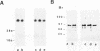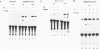Abstract
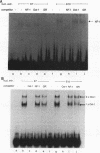
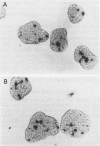
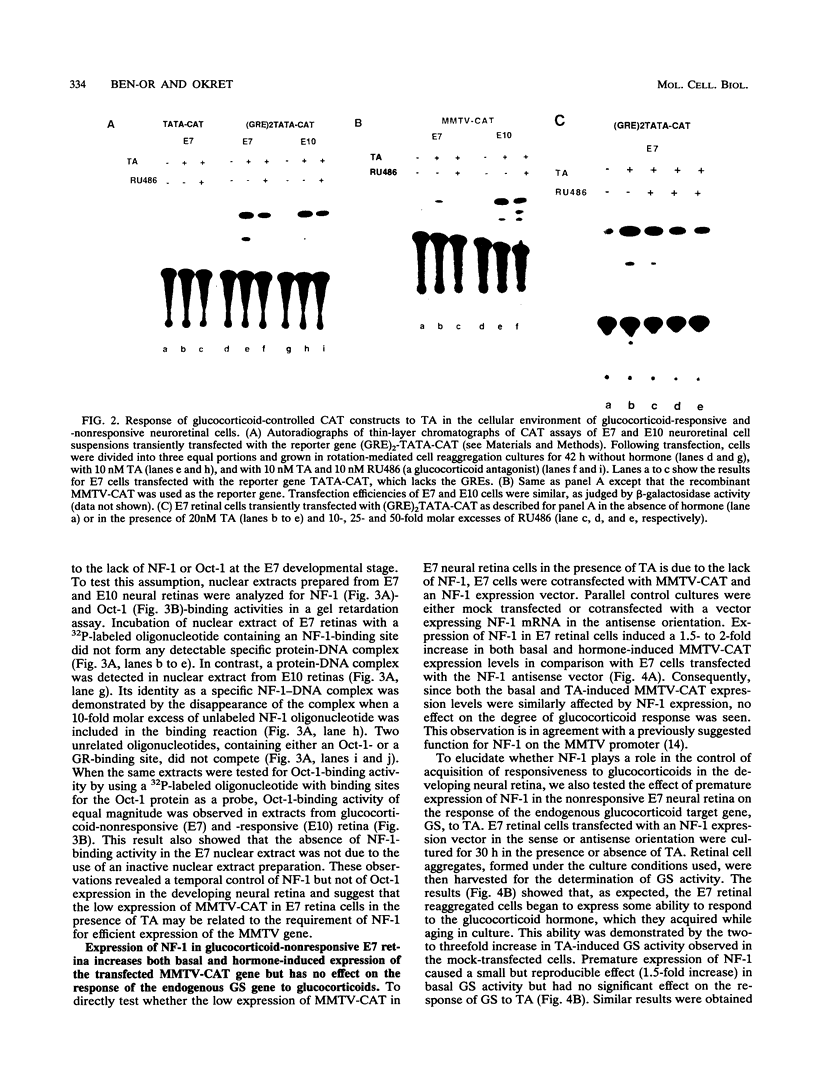
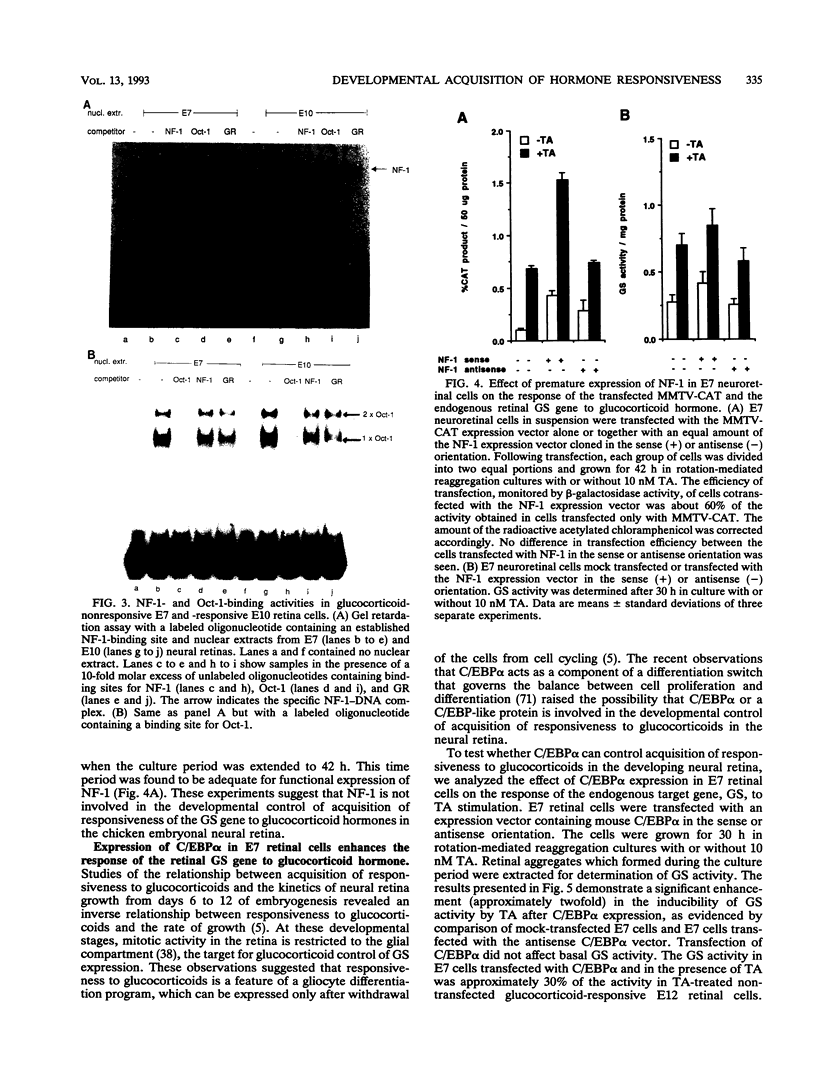
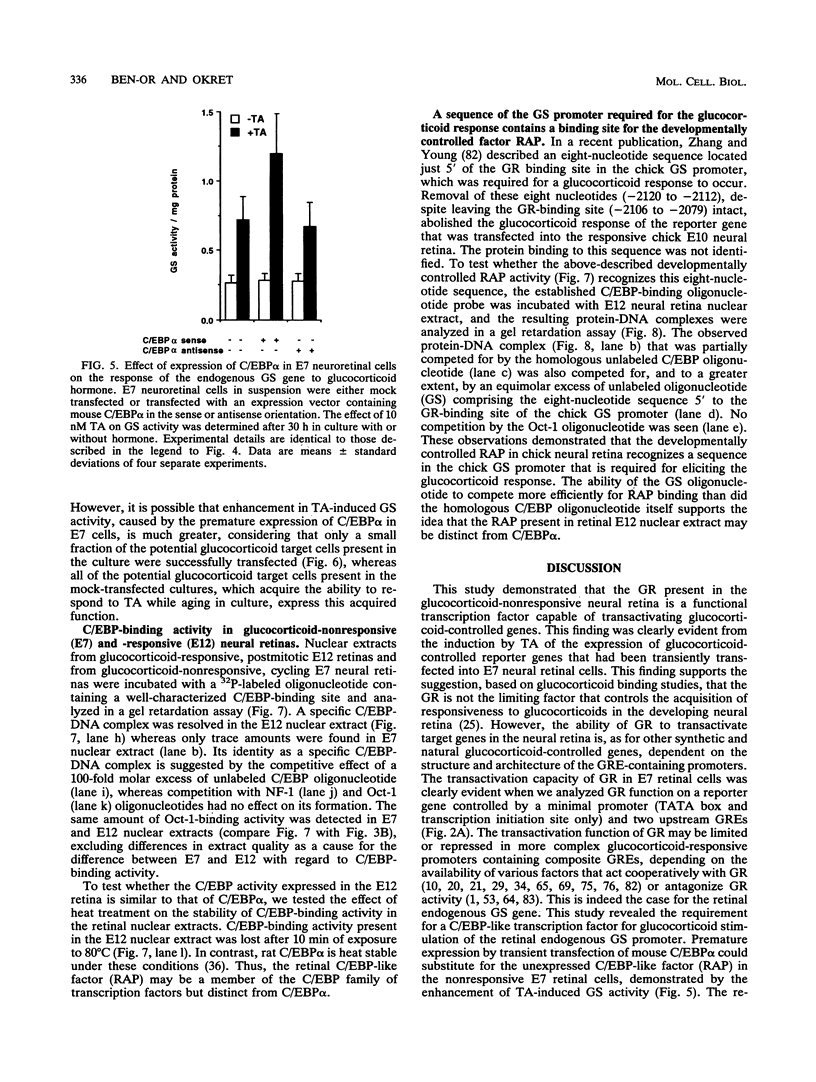
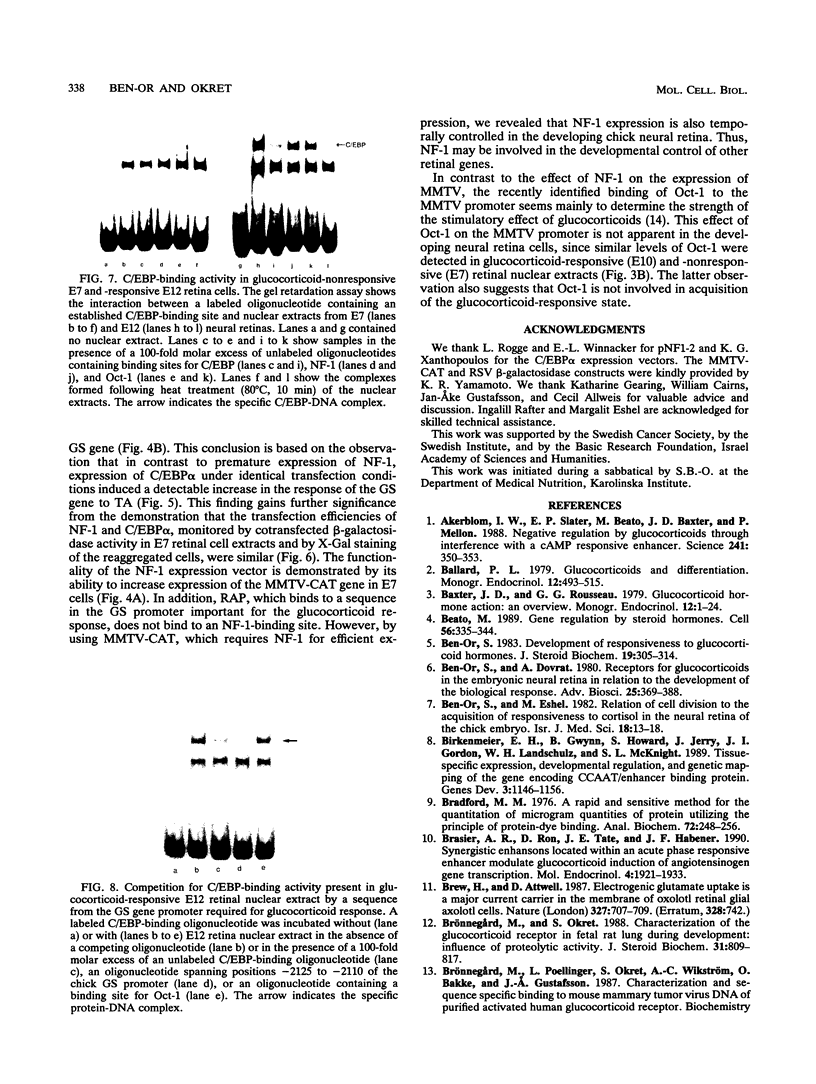
The glucocorticoid receptor in chicken embryonic neural retina is expressed early in ontogeny, yet the tissue's response to the glucocorticoid hormone, i.e., induction of glutamine synthetase (GS), develops later, only during week 2 of ontogeny. Transient transfection of embryonic day 7 (E7) retinal cells, which are nonresponsive to glucocorticoids, with chimeric plasmids containing the chloramphenicol acetyltransferase reporter gene under the control of glucocorticoid-responsive promoters demonstrated that GR in E7 cells is a functional transactivating factor. We show that the limiting transcription factor that controls the developmental acquisition of responsiveness to glucocorticoids is similar to a CCAAT enhancer-binding protein (C/EBP). This protein recognizes a sequence in the promoter of the chick GS gene, which is required for eliciting the glucocorticoid response. Retinal C/EBP-like protein was not detected in the glucocorticoid-nonresponsive (E7) proliferating glioblasts but was found to be present in the glucocorticoid-responsive (E12) postmitotic cells. Premature expression of C/EBP in the nonresponsive E7 cells by transfection was shown to enhance the developmental acquisition of responsiveness to the glucocorticoid hormone, as deduced from the level of GS inducibility.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerblom I. E., Slater E. P., Beato M., Baxter J. D., Mellon P. L. Negative regulation by glucocorticoids through interference with a cAMP responsive enhancer. Science. 1988 Jul 15;241(4863):350–353. doi: 10.1126/science.2838908. [DOI] [PubMed] [Google Scholar]
- Ballard P. L. Glucocorticoids and differentiation. Monogr Endocrinol. 1979;12:493–515. doi: 10.1007/978-3-642-81265-1_26. [DOI] [PubMed] [Google Scholar]
- Baxter J. D., Rousseau G. G. Glucocorticoid hormone action: an overview. Monogr Endocrinol. 1979;12:1–24. doi: 10.1007/978-3-642-81265-1_1. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Ben-Or S. Development of responsiveness to glucocorticoid hormones. J Steroid Biochem. 1983 Jul;19(1A):305–314. [PubMed] [Google Scholar]
- Ben-Or S., Eshel M. Relation of cell division to the acquisition of responsiveness to cortisol in the neural retina of the chick embryo. Isr J Med Sci. 1982 Jan;18(1):13–18. [PubMed] [Google Scholar]
- Birkenmeier E. H., Gwynn B., Howard S., Jerry J., Gordon J. I., Landschulz W. H., McKnight S. L. Tissue-specific expression, developmental regulation, and genetic mapping of the gene encoding CCAAT/enhancer binding protein. Genes Dev. 1989 Aug;3(8):1146–1156. doi: 10.1101/gad.3.8.1146. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brasier A. R., Ron D., Tate J. E., Habener J. F. Synergistic enhansons located within an acute phase responsive enhancer modulate glucocorticoid induction of angiotensinogen gene transcription. Mol Endocrinol. 1990 Dec;4(12):1921–1933. doi: 10.1210/mend-4-12-1921. [DOI] [PubMed] [Google Scholar]
- Brew H., Attwell D. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. 1987 Jun 25-Jul 1Nature. 327(6124):707–709. doi: 10.1038/327707a0. [DOI] [PubMed] [Google Scholar]
- Brönnegård M., Okret S. Characterization of the glucocorticoid receptor in fetal rat lung during development: influence of proteolytic activity. J Steroid Biochem. 1988 Nov;31(5):809–817. doi: 10.1016/0022-4731(88)90290-7. [DOI] [PubMed] [Google Scholar]
- Brüggemeier U., Kalff M., Franke S., Scheidereit C., Beato M. Ubiquitous transcription factor OTF-1 mediates induction of the MMTV promoter through synergistic interaction with hormone receptors. Cell. 1991 Feb 8;64(3):565–572. doi: 10.1016/0092-8674(91)90240-y. [DOI] [PubMed] [Google Scholar]
- Brüggemeier U., Rogge L., Winnacker E. L., Beato M. Nuclear factor I acts as a transcription factor on the MMTV promoter but competes with steroid hormone receptors for DNA binding. EMBO J. 1990 Jul;9(7):2233–2239. doi: 10.1002/j.1460-2075.1990.tb07393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetti E., Kühnel B. Distinct sequence elements involved in the glucocorticoid regulation of the mouse mammary tumor virus promoter identified by linker scanning mutagenesis. J Mol Biol. 1986 Aug 5;190(3):379–389. doi: 10.1016/0022-2836(86)90009-4. [DOI] [PubMed] [Google Scholar]
- Cao Z., Umek R. M., McKnight S. L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991 Sep;5(9):1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- Chodosh L. A., Baldwin A. S., Carthew R. W., Sharp P. A. Human CCAAT-binding proteins have heterologous subunits. Cell. 1988 Apr 8;53(1):11–24. doi: 10.1016/0092-8674(88)90483-7. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Grayson D. R., Xanthopoulos K. G., Darnell J. E., Jr A liver-specific DNA-binding protein recognizes multiple nucleotide sites in regulatory regions of transthyretin, alpha 1-antitrypsin, albumin, and simian virus 40 genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3840–3844. doi: 10.1073/pnas.85.11.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesch U., Gloss B., Schmid W., Schütz G., Schüle R., Renkawitz R. Glucocorticoid induction of the rat tryptophan oxygenase gene is mediated by two widely separated glucocorticoid-responsive elements. EMBO J. 1987 Mar;6(3):625–630. doi: 10.1002/j.1460-2075.1987.tb04800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M. I., Miner J. N., Yoshinaga S. K., Yamamoto K. R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990 Sep 14;249(4974):1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- Distel R. J., Ro H. S., Rosen B. S., Groves D. L., Spiegelman B. M. Nucleoprotein complexes that regulate gene expression in adipocyte differentiation: direct participation of c-fos. Cell. 1987 Jun 19;49(6):835–844. doi: 10.1016/0092-8674(87)90621-0. [DOI] [PubMed] [Google Scholar]
- Dong Y., Poellinger L., Gustafsson J. A., Okret S. Regulation of glucocorticoid receptor expression: evidence for transcriptional and posttranslational mechanisms. Mol Endocrinol. 1988 Dec;2(12):1256–1264. doi: 10.1210/mend-2-12-1256. [DOI] [PubMed] [Google Scholar]
- Eshel M., Ben-Or S. The relation between cortisol binding and glutamine synthetase induction in the neural retina of the chick embryo. Endocrinology. 1980 Jan;106(1):284–290. doi: 10.1210/endo-106-1-284. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange T., Roux J., Rigaud G., Pictet R. Cell-type specific activity of two glucocorticoid responsive units of rat tyrosine aminotransferase gene is associated with multiple binding sites for C/EBP and a novel liver-specific nuclear factor. Nucleic Acids Res. 1991 Jan 11;19(1):131–139. doi: 10.1093/nar/19.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson J. A., Carlstedt-Duke J., Poellinger L., Okret S., Wikström A. C., Brönnegård M., Gillner M., Dong Y., Fuxe K., Cintra A. Biochemistry, molecular biology, and physiology of the glucocorticoid receptor. Endocr Rev. 1987 May;8(2):185–234. doi: 10.1210/edrv-8-2-185. [DOI] [PubMed] [Google Scholar]
- Hayward B. E., Hussain A., Wilson R. H., Lyons A., Woodcock V., McIntosh B., Harris T. J. The cloning and nucleotide sequence of cDNA for an amplified glutamine synthetase gene from the Chinese hamster. Nucleic Acids Res. 1986 Jan 24;14(2):999–1008. doi: 10.1093/nar/14.2.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera R., Ro H. S., Robinson G. S., Xanthopoulos K. G., Spiegelman B. M. A direct role for C/EBP and the AP-I-binding site in gene expression linked to adipocyte differentiation. Mol Cell Biol. 1989 Dec;9(12):5331–5339. doi: 10.1128/mcb.9.12.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai E., Stromstedt P. E., Quinn P. G., Carlstedt-Duke J., Gustafsson J. A., Granner D. K. Characterization of a complex glucocorticoid response unit in the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol. 1990 Sep;10(9):4712–4719. doi: 10.1128/mcb.10.9.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen H. M., Strähle U., Gloss B., Stewart F., Schmid W., Boshart M., Miksicek R., Schütz G. Cooperativity of glucocorticoid response elements located far upstream of the tyrosine aminotransferase gene. Cell. 1987 Apr 10;49(1):29–38. doi: 10.1016/0092-8674(87)90752-5. [DOI] [PubMed] [Google Scholar]
- Johnson P. F., Landschulz W. H., Graves B. J., McKnight S. L. Identification of a rat liver nuclear protein that binds to the enhancer core element of three animal viruses. Genes Dev. 1987 Apr;1(2):133–146. doi: 10.1101/gad.1.2.133. [DOI] [PubMed] [Google Scholar]
- Jonat C., Rahmsdorf H. J., Park K. K., Cato A. C., Gebel S., Ponta H., Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990 Sep 21;62(6):1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Kahn A. J. An autoradiographic analysis of the time of appearance of neurons in the developing chick neural retina. Dev Biol. 1974 May;38(1):30–40. doi: 10.1016/0012-1606(74)90256-5. [DOI] [PubMed] [Google Scholar]
- Koehler D. E., Moscona A. A. Corticosteroid receptors in the neural retina and other tissues of the chick embryo. Arch Biochem Biophys. 1975 Sep;170(1):102–113. doi: 10.1016/0003-9861(75)90101-0. [DOI] [PubMed] [Google Scholar]
- Kuo C. F., Darnell J. E., Jr Mouse glutamine synthetase is encoded by a single gene that can be expressed in a localized fashion. J Mol Biol. 1989 Jul 5;208(1):45–56. doi: 10.1016/0022-2836(89)90086-7. [DOI] [PubMed] [Google Scholar]
- Lamph W. W., Wamsley P., Sassone-Corsi P., Verma I. M. Induction of proto-oncogene JUN/AP-1 by serum and TPA. Nature. 1988 Aug 18;334(6183):629–631. doi: 10.1038/334629a0. [DOI] [PubMed] [Google Scholar]
- Linser P. J., Perkins M. S. Regulatory aspects of the in vitro development of retinal Müller glial cells. Cell Differ. 1987 Mar;20(2-3):189–196. doi: 10.1016/0045-6039(87)90433-7. [DOI] [PubMed] [Google Scholar]
- Linser P., Moscona A. A. Induction of glutamine synthetase in embryonic neural retina: localization in Müller fibers and dependence on cell interactions. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6476–6480. doi: 10.1073/pnas.76.12.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucibello F. C., Slater E. P., Jooss K. U., Beato M., Müller R. Mutual transrepression of Fos and the glucocorticoid receptor: involvement of a functional domain in Fos which is absent in FosB. EMBO J. 1990 Sep;9(9):2827–2834. doi: 10.1002/j.1460-2075.1990.tb07471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSCONA A. Rotation-mediated histogenetic aggregation of dissociated cells. A quantifiable approach to cell interactions in vitro. Exp Cell Res. 1961 Jan;22:455–475. doi: 10.1016/0014-4827(61)90122-7. [DOI] [PubMed] [Google Scholar]
- Mao J., Regelson W., Kalimi M. Molecular mechanism of RU 486 action: a review. Mol Cell Biochem. 1992 Jan 15;109(1):1–8. doi: 10.1007/BF00230867. [DOI] [PubMed] [Google Scholar]
- Mearow K. M., Mill J. F., Freese E. Neuron-glial interactions involved in the regulation of glutamine synthetase. Glia. 1990;3(5):385–392. doi: 10.1002/glia.440030510. [DOI] [PubMed] [Google Scholar]
- Meister A. On the synthesis and utilization of glutamine. Harvey Lect. 1969;63:139–178. [PubMed] [Google Scholar]
- Meisterernst M., Rogge L., Foeckler R., Karaghiosoff M., Winnacker E. L. Structural and functional organization of a porcine gene coding for nuclear factor I. Biochemistry. 1989 Oct 3;28(20):8191–8200. doi: 10.1021/bi00446a034. [DOI] [PubMed] [Google Scholar]
- Miesfeld R., Rusconi S., Godowski P. J., Maler B. A., Okret S., Wikström A. C., Gustafsson J. A., Yamamoto K. R. Genetic complementation of a glucocorticoid receptor deficiency by expression of cloned receptor cDNA. Cell. 1986 Aug 1;46(3):389–399. doi: 10.1016/0092-8674(86)90659-8. [DOI] [PubMed] [Google Scholar]
- Mill J. F., Mearow K. M., Purohit H. J., Haleem-Smith H., King R., Freese E. Cloning and functional characterization of the rat glutamine synthetase gene. Brain Res Mol Brain Res. 1991 Feb;9(3):197–207. doi: 10.1016/0169-328x(91)90003-g. [DOI] [PubMed] [Google Scholar]
- Miner J. N., Yamamoto K. R. Regulatory crosstalk at composite response elements. Trends Biochem Sci. 1991 Nov;16(11):423–426. doi: 10.1016/0968-0004(91)90168-u. [DOI] [PubMed] [Google Scholar]
- Moscona M., Moscona A. A. The development of inducibility for glutamine synthetase in embryonic neural retina: inhibition by BrdU. Differentiation. 1979;13(3):165–172. doi: 10.1111/j.1432-0436.1979.tb01579.x. [DOI] [PubMed] [Google Scholar]
- Okret S., Dong Y., Brönnegård M., Gustafsson J. A. Regulation of glucocorticoid receptor expression. Biochimie. 1991 Jan;73(1):51–59. doi: 10.1016/0300-9084(91)90074-b. [DOI] [PubMed] [Google Scholar]
- Okret S., Wikström A. C., Wrange O., Andersson B., Gustafsson J. A. Monoclonal antibodies against the rat liver glucocorticoid receptor. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1609–1613. doi: 10.1073/pnas.81.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patejunas G., Young A. P. Tissue-specific regulation of avian glutamine synthetase expression during development and in response to glucocorticoid hormones. Mol Cell Biol. 1987 Mar;7(3):1070–1077. doi: 10.1128/mcb.7.3.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poellinger L., Yoza B. K., Roeder R. G. Functional cooperativity between protein molecules bound at two distinct sequence elements of the immunoglobulin heavy-chain promoter. Nature. 1989 Feb 9;337(6207):573–576. doi: 10.1038/337573a0. [DOI] [PubMed] [Google Scholar]
- Pu H. F., Young A. P. The structure of the chicken glutamine synthetase-encoding gene. Gene. 1989 Sep 1;81(1):169–175. doi: 10.1016/0378-1119(89)90348-x. [DOI] [PubMed] [Google Scholar]
- Saad A. D., Moscona A. A. Cortisol receptors and inducibility of glutamine synthetase in embryonic retina. Cell Differ. 1985 Jun;16(4):241–250. doi: 10.1016/0045-6039(85)90574-3. [DOI] [PubMed] [Google Scholar]
- Schüle R., Evans R. M. Cross-coupling of signal transduction pathways: zinc finger meets leucine zipper. Trends Genet. 1991 Nov-Dec;7(11-12):377–381. doi: 10.1016/0168-9525(91)90259-s. [DOI] [PubMed] [Google Scholar]
- Schüle R., Muller M., Kaltschmidt C., Renkawitz R. Many transcription factors interact synergistically with steroid receptors. Science. 1988 Dec 9;242(4884):1418–1420. doi: 10.1126/science.3201230. [DOI] [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Kliewer S., Ransone L. J., Bolado J., Yang N., Verma I. M., Evans R. M. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990 Sep 21;62(6):1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Sharp P. A., Wahli W. W., Keller M. J. A high-efficiency HeLa cell nuclear transcription extract. DNA. 1988 Jan-Feb;7(1):47–55. doi: 10.1089/dna.1988.7.47. [DOI] [PubMed] [Google Scholar]
- Shemshedini L., Knauthe R., Sassone-Corsi P., Pornon A., Gronemeyer H. Cell-specific inhibitory and stimulatory effects of Fos and Jun on transcription activation by nuclear receptors. EMBO J. 1991 Dec;10(12):3839–3849. doi: 10.1002/j.1460-2075.1991.tb04953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strähle U., Schmid W., Schütz G. Synergistic action of the glucocorticoid receptor with transcription factors. EMBO J. 1988 Nov;7(11):3389–3395. doi: 10.1002/j.1460-2075.1988.tb03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. M., Jones P. A. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979 Aug;17(4):771–779. doi: 10.1016/0092-8674(79)90317-9. [DOI] [PubMed] [Google Scholar]
- Umek R. M., Friedman A. D., McKnight S. L. CCAAT-enhancer binding protein: a component of a differentiation switch. Science. 1991 Jan 18;251(4991):288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- Vanderbilt J. N., Miesfeld R., Maler B. A., Yamamoto K. R. Intracellular receptor concentration limits glucocorticoid-dependent enhancer activity. Mol Endocrinol. 1987 Jan;1(1):68–74. doi: 10.1210/mend-1-1-68. [DOI] [PubMed] [Google Scholar]
- Vardimon L., Fox L. L., Degenstein L., Moscona A. A. Cell contacts are required for induction by cortisol of glutamine synthetase gene transcription in the retina. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5981–5985. doi: 10.1073/pnas.85.16.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland S., Döbbeling U., Rusconi S. Interference and synergism of glucocorticoid receptor and octamer factors. EMBO J. 1991 Sep;10(9):2513–2521. doi: 10.1002/j.1460-2075.1991.tb07791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P., Ratajczak T., Lee S. C., Ringold G. M. AGP/EBP(LAP) expressed in rat hepatoma cells interacts with multiple promoter sites and is necessary for maximal glucocorticoid induction of the rat alpha-1 acid glycoprotein gene. Mol Cell Biol. 1991 Oct;11(10):4959–4965. doi: 10.1128/mcb.11.10.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. C., Cantwell C. A., Johnson P. F. A family of C/EBP-related proteins capable of forming covalently linked leucine zipper dimers in vitro. Genes Dev. 1991 Sep;5(9):1553–1567. doi: 10.1101/gad.5.9.1553. [DOI] [PubMed] [Google Scholar]
- Wrange O., Okret S., Radojćić M., Carlstedt-Duke J., Gustafsson J. A. Characterization of the purified activated glucocorticoid receptor from rat liver cytosol. J Biol Chem. 1984 Apr 10;259(7):4534–4541. [PubMed] [Google Scholar]
- Wu D. K., Scully S., de Vellis J. Induction of glutamine synthetase in rat astrocytes by co-cultivation with embryonic chick neurons. J Neurochem. 1988 Mar;50(3):929–935. doi: 10.1111/j.1471-4159.1988.tb03001.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- Yang-Yen H. F., Chambard J. C., Sun Y. L., Smeal T., Schmidt T. J., Drouin J., Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990 Sep 21;62(6):1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- Zhang H. Y., Young A. P. A single upstream glucocorticoid response element juxtaposed to an AP1/ATF/CRE-like site renders the chicken glutamine synthetase gene hormonally inducible in transfected retina. J Biol Chem. 1991 Dec 25;266(36):24332–24338. [PubMed] [Google Scholar]
- Zhang X. K., Dong J. M., Chiu J. F. Regulation of alpha-fetoprotein gene expression by antagonism between AP-1 and the glucocorticoid receptor at their overlapping binding site. J Biol Chem. 1991 May 5;266(13):8248–8254. [PubMed] [Google Scholar]
- van de Zande L., Labruyère W. T., Arnberg A. C., Wilson R. H., van den Bogaert A. J., Das A. T., van Oorschot D. A., Frijters C., Charles R., Moorman A. F. Isolation and characterization of the rat glutamine synthetase-encoding gene. Gene. 1990 Mar 15;87(2):225–232. doi: 10.1016/0378-1119(90)90306-c. [DOI] [PubMed] [Google Scholar]