMed] [Google Scholar]
- 38.Desai TK, Stecevic V, Chang CH, Goldstein NS, Badizadegan K, Furuta GT. Association of eosinophilic inflammation with esophageal food impaction in adults. Gastrointest Endosc. 2005;61:795–801. doi: 10.1016/s0016-5107(05)00313-5. [DOI] [PubMed] [Google Scholar]
- 39.Kerlin P, Jones D, Remedios M, Campbell C. Prevalence of eosinophilic esophagitis in adults with food bolus obstruction of the esophagus. J Clin Gastroenterol. 2007;41:356–61. doi: 10.1097/01.mcg.0000225590.08825.77. [DOI] [PubMed] [Google Scholar]
- 40.Sperry SL, Crockett SD, Miller CB, Shaheen NJ, Dellon ES. Esophageal foreign-body impactions: epidemiology, time trends, and the impact of the increasing prevalence of eosinophilic esophagitis. Gastrointest Endosc. 2011;74:985–91. doi: 10.1016/j.gie.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foroutan M, Norouzi A, Molaei M, et al. Eosinophilic Esophagitis in Patients with Refractory Gastroesophageal Reflux Disease. Dig Dis Sci. 2010;55:28–31. doi: 10.1007/s10620-008-0706-z. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Compean D, Gonzalez Gonzalez JA, Marrufo Garcia CA, et al. Prevalence of eosinophilic esophagitis in patients with refractory gastroesophageal reflux disease symptoms: A prospective study. Dig Liver Dis. 2011;43:204–8. doi: 10.1016/j.dld.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Poh CH, Gasiorowska A, Navarro-Rodriguez T, et al. Upper GI tract findings in patients with heartburn in whom proton pump inhibitor treatment failed versus those not receiving antireflux treatment. Gastrointest Endosc. 2010;71:28–34. doi: 10.1016/j.gie.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 44.Rodrigo S, Abboud G, Oh D, et al. High intraepithelial eosinophil counts in esophageal squamous epithelium are not specific for eosinophilic esophagitis in adults. Am J Gastroenterol. 2008;103:435–42. doi: 10.1111/j.1572-0241.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- 45.Garrean CP, Gonsalves N, Hirano I. Epidemiologic implications of symptom onset in adults with eosinophilic esophagitis. Gastroenterology. 2009;136(Suppl 1):AB–S1875. [Google Scholar]
- 46.Assa’ad AH, Putnam PE, Collins MH, et al. Pediatric patients with eosinophilic esophagitis: an 8-year follow-up. J Allergy Clin Immunol. 2007;119:731–8. doi: 10.1016/j.jaci.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 47.Chehade M, Aceves SS. Food allergy and eosinophilic esophagitis. Curr Opin Allergy Clin Immunol. 2010;10:231–7. doi: 10.1097/ACI.0b013e328338cbab. [DOI] [PubMed] [Google Scholar]
- 48.Penfield JD, Lang DM, Goldblum JR, Lopez R, Falk GW. The Role of Allergy Evaluation in Adults With Eosinophilic Esophagitis. J Clin Gastroenterol. 2010;44:22–7. doi: 10.1097/MCG.0b013e3181a1bee5. [DOI] [PubMed] [Google Scholar]
- 49.Roy-Ghanta S, Larosa DF, Katzka DA. Atopic Characteristics of Adult Patients With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2008;6:531–5. doi: 10.1016/j.cgh.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 50.Almansa C, Devault KR, Achem SR. A comprehensive review of eosinophilic esophagitis in adults. J Clin Gastroenterol. 2011;45:658–64. doi: 10.1097/MCG.0b013e318211f95b. [DOI] [PubMed] [Google Scholar]
- 51.Hurrell JM, Genta RM, Dellon ES. Prevalence of esophageal eosinophilia varies by climate zone in the United States. Am J Gastroenterol. 2012;107:698–706. doi: 10.1038/ajg.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Straumann A, Rossi L, Simon HU, Heer P, Spichtin HP, Beglinger C. Fragility of the esophageal mucosa: a pathognomonic endoscopic sign of primary eosinophilic esophagitis? Gastrointest Endosc. 2003;57:407–12. doi: 10.1067/mge.2003.123. [DOI] [PubMed] [Google Scholar]
- 53.Vasilopoulos S, Murphy P, Auerbach A, et al. The small-caliber esophagus: an unappreciated cause of dysphagia for solids in patients with eosinophilic esophagitis. Gastrointest Endosc. 2002;55:99–106. doi: 10.1067/mge.2002.118645. [DOI] [PubMed] [Google Scholar]
- 54.Straumann A, Spichtin HP, Bucher KA, Heer P, Simon HU. Eosinophilic esophagitis: red on microscopy, white on endoscopy. Digestion. 2004;70:109–16. doi: 10.1159/000080934. [DOI] [PubMed] [Google Scholar]
- 55.Kim HP, Vance RB, Shaheen NJ, Dellon ES. The Prevalence and Diagnostic Utility of Endoscopic Features of Eosinophilic Esophagitis: A Meta-Analysis. Clin Gastroenterol Hepatol. 2012;10:988–96.e5. doi: 10.1016/j.cgh.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peery AF, Cao H, Dominik R, Shaheen NJ, Dellon ES. Variable reliability of endoscopic findings with white-light and narrow-band imaging for patients with suspected eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2011;9:475–80. doi: 10.1016/j.cgh.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moy N, Heckman MG, Gonsalves N, Achem SR, Hirano I. Inter-observer agreement on endoscopic esopahgeal findings in eosinophilic esophagitis. Gastroenterology. 2011;140(Suppl 1):S236. Ab Sa1146. [Google Scholar]
- 58.Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2012 doi: 10.1136/gutjnl-2011-301817. [DOI] [PubMed] [Google Scholar]
- 59.Toto E, Kern E, Moy N, Kwansy M, Gonsalves N, Hirano I. Duration of dysphagia is associated with increased frequency of dysphagia and food impaction in adults with eosinophilic esophagitis. Gastroenterology. 2010;138(Suppl 1):AB S1088. [Google Scholar]
- 60.Schoepfer A, Safroneeva E, Bussmann C, Netzer P, Portmann S, Straumann A. Fixed rings and strictures in eosinophilic esophagitis develop due to continuing inflammation over time. Gastroenterology. 2012;142(Suppl 2):AB 1032. [Google Scholar]
- 61.Saffari H, Peterson KA, Fang JC, Teman C, Gleich GJ, Pease LF., 3rd Patchy eosinophil distributions in an esophagectomy specimen from a patient with eosinophilic esophagitis: Implications for endoscopic biopsy. J Allergy Clin Immunol. 2012;130:798–800. doi: 10.1016/j.jaci.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dellon ES, Speck O, Woodward K, Woosley JT, Shaheen NJ. The patchy nature of esophageal eosinophilia in eosinophilic esophagitis: Insights from pathology samples from a clinical trial. Gastroenterology. 2012;142(Suppl 2):Ab Su1129. [Google Scholar]
- 63.Gonsalves N, Policarpio-Nicolas M, Zhang Q, Rao MS, Hirano I. Histopathologic variability and endoscopic correlates in adults with eosinophilic esophagitis. Gastrointest Endosc. 2006;64:313–9. doi: 10.1016/j.gie.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 64.Shah A, Kagalwalla AF, Gonsalves N, Melin-Aldana H, Li BU, Hirano I. Histopathologic variability in children with eosinophilic esophagitis. Am J Gastroenterol. 2009;104:716–21. doi: 10.1038/ajg.2008.117. [DOI] [PubMed] [Google Scholar]
- 65.Collins MH. Histopathologic features of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:59–71. viii–ix. doi: 10.1016/j.giec.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 66.Odze RD. Pathology of eosinophilic esophagitis: what the clinician needs to know. Am J Gastroenterol. 2009;104:485–90. doi: 10.1038/ajg.2008.40. [DOI] [PubMed] [Google Scholar]
- 67.Peery AF, Shaheen NJ, Dellon ES. Practice patterns for the evaluation and treatment of eosinophilic oesophagitis. Aliment Pharmacol Ther. 2010;32:1373–82. doi: 10.1111/j.1365-2036.2010.04476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sperry SL, Shaheen NJ, Dellon ES. Toward uniformity in the diagnosis of eosinophilic esophagitis (EoE): the effect of guidelines on variability of diagnostic criteria for EoE. Am J Gastroenterol. 2011;106:824–32. doi: 10.1038/ajg.2011.10. quiz 33. [DOI] [PubMed] [Google Scholar]
- 69.Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–63. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 70.Dellon ES, Fritchie KJ, Rubinas TC, Woosley JT, Shaheen NJ. Inter- and intraobserver reliability and validation of a new method for determination of eosinophil counts in patients with esophageal eosinophilia. Dig Dis Sci. 2010;55:1940–9. doi: 10.1007/s10620-009-1005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spechler SJ, Genta RM, Souza RF. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007;102:1301–6. doi: 10.1111/j.1572-0241.2007.01179.x. [DOI] [PubMed] [Google Scholar]
- 72.Dranove JE, Horn DS, Davis MA, Kernek KM, Gupta SK. Predictors of response to proton pump inhibitor therapy among children with significant esophageal eosinophilia. J Pediatr. 2009;154:96–100. doi: 10.1016/j.jpeds.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 73.Sayej WN, Patel R, Baker RD, Tron E, Baker SS. Treatment With High-dose Proton Pump Inhibitors Helps Distinguish Eosinophilic Esophagitis From Noneosinophilic Esophagitis. J Pediatr Gastroenterol Nutr. 2009;49:393–9. doi: 10.1097/MPG.0b013e31819c4b3e. [DOI] [PubMed] [Google Scholar]
- 74.Moawad FJ, Dias JA, Veerappan GR, Baker T, Maydonovitch CL. Comparison of Aerosolized Swallowed Fluticasone to Esomeprazole for the Treatment of Eosinophilic Esophagitis. Am J Gastroenterol. 2011;S12:AB 30. doi: 10.1038/ajg.2012.443. [DOI] [PubMed] [Google Scholar]
- 75.Molina-Infante J, Ferrando-Lamana L, Ripoll C, et al. Esophageal Eosinophilic Infiltration Responds to Proton Pump Inhibition in Most Adults. Clin Gastroenterol Hepatol. 2011;9:110–7. doi: 10.1016/j.cgh.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 76.Peterson KA, Thomas KL, Hilden K, Emerson LL, Wills JC, Fang JC. Comparison of esomeprazole to aerosolized, swallowed fluticasone for eosinophilic esophagitis. Dig Dis Sci. 2010;55:1313–9. doi: 10.1007/s10620-009-0859-4. [DOI] [PubMed] [Google Scholar]
- 77.Dellon ES, Speck O, Woodward K, et al. Prospective determination of the prevalence of PPI-responsive esophageal eosinophilia in patients with dysphagia undergoing upper endoscopy. Am J Gastroenterol. 2012 Abstract in press. [Google Scholar]
- 78.Aceves SS, Newbury RO, Dohil MA, Bastian JF, Dohil R. A symptom scoring tool for identifying pediatric patients with eosinophilic esophagitis and correlating symptoms with inflammation. Ann Allergy Asthma Immunol. 2009;103:401–6. doi: 10.1016/S1081-1206(10)60359-6. [DOI] [PubMed] [Google Scholar]
- 79.von Arnim U, Wex T, Rohl FW, et al. Identification of clinical and laboratory markers for predicting eosinophilic esophagitis in adults. Digestion. 2011;84:323–7. doi: 10.1159/000331142. [DOI] [PubMed] [Google Scholar]
- 80.Yoo H, Kang D, Katz AJ, et al. Reflectance confocal microscopy for the diagnosis of eosinophilic esophagitis: a pilot study conducted on biopsy specimens. Gastrointest Endosc. 2011;74:992–1000. doi: 10.1016/j.gie.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Safdarian N, Liu Z, Zhou X, et al. Quantifying human eosinophils using three-dimensional volumetric images collected with multiphoton fluorescence microscopy. Gastroenterology. 2012;142:15–20 e1. doi: 10.1053/j.gastro.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kwiatek MA, Hirano I, Kahrilas PJ, Rothe J, Luger D, Pandolfino JE. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology. 2011;140:82–90. doi: 10.1053/j.gastro.2010.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Protheroe C, Woodruff SA, de Petris G, et al. A novel histologic scoring system to evaluate mucosal biopsies from patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2009;7:749–55 e11. doi: 10.1016/j.cgh.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kephart GM, Alexander JA, Arora AS, et al. Marked deposition of eosinophil-derived neurotoxin in adult patients with eosinophilic esophagitis. Am J Gastroenterol. 2010;105:298–307. doi: 10.1038/ajg.2009.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blanchard C, Stucke EM, Rodriguez-Jimenez B, et al. A striking local esophageal cytokine expression profile in eosinophilic esophagitis. J Allergy Clin Immunol. 2011;127:208–17. doi: 10.1016/j.jaci.2010.10.039. 17 e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dellon ES, Chen X, Miller CR, et al. Tryptase staining of mast cells may differentiate eosinophilic esophagitis from gastroesophageal reflux disease. Am J Gastroenterol. 2011;106:264–71. doi: 10.1038/ajg.2010.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dellon ES, Chen X, Miller CR, Woosley JT, Shaheen NJ. Diagnostic utility of major basic protein, eotaxin-3, and leukotriene enzyme staining in eosinophilic esophagitis. Am J Gastroenterol. 2012 doi: 10.1038/ajg.2012.202. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saffari H, Gleich GJ, Pease L, Peterson K. A new approach to image and enhance diagnosis of EoE: Radiolobeled conrast agents. Gastroenterology. 2012;142(Suppl 2):AB Sa1836. [Google Scholar]
- 89.Furuta GT, Kagalwalla AF, Lee JJ, et al. The oesophageal string test: a novel, minimally invasive method measures mucosal inflammation in eosinophilic oesophagitis. Gut. 2012 doi: 10.1136/gutjnl-2012-303171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gupta SK, Fitzgerald JF, Kondratyuk T, HogenEsch H. Cytokine expression in normal and inflamed esophageal mucosa: a study into the pathogenesis of allergic eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2006;42:22–6. doi: 10.1097/01.mpg.0000188740.38757.d2. [DOI] [PubMed] [Google Scholar]
- 91.Konikoff MR, Blanchard C, Kirby C, et al. Potential of blood eosinophils, eosinophil-derived neurotoxin, and eotaxin-3 as biomarkers of eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4:1328–36. doi: 10.1016/j.cgh.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 92.Subbarao G, Rosenman MB, Ohnuki L, et al. Exploring potential noninvasive biomarkers in eosinophilic esophagitis in children. J Pediatr Gastroenterol Nutr. 2011;53:651–8. doi: 10.1097/MPG.0b013e318228cee6. [DOI] [PubMed] [Google Scholar]
- 93.Blanchard C, Mingler MK, Vicario M, et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol. 2007;120:1292–300. doi: 10.1016/j.jaci.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 94.Hirano I. Therapeutic End Points in Eosinophilic Esophagitis: Is Elimination of Esophageal Eosinophils Enough? Clin Gastroenterol Hepatol. 2012;10:750–2. doi: 10.1016/j.cgh.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 95.Liacouras CA, Wenner WJ, Brown K, Ruchelli E. Primary eosinophilic esophagitis in children: successful treatment with oral corticosteroids. J Pediatr Gastroenterol Nutr. 1998;26:380–5. doi: 10.1097/00005176-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 96.Helou EF, Simonson J, Arora AS. 3-Yr-Follow-Up of Topical Corticosteroid Treatment for Eosinophilic Esophagitis in Adults. Am J Gastroenterol. 2008;103:2194–9. doi: 10.1111/j.1572-0241.2008.01989.x. [DOI] [PubMed] [Google Scholar]
- 97.Straumann A, Conus S, Degen L, et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2011;9:400–9 e1. doi: 10.1016/j.cgh.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 98.Picus D, Frank PH. Eosinophilic esophagitis. AJR Am J Roentgenol. 1981;136:1001–3. doi: 10.2214/ajr.136.5.1001. [DOI] [PubMed] [Google Scholar]
- 99.Faubion WA, Jr., Perrault J, Burgart LJ, Zein NN, Clawson M, Freese DK. Treatment of eosinophilic esophagitis with inhaled corticosteroids. J Pediatr Gastroenterol Nutr. 1998;27:90–3. doi: 10.1097/00005176-199807000-00016. [DOI] [PubMed] [Google Scholar]
- 100.Teitelbaum JE, Fox VL, Twarog FJ, et al. Eosinophilic esophagitis in children: immunopathological analysis and response to fluticasone propionate. Gastroenterology. 2002;122:1216–25. doi: 10.1053/gast.2002.32998. [DOI] [PubMed] [Google Scholar]
- 101.Arora AS, Perrault J, Smyrk TC. Topical corticosteroid treatment of dysphagia due to eosinophilic esophagitis in adults. Mayo Clin Proc. 2003;78:830–5. doi: 10.4065/78.7.830. [DOI] [PubMed] [Google Scholar]
- 102.Noel RJ, Putnam PE, Collins MH, et al. Clinical and immunopathologic effects of swallowed fluticasone for eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2004;2:568–75. doi: 10.1016/s1542-3565(04)00240-x. [DOI] [PubMed] [Google Scholar]
- 103.Remedios M, Campbell C, Jones DM, Kerlin P. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc. 2006;63:3–12. doi: 10.1016/j.gie.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 104.Aceves SS, Bastian JF, Newbury RO, Dohil R. Oral Viscous Budesonide: A Potential New Therapy for Eosinophilic Esophagitis in Children. Am J Gastroenterol. 2007;102:2271–9. doi: 10.1111/j.1572-0241.2007.01379.x. [DOI] [PubMed] [Google Scholar]
- 105.Lucendo AJ, Pascual-Turrion JM, Navarro M, et al. Endoscopic, bioptic, and manometric findings in eosinophilic esophagitis before and after steroid therapy: a case series. Endoscopy. 2007;39:765–71. doi: 10.1055/s-2007-966738. [DOI] [PubMed] [Google Scholar]
- 106.Bergquist H, Larsson H, Johansson L, Bove M. Dysphagia and quality of life may improve with mometasone treatment in patients with eosinophilic esophagitis: a pilot study. Otolaryngol Head Neck Surg. 2011;145:551–6. doi: 10.1177/0194599811409857. [DOI] [PubMed] [Google Scholar]
- 107.Schroeder S, Fleischer DM, Masterson JC, Gelfand E, Furuta GT, Atkins D. Successful treatment of eosinophilic esophagitis with ciclesonide. J Allergy Clin Immunol. 2012;129:1419–21. doi: 10.1016/j.jaci.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schaefer ET, Fitzgerald JF, Molleston JP, et al. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: a randomized trial in children. Clin Gastroenterol Hepatol. 2008;6:165–73. doi: 10.1016/j.cgh.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 109.Konikoff MR, Noel RJ, Blanchard C, et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology. 2006;131:1381–91. doi: 10.1053/j.gastro.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 110.Alexander JA, Jung KW, Arora AS, et al. Swallowed Fluticasone Improves Histologic but Not Symptomatic Responses of Adults with Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2012;10:742–9.e1. doi: 10.1016/j.cgh.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 111.Aceves SS, Dohil R, Newbury RO, Bastian JF. Topical viscous budesonide suspension for treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2005;116:705–6. doi: 10.1016/j.jaci.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 112.Gupta SK, Collins MH, Lewis JD, Farber RH. Efficacy and Safety of Oral Budesonide Suspension (OBS) in Pediatric Subjects With Eosinophilic Esophagitis (EoE): Results From the Double-Blind. Placebo-Controlled PEER Study Gastroenterology. 2011;140(Suppl 1):S179. [Google Scholar]
- 113.Straumann A, Conus S, Degen L, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology. 2010;139:1526–37. doi: 10.1053/j.gastro.2010.07.048. 37 e1. [DOI] [PubMed] [Google Scholar]
- 114.Dellon ES, Sheikh A, Speck O, et al. Viscous Topical is More Effective than Nebulized Steroid Therapy for Patients with Eosinophilic Esophagitis. Gastroenterology. 2012;143:321–4e1. doi: 10.1053/j.gastro.2012.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dohil R, Newbury R, Fox L, Bastian J, Aceves S. Oral Viscous Budesonide Is Effective in Children With Eosinophilic Esophagitis in a Randomized, Placebo-controlled Trial. Gastroenterology. 2010;139:418–29. doi: 10.1053/j.gastro.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 116.Lindberg GM, Van Eldik R, Saboorian MH. A case of herpes esophagitis after fluticasone propionate for eosinophilic esophagitis. Nat Clin Pract Gastroenterol Hepatol. 2008;5:527–30. doi: 10.1038/ncpgasthep1225. [DOI] [PubMed] [Google Scholar]
- 117.Straumann A, Conus S, Grzonka P, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2010;59:21–30. doi: 10.1136/gut.2009.178558. [DOI] [PubMed] [Google Scholar]
- 118.Assa’ad AH, Gupta SK, Collins MH, et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141:1593–604. doi: 10.1053/j.gastro.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 119.Spergel JM, Rothenberg ME, Collins MH, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: Results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129:456–63. doi: 10.1016/j.jaci.2011.11.044. 63.e1-3. [DOI] [PubMed] [Google Scholar]
- 120.Straumann A, Bussmann C, Conus S, Beglinger C, Simon HU. Anti-TNF-alpha (infliximab) therapy for severe adult eosinophilic esophagitis. J Allergy Clin Immunol. 2008;122:425–7. doi: 10.1016/j.jaci.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 121.Fang JC, Hilden K, Gleich GJ, et al. A Pilot Study of the Treatment of Eosinophilic Esophagitis With Omalizumab. Gastroenterology. 2011;140(Suppl 1):AB Sa1143. [Google Scholar]
- 122.Attwood SE, Lewis CJ, Bronder CS, Morris CD, Armstrong GR, Whittam J. Eosinophilic oesophagitis: a novel treatment using Montelukast. Gut. 2003;52:181–5. doi: 10.1136/gut.52.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stumphy J, Al-Zubeidi D, Guerin L, Mitros F, Rahhal R. Observations on use of montelukast in pediatric eosinophilic esophagitis: insights for the future. Dis Esophagus. 2011;24:229–34. doi: 10.1111/j.1442-2050.2010.01134.x. [DOI] [PubMed] [Google Scholar]
- 124.Lucendo AJ, De Rezende LC, Jimenez-Contreras S, et al. Montelukast was inefficient in maintaining steroid-induced remission in adult eosinophilic esophagitis. Dig Dis Sci. 2011;56:3551–8. doi: 10.1007/s10620-011-1775-y. [DOI] [PubMed] [Google Scholar]
- 125.Netzer P, Gschossmann JM, Straumann A, Sendensky A, Weimann R, Schoepfer AM. Corticosteroid-dependent eosinophilic oesophagitis: azathioprine and 6-mercaptopurine can induce and maintain long-term remission. Eur J Gastroenterol Hepatol. 2007;19:865–9. doi: 10.1097/MEG.0b013e32825a6ab4. [DOI] [PubMed] [Google Scholar]
- 126.Straumann A, Bussmann C, Perkins MC, et al. Treatment of eosinophilic esophagitis with the CRTH2-antagonist OCT000459: A novel therapeutic principle. Gastroenterology. 2012;142(Suppl 2):AB 856. [Google Scholar]
- 127.Markowitz JE, Spergel JM, Ruchelli E, Liacouras CA. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol. 2003;98:777–82. doi: 10.1111/j.1572-0241.2003.07390.x. [DOI] [PubMed] [Google Scholar]
- 128.Henderson CJ, Abonia JP, King EC, et al. Comparative dietary therapy effectiveness in remission of pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2012;129:1570–8. doi: 10.1016/j.jaci.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Peterson K, Clayton F, Vinson LA, et al. Utility of an Elemental Diet in Adult Eosinophilic Esophagitis. Gastroenterology. 2011;140(Suppl 1):AB 1080. [Google Scholar]
- 130.Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of Six-Food Elimination Diet on Clinical and Histologic Outcomes in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2006;4:1097–102. doi: 10.1016/j.cgh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 131.Kagalwalla AF, Shah A, Li BU, et al. Identification of specific foods responsible for inflammation in children with eosinophilic esophagitis successfully treated with empiric elimination diet. J Pediatr Gastroenterol Nutr. 2011;53:145–9. doi: 10.1097/MPG.0b013e31821cf503. [DOI] [PubMed] [Google Scholar]
- 132.Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination Diet Effectively Treats Eosinophilic Esophagitis in Adults; Food Reintroduction Identifies Causative Factors. Gastroenterology. 2012;142:1451–9.e1. doi: 10.1053/j.gastro.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 133.Spergel JM, Brown-Whitehorn T, Beausoleil JL, Shuker M, Liacouras CA. Predictive values for skin prick test and atopy patch test for eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119:509–11. doi: 10.1016/j.jaci.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 134.Spergel JM, Beausoleil JL, Mascarenhas M, Liacouras CA. The use of skin prick tests and patch tests to identify causative foods in eosinophilic esophagitis. J Allergy Clin Immunol. 2002;109:363–8. doi: 10.1067/mai.2002.121458. [DOI] [PubMed] [Google Scholar]
- 135.Spergel JM, Brown-Whitehorn TF, Cianferoni A, et al. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J Allergy Clin Immunol. 2012;130:461–7 e5. doi: 10.1016/j.jaci.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 136.Spergel JM, Andrews T, Brown-Whitehorn TF, Beausoleil JL, Liacouras CA. Treatment of eosinophilic esophagitis with specific food elimination diet directed by a combination of skin prick and patch tests. Ann Allergy Asthma Immunol. 2005;95:336–43. doi: 10.1016/S1081-1206(10)61151-9. [DOI] [PubMed] [Google Scholar]
- 137.Molina-Infante J, Martin-Noguerol E, Alvarado-Arenas M, Porcel-Carreno SL, Jimenez-Timon S, Hernandez-Arbeiza FJ. Selective elimination diet based on skin testing has suboptimal efficacy for adult eosinophilic esophagitis. J Allergy Clin Immunol. 2012 doi: 10.1016/j.jaci.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 138.Simon D, Straumann A, Wenk A, Spichtin H, Simon HU, Braathen LR. Eosinophilic esophagitis in adults--no clinical relevance of wheat and rye sensitizations. Allergy. 2006;61:1480–3. doi: 10.1111/j.1398-9995.2006.01224.x. [DOI] [PubMed] [Google Scholar]
- 139.Croese J, Fairley SK, Masson JW, et al. Clinical and endoscopic features of eosinophilic esophagitis in adults. Gastrointest Endosc. 2003;58:516–22. doi: 10.1067/s0016-5107(03)01870-4. [DOI] [PubMed] [Google Scholar]
- 140.Kaplan M, Mutlu EA, Jakate S, et al. Endoscopy in eosinophilic esophagitis: “feline” esophagus and perforation risk. Clin Gastroenterol Hepatol. 2003;1:433–7. doi: 10.1016/s1542-3565(03)00222-2. [DOI] [PubMed] [Google Scholar]
- 141.Potter JW, Saeian K, Staff D, et al. Eosinophilic esophagitis in adults: an emerging problem with unique esophageal features. Gastrointest Endosc. 2004;59:355–61. doi: 10.1016/s0016-5107(03)02713-5. [DOI] [PubMed] [Google Scholar]
- 142.Cohen MS, Kaufman AB, Palazzo JP, Nevin D, Dimarino AJ, Jr., Cohen S. An audit of endoscopic complications in adult eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2007;5:1149–53. doi: 10.1016/j.cgh.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 143.Gonsalves N, Karmali K, Hirano I. Safety and response of esophageal dilation in adults with eosinophilic esophagitis: A single center experience of 81 patients. Gastroenterology. 2007;132(Suppl 2):A607. [Google Scholar]
- 144.Schoepfer AM, Gschossmann J, Scheurer U, Seibold F, Straumann A. Esophageal strictures in adult eosinophilic esophagitis: dilation is an effective and safe alternative after failure of topical corticosteroids. Endoscopy. 2008;40:161–4. doi: 10.1055/s-2007-995345. [DOI] [PubMed] [Google Scholar]
- 145.Straumann A, Bussmann C, Zuber M, Vannini S, Simon HU, Schoepfer A. Eosinophilic esophagitis: analysis of food impaction and perforation in 251 adolescent and adult patients. Clin Gastroenterol Hepatol. 2008;6:598–600. doi: 10.1016/j.cgh.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 146.Dellon ES, Gibbs WB, Rubinas TC, et al. Esophageal dilation in eosinophilic esophagitis: Safety and predictors of clinical response and complications. Gastrointest Endosc. 2010;71:706–12. doi: 10.1016/j.gie.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 147.Schoepfer AM, Gonsalves N, Bussmann C, et al. Esophageal dilation in eosinophilic esophagitis: effectiveness, safety, and impact on the underlying inflammation. Am J Gastroenterol. 2010;105:1062–70. doi: 10.1038/ajg.2009.657. [DOI] [PubMed] [Google Scholar]
- 148.Bohm M, Richter JE, Kelsen S, Thomas R. Esophageal dilation: simple and effective treatment for adults with eosinophilic esophagitis and esophageal rings and narrowing. Dis Esophagus. 2010;23:377–85. doi: 10.1111/j.1442-2050.2010.01051.x. [DOI] [PubMed] [Google Scholar]
- 149.Jung KW, Gundersen N, Kopacova J, et al. Occurrence of and risk factors for complications after endoscopic dilation in eosinophilic esophagitis. Gastrointest Endosc. 2011;73:15–21. doi: 10.1016/j.gie.2010.09.036. [DOI] [PubMed] [Google Scholar]
- 150.Jacobs JW, Jr., Spechler SJ. A systematic review of the risk of perforation during esophageal dilation for patients with eosinophilic esophagitis. Dig Dis Sci. 2010;55:1512–5. doi: 10.1007/s10620-010-1165-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bohm ME, Richter JE. Review article: oesophageal dilation in adults with eosinophilic oesophagitis. Aliment Pharmacol Ther. 2011;33:748–57. doi: 10.1111/j.1365-2036.2011.04593.x. [DOI] [PubMed] [Google Scholar]
- 152.Egan JV, Baron TH, Adler DG, et al. Esophageal dilation. Gastrointest Endosc. 2006;63:755–60. doi: 10.1016/j.gie.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 153.Madanick RD, Shaheen NJ, Dellon ES. A novel balloon pull-through technique for esophageal dilation in eosinophilic esophagitis (with video) Gastrointest Endosc. 2011;73:138–42. doi: 10.1016/j.gie.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 154.Ally MR, Dias J, Veerappan GR, Maydonovitch CL, Wong RK, Moawad FJ. Safety of dilation in adults with eosinophilic esophagitis. Dis Esophagus. 2012 doi: 10.1111/j.1442-2050.2012.01363.x. [DOI] [PubMed] [Google Scholar]
- 155.Korsapati HR, Babaei A, Bhargava V, Dohil R, Quin AM, Mittal RK. Dysfunction of the Longitudinal Muscles of the Esophagus in Eosinophilic Esophagitis. Gut. 2009;58:1056–62. doi: 10.1136/gut.2008.168146. [DOI] [PubMed] [Google Scholar]
- 156.Nurko S, Rosen R, Furuta GT. Esophageal Dysmotility in Children With Eosinophilic Esophagitis: A Study Using Prolonged Esophageal Manometry. Am J Gastroenterol. 2009;104:3050–7. doi: 10.1038/ajg.2009.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Roman S, Hirano I, Kwiatek MA, et al. Manometric features of eosinophilic esophagitis in esophageal pressure topography. Neurogastroenterol Motil. 2011;23:208–14. e111. doi: 10.1111/j.1365-2982.2010.01633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]