Abstract
DNA damage as a result of environmental stress is recognized by sensor proteins that trigger repair mechanisms, or, if repair is unsuccessful, initiate apoptosis. Defects in DNA damage-induced apoptosis promote genomic instability and tumorigenesis. The protein ataxia-telangiectasia mutated (ATM) is activated by DNA double strand breaks and regulates apoptosis via p53. Here we show that FOXO3 interacts with the ATM-Chk2-p53 complex, augments phosphorylation of the complex and induces the formation of nuclear foci in cells upon DNA damage. FOXO3 is essential for DNA damage-induced apoptosis and conversely FOXO3 requires ATM, Chk2, and phosphorylated p53 isoforms to trigger apoptosis as a result of DNA damage. Under these conditions FOXO3 may also play a role in regulating chromatin retention of phosphorylated p53. These results suggest an essential link between FOXO3 and the ATM-Chk2-p53-mediated apoptotic program following DNA damage.
All living organisms are inexorably exposed to environmental stress that cause DNA double-strand breaks (DSBs), which pose the greatest challenge to the maintenance of genomic stability in cells. Many anti-cancer agents also induce DSBs in cancer cells that promote cellular apoptosis. DSBs are generally recognized by sensors and transducers, containing signaling kinases, which can signal repair mechanisms or, if this fails, they trigger apoptosis. One of the most important proteins is ATM, a crucial tumor suppressor, which is activated by DSBs though autophosphorylation at Serine-1981 (ATM-pS1981)1. Once activated, ATM-pS1981 phosphorylates various downstream substrates such as histone H2AX (H2AX), nibrin (Nbs1), BRCA1, cell-cycle checkpoint kinases Chk1 and Chk2, and p53 that exert vital DNA-damage functions including regulation of cell-cycle checkpoints or inducing repair of damaged DNA or promoting apoptosis through p532,3.
The p53 tumor suppressor protein plays a central role in the decision of a cell to undergo either cell-cycle arrest or apoptosis after diverse stresses, including DNA damage, hypoxia and the activation of oncogenes4–6. The amount and transcriptional activity of p53 is regulated by post-translational modification, such as phosphorylation, sumoylation, neddation and acetylation7. Under normal conditions, p53 protein levels are low owing to Hdm2 (human Mdm2)-mediated ubiquitylation and degradation through the proteasome pathway. Upon DNA damage, DSBs-activated ATM phosphorylates p53 at Serine-15 (p53-pS15)8 that may inhibit the interaction of p53 with Hdm29, resulting in p53 stabilization. In addition, ATM can activate Chk2 and homeodomain interacting protein kinase 2 (HIPK2) to phosphorylate p53 at Serine-20 (p53-pS20)10 and Serine-46 (p53-pS46)11,12, respectively, in its transactivation domain, which in turn leads to upregulation of the expression of pro-apoptotic genes such as Bax (Bcl2-associated X protein) and Puma (p53-upregulated modulator of apoptosis)13. HIPK2 plays a critical role in regulating the apoptotic program induced by DNA damage11,12,14. Notably it has been shown that mutations in Serine-15 and Serine-20 of human p53 impair its apoptotic activity15, and Serine-46 phosphorylation is crucial for p53-mediated apoptosis after DNA damage16. Thus, the regulation of ATM–p53 activation is certainly important in DNA damage-triggered apoptosis. However, the upstream signaling mechanisms that regulate p53 stabilization and activity by ATM-mediated phosphorylation are largely unclear.
Interestingly, molecular interactions between p53 and FOXO3 (or FOXO3a) have been discovered17–20. FOXO3 is a pivotal transcription factor that regulates the transcription of a number of genes important for modulating cell cycle control21, DNA damage response22,23, oxidative and nutritional stress17,24, aging and longevity25–27, cellular apoptosis28–30, and suppression of cancer31–33 in animal and human cells. Gene knockout findings support FOXOs’ essential functions in tumor suppression34 and the maintenance of the hematopoietic stem cell pool35. While multiple mechanisms have been shown to regulate FOXO3 activity, phosphorylation inhibits FOXO3 nuclear translocation that is essential for its regulation and function. This nuclear exclusion and translocation of FOXO3 into the cytoplasm inhibits FOXO3-dependent transcription. Loss of function of FOXO3 through phosphorylation has been linked to tumorigenesis and poor patient survival in breast cancer31,32, suggesting that FOXO3 is a key tumor suppressor.
Notably FOXO3 and p53 share a number of similarities in their functions and regulations. Not only do they interact with each other but they regulate each other’s activity or stability. For instance, it has been reported that activation of p53 can suppress FOXO3 transcriptional activity through serum/glucocorticoid-inducible kinase 1 (SGK1)36 or induce FOXO3 degradation through Mdm237. Under oxidative stress, p53 can inhibit FOXO3 transcriptional activity38. Conversely it has been shown that activated FOXO3 can stabilize p53 and promote p53-dependent apoptosis18. However, during DNA damage-induced apoptosis, the regulation of functional interaction between FOXO3 and p53 to coordinate with crucial DNA damage response proteins such as ATM and Chk2 remains largely unknown.
Although several important proteins responsible for activation of the p53-mediated apoptosis have been identified, the mechanism by which ATM activates p53 apoptotic signaling has remained elusive. Our recent findings suggest that FOXO3 is essential for activation of the ATM-BRCA1-Rad17 complex that signals the ATM-mediated repair mechanism at early stages of DNA damage23. In addition, it has been found that FOXO3 is necessary for the regulation of normal ATM expression to maintain homeostasis of hematopoietic stem cells39. The fact that prolonged activation of FOXO3 by anti-cancer agents can trigger apoptosis29,30 suggests that FOXO3 may be involved in regulating the ATM-mediated p53 apoptotic signaling pathway at late stages of DNA damage. Because camptothecin (CPT), a topoisomerase-1 inhibitor, can induce DNA damage and activate the ATM-Chk2 apoptotic pathway in cancer cells40,41, we set out to determine whether FOXO3 plays a key role in linking ATM and Chk2 to the p53-mediated apoptosis after CPT-induced DNA damage, and if so, how the ATM-p53 apoptotic machinery is regulated.
Here we show that FOXO3 is functionally associated with the ATM-Chk2-p53 complex, and regulates phosphorylation and the formation of ATM-Chk2-p53 complexes at sites of DNA damage that induce apoptosis. Collectively, we suggest a novel network between FOXO3 and the ATM-Chk2-p53-mediated apoptotic program after DNA damage.
RESULTS
FOXO3 interacts with the ATM-Chk2-p53 complex
Because p53-pS15 is phosphorylated directly by ATM-pS1981 and our and other recent studies suggest that FOXO3 may be necessary for the regulation of ATM activation upon DNA damage23,39, we investigated whether FOXO3 has an essential role in the regulation of the ATM-mediated Chk2-p53 apoptotic signaling pathway after DNA damage induced by CPT.
To assess the effect of CPT on endogenous FOXO3 expression and the levels of p53-pS15/pS20/pS46, ATM-pS1981, Chk2-pT68, and phosphorylated H2AX (γ-H2AX), we treated MCF-7 cells with CPT or control (DMSO) and analyzed the levels of these proteins in cell lysates by immunoblotting (IB). We found that the levels of FOXO3, p53-pS15/pS20/pS46, ATM-pS1981, Chk2-pT68, and γ-H2AX were increased significantly 1–4 hours post treatment with CPT, while total expressions of Chk2, ATM, and H2AX remained unchanged and the control vehicle did not alter the levels of the tested proteins (Supplementary Fig. S1). In addition, other DNA damaging agents such as epotoside (VP16), ionizing radiation (IR), and ultraviolet showed similar results to those of CPT (Supplementary Fig. S1).
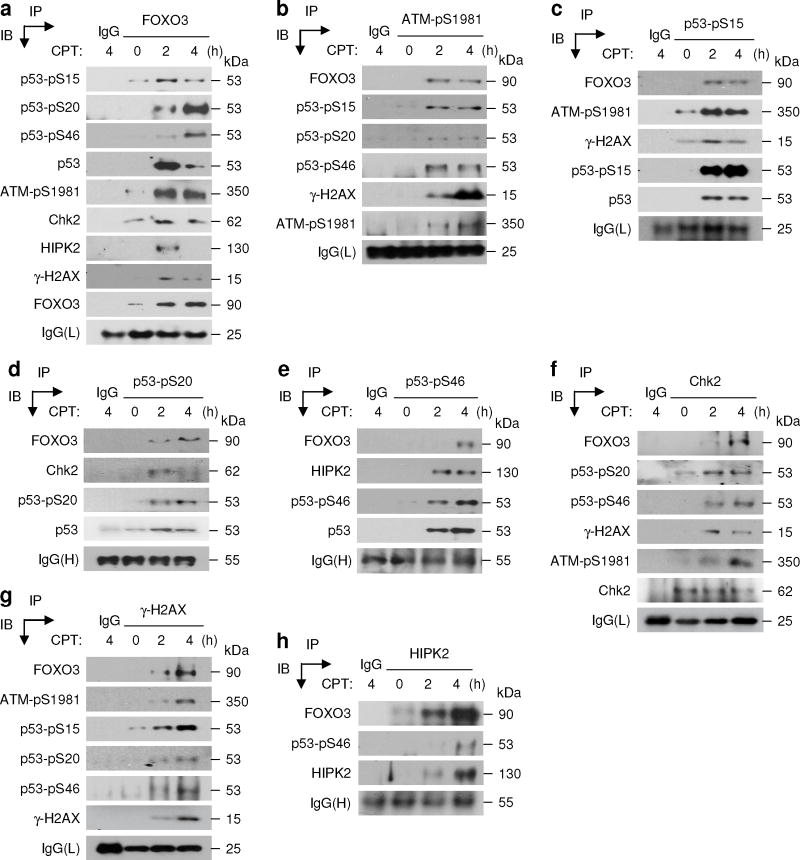
To validate the interaction between FOXO3 and Chk2 or p53 after DNA damage, we compared the levels of FOXO3, Chk2, and p53 using co-immunoprecipitation (co-IP) and IB assays with cell lysates obtained from MCF-7 cells treated for 2–4 hours with CPT. We found that FOXO3 was associated with not only Chk2 and p53 but also p53-pS15 specifically, a direct phosphorylation target of activated ATM, suggesting that FOXO3 may interact with ATM-pS1981 also. Indeed, we showed that FOXO3 was physically associated with p53-pS15, p53-pS20, p53-pS46 (p53-pS15/pS20/pS46), ATM-pS1981, Chk2, HIPK2, and γ-H2AX in cells treated with CPT (Fig. 1). In addition, the expression of HIPK2 was induced after CPT treatment. Collectively these results suggest that FOXO3 may have a role in regulating the ATM-Chk2-p53 pathway after critical DNA damage.
Fig. 1. FOXO3 interacts with the ATM-Chk2-p53-H2AX complex.
(a) Whole cell lysates of MCF-7 treated with CPT (1 μM) for 2 or 4 hours (h) or DMSO (0, control) were subjected to immunoprecipitation (IP) with an anti-FOXO3 antibody (Ab) or an isotype IgG (control) followed by immunoblotting (IB) with Abs as indicated, or an anti-FOXO3 (IP control) or a control Ab against IgG light chain (L). (b–h) Similarly, cell lysates were subjected to IP with an Ab against ATM-pS1981 (b) or p53-pS15 (c) or p53-pS20 (d) or p53-pS46 (e) or Chk2 (f) or γ-H2AX (g) or HIPK2 (h) or an isotype IgG followed by IB with the indicated Abs or a control Ab against IgG(L) or heavy chain (H).
To confirm the sub-cellular locations of FOXO3, p53-pS15/pS20/pS46, ATM-pS1981, Chk2-pT68, and γ-H2AX in the CPT-treated cells, we performed IB analysis with cytoplamic and nuclear extracts from cells treated with CPT and showed that the levels of FOXO3, p53-pS15/pS20/pS46, ATM-pS1981, and γ-H2AX were elevated in the nucleus (Supplementary Fig. S2). These results suggest that DNA damaging agents such as CPT can substantially induce FOXO3 expression and promote ATM and p53 activation in the nucleus concurrently.
FOXO3 is colocalized with ATM-Chk2-p53-H2AX nuclear foci
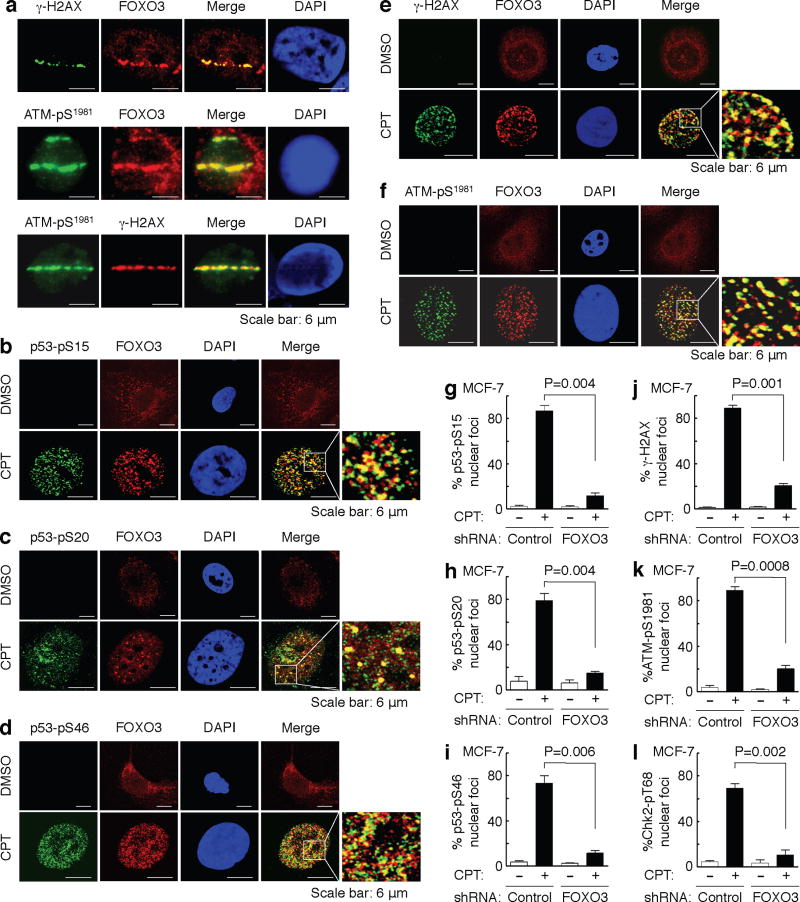
An important question in this study is the cellular location of DNA damage-induced FOXO3. In light of the significance of DNA damage-induced FOXO3 interaction with p53-pS15/pS20/pS46, γ-H2AX, ATM-pS1981, and Chk2-pT68, it was plausible to examine whether FOXO3 was recruited to the damaged DNA sites after DNA damage. Thus, we induced spatially localized DSBs in MCF-7 cells by using focused laser micro-irradiation as described previously42,43. This technique allowed us to detect the recruitment of a unique fraction of FOXO3 and γ-H2AX and/or ATM-pS1981 to the laser-induced DNA damage tracks (Fig. 2a). In addition, to determine the physical associations between FOXO3 and p53-pS15/pS20/pS46 or γ-H2AX or ATM-pS1981 or Chk2-pT68 at the sites of DNA lesions, we assessed whether FOXO3 localized to the potential sites of DSBs by co-localization with p53-pS15/pS20/pS46 or γ-H2AX or ATM-pS1981 or Chk2-pT68 following CPT-induced DNA damage. MCF-7 cells were treated with CPT or control and stained with specific antibodies. We found that approximately 70–80% of FOXO3 located in the nucleus and co-localized with p53-pS15/pS20/pS46, ATM-pS1981, γ-H2AX, and Chk2-pT68 to form nuclear foci after exposure to a low dose of CPT for 2 hours, while FOXO3 primarily resided in the cytoplasm of cells treated with control (Fig. 2b–f and Supplementary Fig. S3). To generalize these results, we performed the same experiments in another p53 wild-type (WT) cell line A549 and showed that FOXO3 co-localized with p53-pS15/pS20/pS46, ATM-pS1981, γ-H2AX, and Chk2-pT68 to form nuclear foci in A549 cells after CPT treatment (Supplementary Fig. S3 and S4), suggesting that FOXO3 may co-localize with p53-pS15/pS20/pS46, ATM-pS1981, γ-H2AX, and Chk2-pT68 to sites of DNA breaks in response to DNA damage.
Fig. 2. FOXO3 is essential for the formation of ATM-Chk2-p53-H2AX nuclear foci.
(a) Accumulation of a fraction of FOXO3 at laser-induced damage was detected in MCF-7 cells 30 min after focused laser micro-irradiation. The cells were stained with Abs against FOXO3 and γ-H2AX or ATM-pS1981, followed by fluorescence microscopy as described in the section of Immunofluorescence under “Methods”. DAPI was used to show the nuclei, and co-localizations of FOXO3 with ATM-pS1981 were shown as the merged images. Scale bar: 6 μm. (b–f) MCF-7 cells were treated with CPT (1 μM) or DMSO control for 2 h, and co-localizations between FOXO3 and p53-pS15 (b) or p53-pS20 (c) or p53-pS46 (d) or γ-H2AX (e) or ATM-pS1981 (f) were detected using Abs as indicated and followed by fluorescence microscopy. Scale bar: 6 μm. (g–l) FOXO3 is necessary for the formation of p53-pS15, p53-pS20, and p53-pS46, γ-H2AX, ATM-pS1981, and Chk2-pT68 nuclear foci upon CPT-induced DNA damage. MCF-7 stable cell lines transfected with FOXO3-shRNA or control-shRNA were treated with CPT (1 μM) or DMSO for 2 h, and then the subcellular localizations and co-localization of FOXO3 and p53-pS15 (g) or p53-pS20 (h) or p53-pS46 (i) or γ-H2AX (j) or ATM-pS1981 (k) or Chk2-pT68 (l) were detected using specific Abs as indicated. An average of 200 cells with specific nuclear foci in each comparison was determined and shown. The images of nuclear foci of these proteins were shown in Supplementary Fig. S5. The error bars represent standard deviation, and the statistical test is the paired t-test.
FOXO3 is essential for forming ATM-Chk2-p53 nuclear foci
While nuclear foci of p53-pS15, p53-pS20, p53-pS46, γ-H2AX, ATM-pS1981, and Chk2-pT68 were formed significantly (around 65–75%) in the presence of FOXO3 in MCF-7 stable cell line transfected with control-shRNA after exposure to a low dose (1 μM) of CPT for 2 hours, the formations of p53-pS15, p53-pS20, p53-pS46, γ-H2AX, ATM-pS1981, and Chk2-pT68 nuclear foci were greatly reduced in MCF-7 stable cell line transfected with FOXO3-shRNA following the same CPT treatment (Fig. 2g–l and Supplementary Fig. S5). To show the generality of this effect, we performed the same experiments in A549 cells by treating A549 (control-shRNA) and A549 (FOXO3-shRNA) cells with CPT and showed that knockdown of FOXO3 expression in A549 cells abrogated the formations of p53-pS15, p53-pS20, p53-pS46, γ-H2AX, ATM-pS1981, and Chk2-pT68 nuclear foci in A549 cells following CPT treatment (Supplementary Fig. S6 and S7). These results suggest that FOXO3 may be crucial for the formation of p53-pS15, p53-pS20, p53-pS46, γ-H2AX, ATM-pS1981, and Chk2-pT68 nuclear foci after DNA damage induced by CPT.
FOXO3 is required for phosphorylation of ATM-Chk2-p53-H2AX
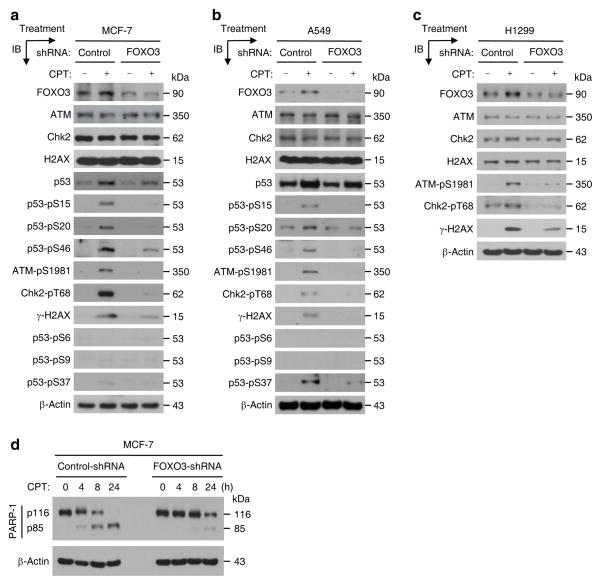
Next, we determined whether silencing of FOXO3 alters the expression or phosphorylation of ATM, Chk2, p53, and H2AX following DNA damage by biochemical approaches. We treated MCF-7 (control-shRNA) and MCF-7 (FOXO3-shRNA) cells with a low dose of CPT for four hours and performed IB analysis. Interestingly, silencing of FOXO3 did not change the expression of ATM, Chk2, H2AX, and p53 total proteins but, significantly, did reduce the levels of phosphorylation (or activation) of ATM, Chk2, p53, and H2AX (Fig. 3a). To demonstrate the generality of this finding, we performed the same experiments in A549 cells by treating A549 (control-shRNA) and A549 (FOXO3-shRNA) cells with CPT and performing IB analysis. In agreement with our results in MCF-7 cells, silencing of FOXO3 expression in A549 cells significantly reduced phosphorylation of ATM, Chk2, p53, and H2AX in A549 cells after CPT treatment (Fig. 3b), suggesting that indeed FOXO3 is required for ATM-Chk2-p53-H2AX activation in A549 cells upon DNA damage. In addition, we have carried out similar experiments in a p53-deficient cell line H1299 by treating H1299 (control-shRNA) and H1299 (FOXO3-shRNA) cells with CPT as described above. We showed that knockdown of FOXO3 expression in H1299 cells diminished phosphorylation of ATM, Chk2, and H2AX in H1299 cells after CPT treatment (Fig. 3c), suggesting that FOXO3 is necessary for ATM-Chk2-H2AX activation in H1299 cells following DNA damage. Collectively, these results suggest that FOXO3 is essential for phosphorylation or activation of ATM, Chk2, p53, and H2AX when DNA damage occurs.
Fig. 3. FOXO3 is required for ATM-Chk2-p53 phosphorylation and PARP-1 cleavage.
(a) MCF-7 stable cell lines transfected with FOXO3-shRNA or control-shRNA were treated with CPT (1 μM) or DMSO (negative control) for 4 h, and whole cell lysates were prepared and the levels of proteins were analyzed by IB with specific Abs as highlighted or an anti-β-actin (loading control). (b, c) A549 (b) and H1299 (c) stable cell lines transfected with FOXO3-shRNA or control-shRNA were treated with CPT (1 μM) or DMSO (negative control) for 4 h, and whole cell lysates were analyzed by IB with specific Abs as indicated or an anti-β-actin. (d) Cell lysates from MCF-7 stable cell lines (FOXO3-shRNA and control-shRNA) treated with CPT (1 μM) for an indicated time course were subjected to IB analysis using Abs against PARP-1 and β-actin (loading control).
FOXO3 is necessary for inducing DNA damage-induced apoptosis
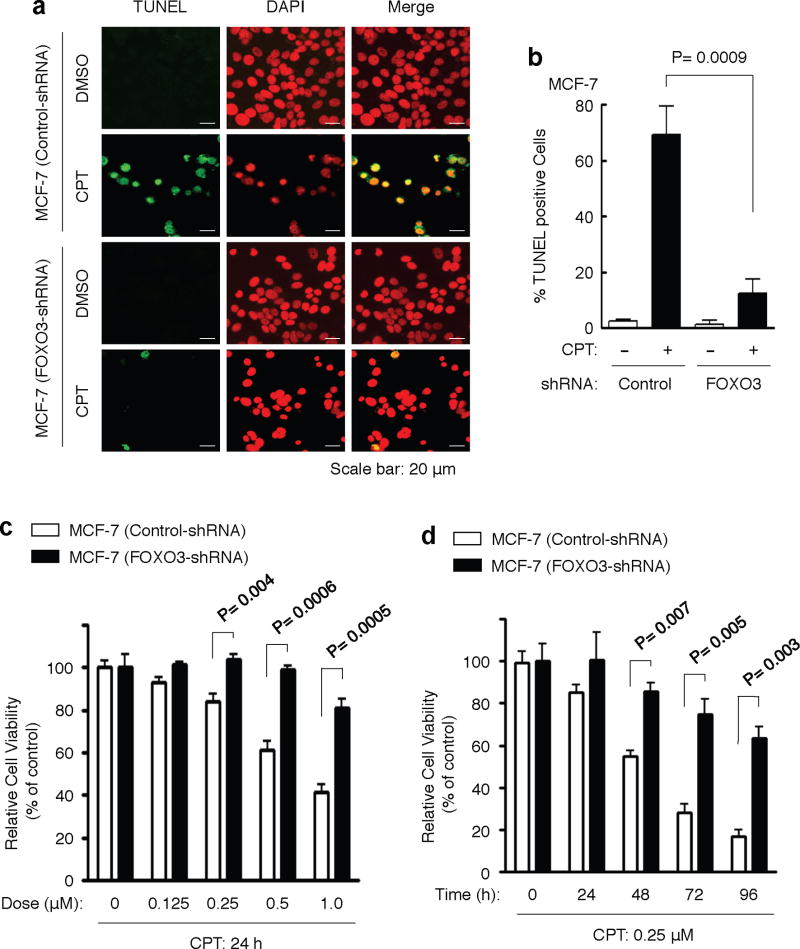
To examine the role of FOXO3 in DNA-damage induced apoptosis, we treated MCF-7 cells with a low dose of CPT and analyzed apoptosis by the standard poly-ADP-ribose polymerase (PARP) degradation, an indicator of apoptosis44, and TUNEL apoptosis assays. We showed that CPT treatment induced significant PARP-1 degradation and TUNEL positive cells approximately 8 hours post drug treatment, respectively (Fig. 3d). Furthermore, we showed that silencing of FOXO3 expression in these cells significantly attenuated this CPT-induced apoptosis (Fig. 3d and Fig. 4a, b), suggesting that FOXO3 may be necessary for promoting apoptosis after DNA damage. To determine whether FOXO3 knockdown also contributes to survival, we treated MCF-7 (control-shRNA) and MCF-7 (FOXO3-shRNA) cells with various doses of CPT or DMSO (control) for 24 hours or with 0.25 μM CPT for a time course, and performed cell survival assays by cell counting or the MTT assays. We found that FOXO3 knockdown in these cells significantly promoted survival after CPT treatment (Fig. 4c, d). To generalize these results, we compared the survival rates in A549 (control-shRNA) versus A549 (FOXO3-shRNA) cells and H1299 (control-shRNA) versus H1299 (FOXO3-shRNA) cells following CPT treatment. We showed that silencing of FOXO3 in these cells significantly enhanced survival after CPT treatment (Supplementary Fig. S8). Taken together, these results suggest that FOXO3 is essential for regulating survival and apoptosis when DNA damage occurred.
Fig. 4. FOXO3 is necessary for inducing DNA damage-induced apoptosis.
(a) MCF-7 stable cell lines (FOXO3-shRNA and control-shRNA) were treated with CPT (1 μM) or DMSO (control) for 48 h, and the cells were fixed on the slides for determining cellular apoptosis by TUNEL assays (Promega). Nuclei were stained with DAPI (color-inverted to red), and merged images (yellow) were considered as apoptotic cells. Scale bar: 20 μm. (b) An average (%) of apoptotic (TUNEL-positive) cells was determined and shown in the diagram; The samples include three biological replicates, the error bars represent standard deviation, and the statistical test is the paired t-test. The significant P values between the FOXO3-shRNA group versus the control group treated with CPT are highlighted. (c, d) MCF-7 (control-shRNA) and MCF-7 (FOXO3-shRNA) cells were treated with various doses of CPT or DMSO (control) for 24 hours (c) or with a low dose CPT (0.25 μM) for a time course as indicated (d), and performed cell survival assays by the MTT assays (c) or cell counting (d). The significant P values between the FOXO3-shRNA group versus the control group treated with CPT are indicated. The number of biological replicates is three, the error bars represent standard deviation, and the statistical test is the paired t-test.
FOXO3 may need ATM-Chk2-p53 activation to lead to apoptosis
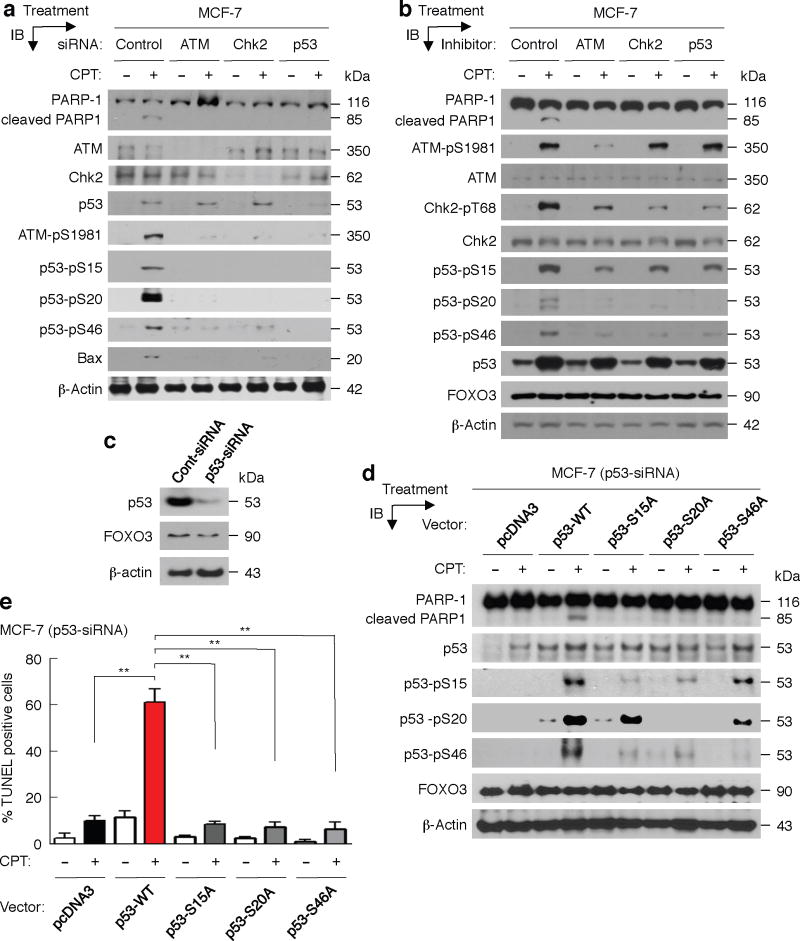
Further, to determine the roles of phosphorylated ATM-Chk2-p53 in the regulation of FOXO3-mediated apoptosis following DNA damage induced by CPT, we transfected MCF-7 cells with control-siRNA or siRNA targeting ATM or Chk2 or p53, and treated these cells with a low dose of CPT. Markedly, silencing the expression of ATM, Chk2, or p53 with specific siRNAs in MCF-7 cells abolished CPT-induced PARP-1 degradation (Fig. 5a), suggesting that ATM-Chk2-p53 proteins are necessary for the FOXO3-mediated apoptosis upon CPT-induced DNA damage. In addition, we treated MCF-7 cells with specific inhibitors of ATM, Chk2, and p53 or control for 6 hours prior to exposure to a low dose of CPT or control, and we showed that inhibiting the activity of ATM, Chk2, or p53 significantly diminished the levels of ATM-pS1981, Chk2-pT68, or p53 phosphorylated isoforms (p53-pS15/pS20/pS46) and abrogated CPT-induced PARP-1 degradation (Fig. 5b). As controls, we showed that these inhibitor treatments did not alter FOXO3 expression significantly; thus, the lack of PARP-1 degradation could not be attributed to attenuation of FOXO3 expression in these cells. These results suggest that the activities of ATM-pS1981, Chk2-pT68, and p53 phosphorylated isoforms (p53-pS15/pS20/pS46) may be essential for FOXO3-mediated apoptosis.
Fig. 5. Phosphorylated ATM-Chk2-p53 may be needed for FOXO3-mediated apoptosis.
(a) MCF-7 cells were transfected with control-siRNA (control) or siRNA (0.3 μM) targeting ATM or Chk2 or p53 and incubated for 48 hours (h), and then these cells were treated with CPT (1 μM) (+) or DMSO (−) for 48 h. Whole cell lysates were prepared from these treated cells and the levels of PARP-1 and its degraded proteins, ATM, Chk2, p53, and their phosphorylated proteins or a p53 target (Bax) were analyzed by IB with specific Abs as indicated or an anti-β-actin (loading control). (b) MCF-7 cells were first treated with ATM inhibitor (Mirin) or Chk2 inhibitor (NSC10955) or p53 inhibitor (Pifithrin), 20 μM/each, or DMSO for 6 h, and then treated with CPT (1 μM) or DMSO for 16 h. Whole cell lysates were prepared from these treated cells and the levels of PARP-1 and its degraded proteins, and the indicated proteins and β-actin were analyzed by IB analysis with specific Abs. (c) MCF-7 cells were transfected with p53-siRNA or Cont-siRNA (Control-siRNA) for 48 h. Whole cell lysates were prepared from these transfected cells and the expressions of p53 and FOXO3 (control) were determined by IB analysis with specific Abs. (d) MCF-7 (p53-siRNA) cells were transfected with pcDNA3 (control) or p53 wild-type (WT) or mutant (p53-S15A or p53-S20A or p53-46A) expression vectors (2 μg DNA/each) for 48 h, and then treated with CPT (1 μM) (+) or DMSO (−) for 48 h. Whole cell lysates were prepared from these treated cells and the levels of PARP-1 and its degraded proteins, and the indicated proteins were analyzed by IB analysis with specific Abs. (e) MCF-7 (p53-siRNA) cells were transfected with control and expression vectors for 24 h, the transfected cells were treated with CPT (1 μM) or DMSO control for 36 h, and cellular apoptosis was determined by TUNEL assays (Promega). Images of apoptotic cells were shown in Supplementary Fig. S9. An average (%) of TUNEL-positive (apoptotic) cells was determined and shown in the diagram. **, P= 0.0001.
Next, we transfected the MCF-7 p53-knockdown, MCF-7 (p53-siRNA) (Fig. 5c), cells with pcDNA3 (control) or p53-WT or mutant (p53-S15A or p53-S20A or p53-46A) expression vectors for 48 hours, and then treated them with a low dose (1 μM) of CPT or control for 48 hours. Interestingly, over-expression of p53-WT promoted CPT-induced PARP-1 degradation and apoptosis, whereas over-expression of p53-S15A or p53-S20A or p53-S46A mutant failed to induce PARP-1 degradation and TUNEL apoptosis after CPT treatment (Fig. 5d, e and Supplementary Fig. S9). As controls, we showed that over-expression of these p53 mutants did not significantly change FOXO3 expression, suggesting that the observed negative effect on PARP-1 degradation or TUNEL apoptosis cannot be ascribed to debilitation of FOXO3 in these cells. In addition, we repeated the same experiments in another p53-null cell line, H1299, and obtained the same reproducible results (Supplementary Fig. S10), confirming that these p53 phosphorylated isoforms (p53-pS15/pS20/pS46) are necessary for FOXO3-mediated apoptosis following DNA damage induced by CPT. Collectively, these results suggest that FOXO3 may require ATM-Chk2-p53 phosphorylated isoforms to lead to apoptosis after DNA damage.
FOXO3 regulates chromatin retention of phosphorylated p53
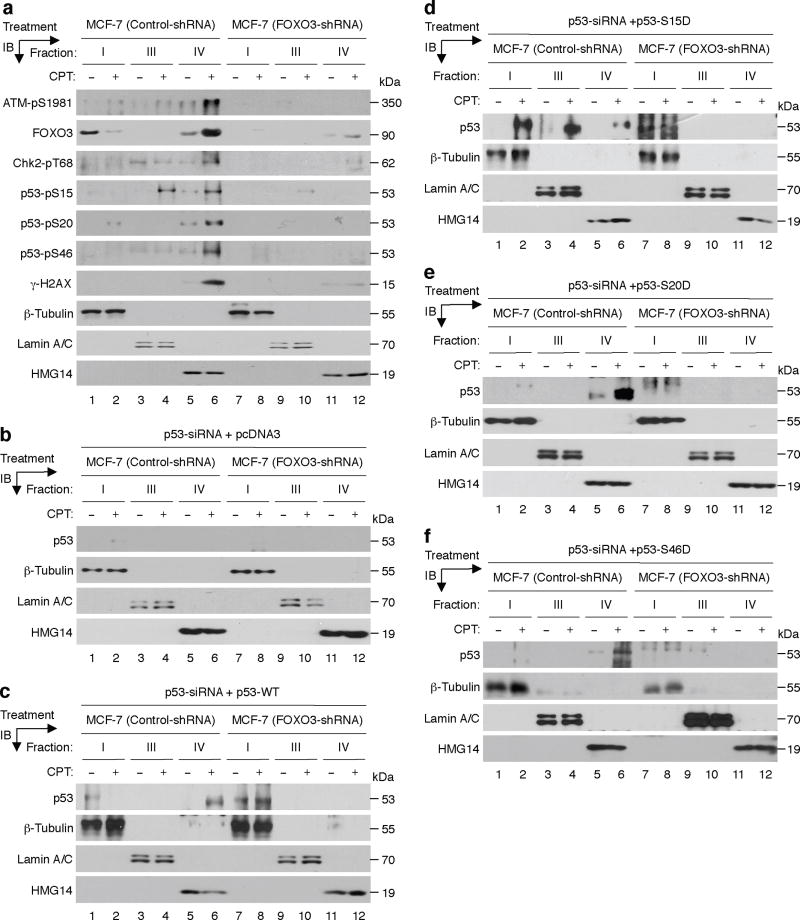
An important question in mechanism is how FOXO3 contributes to the activation of ATM-pS1981, Chk2-pT68, and p53-pS15/pS20/pS46 in cells upon DNA damage. Hence we sought to determine whether FOXO3 is required for binding and retention of ATM-pS1981, Chk2-pT68, and p53-pS15/pS20/pS46 proteins to the chromatin following DNA damage. We treated MCF-7 (control-shRNA) and MCF-7 (FOXO3-shRNA) cells with a low dose of CPT for four hours and performed chromatin retention assays. Remarkably, silencing of FOXO3 expression in MCF-7 cells significantly diminished chromatin retention of ATM-pS1981, Chk2-pT68, p53-pS15/pS20/pS46, and γ-H2AX (Fig. 6a), suggesting that FOXO3 may play a role in regulating chromatin retention of phosphorylated ATM-Chk2-p53-H2AX.
Fig. 6. FOXO3 plays a role in regulating chromatin retention of phosphorylated p53.
(a) MCF-7 stable cell lines transfected with FOXO3-shRNA or control-shRNA were treated with CPT (1 μM) (+) or DMSO (−) (control) for 4 h, and cells were harvested and fractionated with Nonidet P-40 as described in the section of Chromatin Retention Assay under “Methods”. Equal amount (20 μg) of each fraction was analyzed by IB analysis with the highlighted Abs as described above. Proteins β-tubulin, lamin A/C, and HMG14 (high-mobility-group 14, a chromosome binding protein) represent the fractionation and loading controls of the cytosol (fraction I), the nucleoplasm (fraction III) and the chromatin (fraction IV), respectively. (b–f) MCF-7 (control-shRNA) and MCF-7 (FOXO3-shRNA) cells were transfected with p53 siRNA for 48 h, then transfected with pcDNA3 (negative control) (b) or the p53-WT vector (c) or the specific vectors expressing p53-S15D (d), p53-S20D (e), and p53-S46D (f) for 36 h. The transfected cells were treated with CPT (1 μM) or DMSO for 4 h, then cells were harvested and subjected to chromatin fractionation and IB analysis as described above.
To demonstrate the important role of FOXO3 in regulating chromatin retention of the activated p53 proteins in a phosphorylation-independent manner, we silenced endogenous p53 in MCF-7 (control-shRNA) and MCF-7 (FOXO3-shRNA) cells with p53 siRNA as described above. We then transfected these p53-knockdown cells with pcDNA3 (negative control) or the p53-WT vector or the specific vectors expressing p53-S15D, p53-S20D, and p53-S46D, which are the constitutively activated mutant p53 proteins with the specific Serine (S) residue being mutated to Aspartic acid (D) that mimics the phosphorylated-S residue45–47, for 36 hours. We treated these transfected cells with a low dose of CPT for four hours, then harvested cells and performed chromatin fractionation as described above. Remarkably, silencing FOXO3 significantly diminished chromatin retention of these p53-S15D, p53-S20D, and p53-S46D proteins (Fig. 6d-f), suggesting that FOXO3 may play a necessary role in chromatin retention of the activated p53 proteins independent of their phosphorylation.
DISCUSSION
ATM is a key DNA damage sensor that plays a central role in controlling the DNA damage response. A great deal of information has been obtained on the DNA-damage sensor mechanisms detecting DSBs in recent years; however, it is still unclear how specific DNA lesions are recognized and how signaling is delivered downstream to the apoptotic machinery1–3. It also remains largely unknown when the sensor proteins should signal repair mechanisms to repair damaged DNA and when they would trigger apoptosis. Although activation of the p53-mediated apoptosis plays a critical role in tumor suppression, the control of activation of ATM-Chk2-p53 apoptotic signaling is unclear4–12. In this study, we show a critical role of FOXO3 in regulating the formation of ATM-pS1981-Chk2-p53-pS15/pS20/pS46 complexes at sites of DNA breaks, and its role in promoting the ATM-Chk2-p53 apoptotic-signaling pathway after DNA damage. Our findings suggest that FOXO3 may be important for regulating chromatin retention of these phosphorylated (and/or activated) ATM-Chk2-p53 proteins in the nucleus when DNA damage occurs. While it is possible that the general abundance of the proteins examined may regulate the levels of protein-protein interaction and chromatin retention observed here, our data show specific interactions between FOXO3 and p53-pS15 or p53 and the extent of these interactions in co-IP is significantly lower at 4 hours than at 2 hours after CPT treatment (Fig. 1a, c), whereas the levels of FOXO3 and p53-pS15 are considerably higher at 4 hours than at 2 hours post CPT treatment (Supplementary Fig. S1a). Taken together, these results clearly indicate that the formation of specific FOXO3/p53-pS15, FOXO3/p53, FOXO3-Chk2, and other complexes (as shown by co-IP) is not solely dependent on the abundance of these proteins examined.
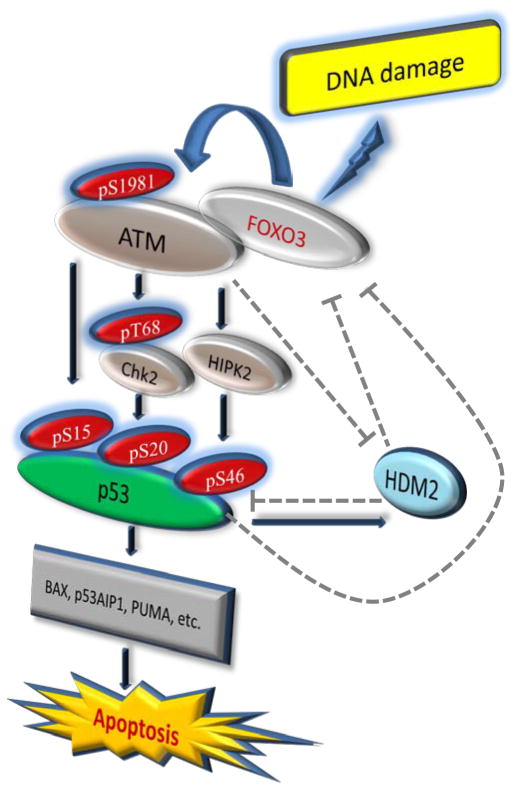
Interestingly, it has been shown that p53 activation can downregulate FOXO3 through induction of SGK1 or Hdm236,37, which in turn promotes ubiquitin-dependent degradation of p5348. These intriguing findings suggest the possible roles of p53 and Hdm2 in downregulation of FOXO3 as negative feedback loops. On the other hand, it has been reported that activation of ATM can enhance p53 stabilization by direct phosphorylation of Hdm249,50 and inhibition of its RING domain oligomerization and E3 ligase51. Collectively, we have proposed a schematic representation of the FOXO3-dependent phosphorylation or activation of ATM-pS1981/p53-pS15, Chk2-pT68/p53-pS20, and HIPK2/p53-pS46 together with their negative feedback loops and the downstream p53 apoptotic-signaling pathways after DNA damage (Fig. 7).
Fig. 7. A link between FOXO3 and the ATM-Chk2-p53-mediated apoptotic program.
A schematic shows the FOXO3-dependent activation of ATM-pS1981, Chk2-pT68, HIPK2, p53-pS15, p53-pS20, p53-pS46, and the possible roles of p53 and Hdm2 in downregulation of FOXO3 as negative feedback loops, and the downstream p53 apoptotic-signaling pathway after DNA damage induced by CPT. The dashed lines denote the inhibitory pathways according to the published literature.
At the early stages of DNA damage with low levels of DSBs, it is possible that only a minor fraction of p53 is activated that is sufficient to drive the transcription of the p21Cip1 gene, resulting in cell-cycle arrest. However, with high levels of DSBs, p53 is activated and accumulated above a particular threshold that upregulates the expression of pro-apoptotic genes such as Bax, Puma, and p53AIP1 (p53-regulated apoptosis-inducing protein 1)13,52. While CPT can significantly upregulate the expression of these p53 downstream target genes in the presence of FOXO3, we have found that the expression levels of Bax, Puma, and p53AIP1 were significantly reduced in the FOXO3-knockdown MCF-7 cells after CPT treatment (data not shown), suggesting that CPT induces these pro-apoptotic proteins in a FOXO3-dependent manner. It has been shown that phosphorylation of p53-pS46 is activated by HIPK2 kinase. Although HIPK2 has been found to be a downstream target of ATM12, it is unclear how DNA damage induces the ATM-HIPK2-p53-pS46 signaling pathway. In this study, we show that CPT-induced DNA damage can induce the FOXO3-dependent phosphorylation of ATM-pS1981 and p53-pS46 and the interactions of FOXO3-HIPK2-p53-pS46, suggesting that FOXO3 may play a critical role in activation of the ATM-HIPK2-p53-pS46 signaling pathway and provide an essential link between ATM-pS1981 and p53-pS15/pS20/pS46 simultaneously upon DNA damage. In addition, we show that FOXO3 is necessary for inducing DNA damage-induced apoptosis. Taken together, these results suggest that FOXO3 plays an essential role in the regulation of ATM-p53-mediated apoptosis after DNA damage.
Moreover, we have found that ATM-pS1981, Chk2-pT68, and p53 phosphorylated isoforms (p53-pS15/pS20/pS46) are essential for FOXO3-mediated apoptosis following DNA damage. To the best of our knowledge, this is the first evidence that FOXO3 requires phosphorylation (or activation) of p53 and its upstream kinases ATM and Chk2 together to lead to apoptosis after DNA damage. Our results are consistent with a recent finding that p53 is necessary for FOXO3 transcriptional upregulation and transactivation in vivo33. Because there is no increase inf FOXO3 levels in MCF-7 cells after CPT treatment for 16 hours (Fig. 5b) and 48 hours (Fig. 5d), we performed IB analysis of FOXO3 and ATM-Chk2-p53 proteins with full time-course of CPT treatment (0–48 hours) in MCF-7 cells, and showed that the amount of FOXO3 protein remains at a similar level between 0 and 16 hours and between 0 and 48 hours after CPT treatment (Supplementary Fig. S11a), indicating that these results do not contradict the FOXO3 increase shown in Supplementary Fig. S1a and Fig. 1a. Together, our results suggest that FOXO3 cooperates with the ATM-Chk2-p53 apoptotic program concurrently, leading to a high degree of activation of the p53-mediated apoptotic pathways following DNA damage.
Although it is known that FOXO3 is physically associated with p5317–20, the molecular mechanisms underlying FOXO3-mediated regulation of the p53-induced apoptotic pathway upon DNA damage and vice versa are largely unknown. Our findings suggest that FOXO3 is required for phosphorylation of p53 isoforms (p53-pS15/pS20/pS46). To further elucidate the mechanism by which FOXO3 regulates p53 activation in the DNA damage response, using the chromatin retention assays, we show that silencing of FOXO3 expression in MCF-7 cells significantly diminishes chromatin retention of p53-pS15/pS20/pS46 upon DNA damage (Fig. 6). Moreover, FOXO3 is triggered to migrate markedly to the chromatin from the cytoplasm in parallel with phosphorylated ATM-Chk2-p53 following DNA damage (Fig. 6a), suggesting that FOXO3 may cooperate with phosphorylated ATM-Chk2-p53 and migrate to the DSB sites on the chromatin concurrently after DNA damage. To determine if FOXO3 is still localized to the chromatin at late time treatment with CPT, we performed the same experiments of Fig. 6a at 24 hours after CPT treatment and showed that the localization of FOXO3 to the chromatin is significantly reduced after 24 hours CPT treatment compared to 4 hours (Supplementary Fig. S11b). Collectively, these results suggest that FOXO3 contributes to the DNA damage response by controlling phosphorylation of ATM-Chk2-p53 or regulating chromatin retention of these phosphorylated proteins in the nucleus or both.
In addition to FOXO3, Mre11-Rad50-Nbs1 (MRN) and other factors can interact with ATM and regulate its kinase activity that enhances its ability to phosphorylate substrates in vitro53,54. However, the fact that ATM can be activated by IR treatment in cells lacking Nbs1 or BRCA1 but cannot be recruited to the DSB sites suggests that the MRN complex may increase the accumulation of ATM at the DSB sites on the chromatin55,56 but other factors can activate ATM activity in an MRN-independent manner. Thus, it is plausible that ATM activity may be regulated by two distinct events, one being the activation of intermolecular autophosphorylation of ATM57 by multiple factors such as FOXO3 and the MRN complex, and the other, recruitment of the activated ATM to the substrate sites. In this respect, the MRN complex induces the ATM DNA-damage response to facilitate the repair of damaged DNA at early stages of DNA damage58, whereas if the damaged DNA cannot be repaired, FOXO3 triggers a cell death program by promoting the ATM-Chk2-p53 apoptotic mechanism. This orchestrated genome maintenance program in response to DNA damage stress mediates not only timely DNA repair to avert the most harmful lesions to the integrity of the genome but an apoptotic mechanism to eliminate cells with heavily damaged genomes, thereby the development of cancerous cells can be prevented59.
METHODS
Cell culture and transfection
All cell lines were grown at 37 °C and 5% CO2 in DMEM/F12 supplemented with L-glutamine, penicilline/streptomycin and 10% fetal bovine serum. All siRNAs against ATM (sc-29761), CHK2 (sc-29271), p53 (sc-29435) and control siRNA (sc-44231) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Four HuSH 29mer shRNA constructs against human FOXO3 (NM_001455) and control HuSH shRNA cloning vector (pRS) using U6 promoter were purchased from Origene (Rockville, MD). The oligonucleotide sequences in the shRNA expression cassettes are shown in Supplementary Table S1. MCF-7 cells were transfected with a combination of four HuSH 29mer shRNA constructs concurrently or control pRS vector by liposome using GenJet™ In Vitro DNA Tranfection Reagent for MCF-7 Cell (SignaGen Laboratories, Gaithersburg, MD). After puromycin selection (1 μg/ml), the MCF-7 FOXO3-knockdown pooled stable clones [designated MCF-7 (FOXO3-shRNA)] and the vector control stable clones [designated MCF-7 (Control-shRNA)] were selected. Similarly, the A549 or H1299 FOXO3-knockdown pooled stable clones [designated A549 (FOXO3-shRNA) or H1299 (FOXO3-shRNA)] and the vector control stable clones [designated A549 (Control-shRNA) or H1299 (Control-shRNA)] were generated and selected as described above. For transfection with siRNA, MCF-7 cells were transfected with specific siRNA or control siRNA as indicated by using DharmaFECT 1 transfection reagent (Thermo Scientific, Rockford, IL) according to the manufacturer’s instructions. Whole cell lysates were prepared 48 hours after transfection as described previously31.
Antibodies and reagents
The DNA damaging agent camptothecin (CPT) was purchased from Sigma (St. Louis, MO). The drug was dissolved in dimethylsulfoxide (DMSO) and stored in aliquots at −20 °C. Antibodies specific to FOXO3 [FKHRL1, H-144 (1:1000 dilution) and N-16, 1:500 dilution], NBS1 (1:1000 dilution), PARP (1:1000 dilution), CHK2 (1:1000 dilution), p53 (DO-1 and FL-393, 1:1000 dilution), phospho-p53 (p53-pS15, p53-pS20, and p53-pS46, 1:1000 dilution), Lamin A/C (1:2000 dilution), and β-Tubulin (1:2000 dilution) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against H2AX (1:2000 dilution), phospho-H2AX Serine-139 (γ-H2AX, 1:1000 dilution), and phospho-ATM Serine-1981 (ATM-pS1981, 1:1000 dilution) were purchased from Millipore (Billerica, MA). Antibodies against FOXO3 (1:1000 dilution), phospho-ATM Serine-1981 (ATM-pS1981, 1:1000 dilution), phospho-Chk2 (Chk2-pT68, 1:1000 dilution), p53 (1:1000 dilution), phospho-p53 (p53-pS6, p53-pS9, p53-pS15, p53-pS20, p53-pS37, and p53-pS46, 1:1000 dilution), p21Cip1 (1:1000 dilution), Bax (1:500 dilution), and Puma (1:500 dilution) were purchased from Cell Signaling Technology (Danvers, MA). Antibodies against ATM (BL116G, 1:500 dilution) were purchased from Bethyl Laboratories (Montgomery, TX). Antibodies against FOXO3 (3280-1, 2071-1, 1:1000 dilution) and phospho-ATM Serine-1981 (1:1000 dilution) were obtained from Epitomics (Burlingame, CA). Antibodies against p21Cip1 (1:1000 dilution) and p27Kip1 (1:1000 dilution) were purchased from Cell Signaling Technology and/or BD PharMingen (San Diego, CA). Antibody against β-Actin (1:3000 dilution) was purchased from Sigma. Antibody against HMG14 (high-mobility-group 14, 1:2000 dilution) was purchased from Abcam (Cambridge, MA). Alexa 488-, Alexa 594, and Alexa-647-conjugated secondary antibodies (1:200 dilution) were obtained from Molecular Probes (Eugene, OR). ATM inhibitor (Mirin) and Chk2 inhibitor (NSC10955) were purchased from Santa Cruz Biotechnology, and p53 inhibitor (Pifithrin) was obtained from Tocris Bioscience. 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) was obtained from Invitrogen and DMSO was purchased from Sigma.
Laser microirradiation and imaging
Live MCF-7 cell imaging combined with laser microirradiation was described previously42,43. Briefly, fluorescence data were acquired with an Axiovert 200 M microscope (Carl Zeiss MicroImaging, Thornwood, NY). A 365 nm pulsed nitrogen laser (Spectra-Physics, Mountain View, CA) was directly coupled to the epifluorescence path of the microscope. DSBs were introduced in the nucleus by microirradiation with 365 nm laser. Images were taken with an AxioCam HRm camera and fluorescence intensities of microirradiated areas were determined by using Axiovision Software, version 4.5 (Carl Zeiss). During microirradiation imaging, cells were maintained in CO2-free medium (Invitrogen) at 37 °C.
Immunofluorescence
MCF-7 and A549 cells were grown on glass coverslips. After treatment with CPT (1 μM) for 2 or 4 hours, cells were fixed with 4 % paraformaldehyde for 10 min and permeabilized with Triton X-100 (0.5 %). Slide culture chambers were washed with PBS and blocked with PBS containing 2% BSA, incubated with an Ab specific to FOXO3 or ATM-pS1981 or γ-H2AX or p53-pS15 or p53-pS20 or p53-pS46 or Chk2-pT68 (1:50 to 1:200 dilution), followed by Alexa 594 or 647 (red)-conjugated anti-rabbit, Alexa 594 (1:200 dilution) or 647 (red)-conjugated anti-goat (1:200 dilution), and Alexa 488 (green)-conjugated anti-mouse (1:200 dilution) or rabbit (1:200 dilution) secondary Abs (Molecular Probes, Eugene, OR). Cells were counterstained with DAPI (SIGMA) to show the nuclei. Specific staining was visualized and images were captured with a Leica SP2 AOBS confocal laser scanning microscope. To analyze quantitative co-localization, we used ~100 cells images randomly captured by confocal microscopy. The volume and percentage of p53-pS20 co-localized with FOXO3 were measured using the Velocity software (ver. 6.1, Improvision, PerkinElmer). To measure foci positive cells, we used ~200 cells randomly captured by confocal microscopy. The percentages of considering foci positive cells were calculated from cells containing at least 5 foci. Each error bar presented is the mean of standard deviation.
Immunoprecipitation and immunoblotting
All IP experiments were performed as described previously23. Briefly, cells were washed twice with phosphate-buffered saline (PBS) and lysed with lysis buffer containing protease inhibitors at 4 °C for 20 min. The lysates were centrifuged at 16,000g for 10 min to remove cell debris. Total protein concentration was determined as described above. Protein samples were first precleared with a nonspecific IgG antibody. Precleared lysates were then incubated with an antibody by rotating at 4 °C overnight followed by the addition of 25 μl of 50% protein A- or Protein G-sepharose slurry and rotating for 1 hour. Protein A/G beads were collected and washed with lysis buffer four times. Immunoprecipitates were resolved by 6% or 8% or 10% or 12% SDS-polyacrylamide gel eletrophoresis (PAGE) and analyzed by IB analysis. For IB analysis, the protein samples were subjected to SDS-PAGE and transferred onto nitrocellulose membranes (Boi-Rad). Membranes were blocked for 1 hour in 3% bovine serum albumin (BSA) in Tris buffered saline containing 0.1% Tween 20 (TBST) and incubated for 1 hour with primary antibody diluted in TBST containing 1% BSA. After three washes with TBST, membranes were incubated for 1 hour with horseradish peroxidase-conjugated secondary antibodies (1:3000 or 1:5000 dilution) in TBST containing 3% BSA. The immunoblots were visualized by an enhanced chemiluminescence kit obtained from Santa Cruz Biotechnology or West-Q ECL Platinum Solution obtained from GenDEPOT.
TUNEL assay
Cells were grown on glass coverslips. After treatment with CPT (1 μM) for 48 or 60 hours, cells were fixed with 4 % paraformaldehyde solution and permeabilized with Triton X-100 (0.2 %). For TUNEL assay, cellular apoptosis assay was determined by enzymatic labeling of DNA strand breaks with a terminal deoxynucleotidyl transferase dUTP nick end–labeling (TUNEL) assay kit (the DeadEnd™ Fluorometric TUNEL System, Promega) according to the manufacturer’s instructions. Nuclei were stained with DAPI (color was inverted to red). Merged images (yellow) were considered as apoptotic cells and counted under a confocal laser scanning microscope (Leica SP2 AOBS).
Chromatin Retention Assay
These experiments were performed as described previously60 with some modifications. Cells (1×107) were suspended for 5 min on ice in 150 μl of fractionation buffer (50 mM Hepes, pH 7.5, 150 mM NaCl, 1 mM EDTA) containing 0.2% Nonidet P-40 (Nonidet P-40), supplemented with protease inhibitors (5 μg/ml each pepstatin, leupeptin, and aprotonin) and phosphatase inhibitors. Following centrifugation at 1000 × g for 5 min, the supernatant was collected (fraction I), and pellets were washed with the same buffer. After the washing step, the washed sample was collected as before (fraction II), and the nuclear pellets were further extracted for 40 min on ice with 150 μl of fractionation buffer containing 0.5% Nonidet P-40. The extracts were clarified by centrifugation at 16,000 × g for 15 min (fraction III). The pellets were finally lysed in fractionation buffer II and boiled for 5 min (fraction IV). After determining concentration of all fractions, samples were separated on 8% or 10% SDS-PAGE gels and blotted onto nitrocellulose membranes (Boi-Rad). Membranes were blocked for 1 hour in 3% BSA in Tris buffered saline containing 0.1% Tween 20 (TBST) and incubated for 1 hour with primary antibody (1:500 or 1:1000) diluted in TBST containing 1% BSA. After three washes with TBST, membranes were incubated for 1 hour with secondary antibodies (1:3000) in TBST containing 3% BSA. The immunoblots were visualized by an enhanced chemiluminescence kit obtained from GenDEPOT.
Statistical analysis
All data are expressed as means and standard deviations from at least three determinations. The statistical significance of difference in nuclear co-localization of proteins examined in cells (by immunofluorescence) and the percentage of apoptotic nuclei (by TUNEL assay) between two groups was analyzed with two-sided unpaired Student’s t tests when the variances were equal with Grahpad PRISM (Ver.4.02) statistical software (San Diego, CA). All statistical tests were two-sided, and P values less than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank T. Unger, D.W. Meek, M.B. Kastan, R.A. DePinho, B. Vogelstein, and L.K. Su for providing reagents or support, and Z. Xu for technical assistance. This work was supported in part by R01 grant CA113859 (to M.C.T.H.) from the National Cancer Institute (NCI), National Institutes of Health (NIH); a grant 02-2010-063 from the Avon Foundation for Women (to M.C.T.H.), a Scientific Scholar Award from the Marsha Rivkin Center for Ovarian Cancer Research (to Y.M.C.), the Ann Schreiber Research Award from the Ovarian Cancer Research Fund (to S.H.P.), NIH grants CA50519 and CA13499 and a grant RP110465 from Cancer Research Institute of Texas (to D.J.C.). The sponsors had no role in the design, conduct, or reporting of the study.
Footnotes
Author contributions
The experiments were conceived and designed by M.C.T.H., Y.M.C. and S.H.P; experiments were performed by Y.M.C., S.H.P., W.B.T. and M.C.T.H; laser microirradiation and imaging was performed by S.Y.W. and supervised by D.J.C.; data were analyzed by Y.M.C., S.H.P. and M.C.T.H; essential resources and reagents were provided by J.S.B. and M.A.I. The paper was written by M.C.T.H. and Y.M.C.
Competing financial interests: The authors declare no competing financial interests.
References
- 1.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 2.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 3.Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3:421–429. doi: 10.1016/s1535-6108(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 4.Giaccia AJ, Kastan MB. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 1998;12:2973–2983. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]
- 5.Prives C, Hall PA. The p53 pathway. J Pathol. 1999;187:112–126. doi: 10.1002/(SICI)1096-9896(199901)187:1<112::AID-PATH250>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Vousden KH. p53: death star. Cell. 2000;103:691–694. doi: 10.1016/s0092-8674(00)00171-9. [DOI] [PubMed] [Google Scholar]
- 7.Appella E, Anderson CW. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem. 2001;268:2764–2772. doi: 10.1046/j.1432-1327.2001.02225.x. [DOI] [PubMed] [Google Scholar]
- 8.Banin S, Moyal L, Shieh S, et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 9.Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 10.Chehab NH, Malikzay A, Stavridi ES, Halazonetis TD. Phosphorylation of Ser-20 mediates stabilization of human p53 in response to DNA damage. Proc Natl Acad Sci USA. 1999;96:13777–13782. doi: 10.1073/pnas.96.24.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Orazi G, et al. Homeodomain-interacting protein kinase-2 phosphorylates p53 at Ser 46 and mediates apoptosis. Nat Cell Biol. 2002;4:11–19. doi: 10.1038/ncb714. [DOI] [PubMed] [Google Scholar]
- 12.Dauth I, Krüger J, Hofmann TG. Homeodomain-interacting protein kinase 2 is the ionizing radiation-activated p53 serine 46 kinase and is regulated by ATM. Cancer Res. 2007;67:2274–2279. doi: 10.1158/0008-5472.CAN-06-2884. [DOI] [PubMed] [Google Scholar]
- 13.Lane DP. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann TG, Möller A, Sirma H, et al. Regulation of p53 activity by its interaction with homeodomain-interacting protein kinase-2. Nature Cell Biol. 2002;4:1–10. doi: 10.1038/ncb715. [DOI] [PubMed] [Google Scholar]
- 15.Unger T, Sionov RV, Moallem E, et al. Mutations in serines 15 and 20 of human p53 impair its apoptotic activity. Oncogene. 1999;18:3205–3212. doi: 10.1038/sj.onc.1202656. [DOI] [PubMed] [Google Scholar]
- 16.Ichwan SJ, Yamada S, Sumrejkanchanakij P, et al. Defect in serine 46 phosphorylation of p53 contributes to acquisition of p53 resistance in oral squamous cell carcinoma cells. Oncogene. 2006;25:1216–1224. doi: 10.1038/sj.onc.1209158. [DOI] [PubMed] [Google Scholar]
- 17.Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306:2105–2108. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- 18.You H, Yamamoto K, Mak TW. Regulation of transactivation-independent proapoptotic activity of p53 by FOXO3a. Proc Natl Acad Sci USA. 2006;103:9051–9056. doi: 10.1073/pnas.0600889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang F, Marshall CB, Yamamoto K. Biochemical and structural characterization of an intramolecular interaction in FOXO3a and its binding with p53. J Mol Biol. 2008;384:590–603. doi: 10.1016/j.jmb.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 20.Miyaguchi Y, Tsuchiya K, Sakamoto K. P53 negatively regulates the transcriptional activity of FOXO3a under oxidative stress. Cell Biol Int. 2009;33:853–860. doi: 10.1016/j.cellbi.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez B, Martinez AC, Burgering BM, Carrera AC. Forkhead transcription factors contribute to execution of the mitotic programme in mammals. Nature. 2001;413:744–747. doi: 10.1038/35099574. [DOI] [PubMed] [Google Scholar]
- 22.Tran H, Brunet A, Grenier JM, et al. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science. 2002;296:530–534. doi: 10.1126/science.1068712. [DOI] [PubMed] [Google Scholar]
- 23.Tsai WB, Chung YM, Takahashi Y, Xu Z, Hu MC. Functional interaction between FOXO3 and ATM regulates DNA damage response. Nat Cell Biol. 2008;10:460–467. doi: 10.1038/ncb1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furukawa-Hibi Y, Kobayashi Y, Chen C, Motoyama N. FOXO transcription factors in cell-cycle regulation and the response to oxidative stress. Antioxid Redox Signal. 2005;7:752–760. doi: 10.1089/ars.2005.7.752. [DOI] [PubMed] [Google Scholar]
- 25.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 26.Willcox BJ, Donlon TA, He Q, et al. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci USA. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flachsbart F, Caliebe A, Kleindorp R, et al. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci USA. 2009;106:2700–2705. doi: 10.1073/pnas.0809594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunet A, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 29.Sunters A, et al. FOXO3 transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem. 2003;278:49795–49805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- 30.Sunters A, et al. Paclitaxel-induced nuclear translocation of FOXO3 in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res. 2006;66:212–220. doi: 10.1158/0008-5472.CAN-05-1997. [DOI] [PubMed] [Google Scholar]
- 31.Hu MC, Lee D-F, Xia W, et al. IκB kinase promotes tumourigenesis through inhibition of Forkhead FOXO3. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 32.Seoane J, Le HV, Shen L, Anderson SA, Massague J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117:211–223. doi: 10.1016/s0092-8674(04)00298-3. [DOI] [PubMed] [Google Scholar]
- 33.Renault VM, Thekkat PU, Hoang KL, et al. The pro-longevity gene FoxO3 is a direct target of the p53 tumor suppressor. Oncogene. 2011;30:3207–3021. doi: 10.1038/onc.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paik JH, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyamoto K, et al. FOXO3 is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 36.You H, Jang Y, You-Ten AI, et al. p53-dependent inhibition of FKHRL1 in response to DNA damage through protein kinase SGK1. Proc Natl Acad Sci USA. 2004;101:14057–14062. doi: 10.1073/pnas.0406286101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu W, Ma Q, Chen L, et al. MDM2 acts downstream of p53 as an E3 ligase to promote FOXO ubiquitination and degradation. J Biol Chem. 2009;284:13987–14000. doi: 10.1074/jbc.M901758200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyaguchi Y, Tsuchiya K, Sakamoto K. P53 negatively regulates the transcriptional activity of FOXO3a under oxidative stress. Cell Biol Int. 2009;33:853–860. doi: 10.1016/j.cellbi.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 39.Yalcin S, Zhang X, Luciano JP, et al. Foxo3 is essential for the regulation of ataxia telangiectasia mutated and oxidative stress-mediated homeostasis of hematopoietic stem cells. J Biol Chem. 2008;283:25692–25705. doi: 10.1074/jbc.M800517200. [DOI] [PubMed] [Google Scholar]
- 40.Takemura H, Rao VA, Sordet O, et al. Defective Mre11-dependent activation of Chk2 by ataxia telangiectasia mutated in colorectal carcinoma cells in response to replication-dependent DNA double strand breaks. J Biol Chem. 2006;281:30814–30823. doi: 10.1074/jbc.M603747200. [DOI] [PubMed] [Google Scholar]
- 41.Zuco V, Benedetti V, Zunino F. ATM- and ATR-mediated response to DNA damage induced by a novel camptothecin, ST1968. Cancer Lett. 2010;292:186–196. doi: 10.1016/j.canlet.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Uematsu N, Weterings E, Yano K, et al. Autophosphorylation of DNA-PKCS regulates its dynamics at DNA doublestrand breaks. J Cell Biol. 2007;177:219–229. doi: 10.1083/jcb.200608077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yano K, Morotomi-Yano K, Wang SY, et al. Ku recruits XLF to DNA double-strand breaks. EMBO Rep. 2008;9:91–96. doi: 10.1038/sj.embor.7401137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang JF, Kieba IR, Korostoff J, et al. Molecular and biochemical mechanisms of Pasteurella haemolytica leukotoxin-induced cell death. Microb Pathog. 1998;25:317–331. doi: 10.1006/mpat.1998.0236. [DOI] [PubMed] [Google Scholar]
- 45.Meek DW. p53 Induction: phosphorylation sites cooperate in regulating. Cancer Biol Ther. 2002;1:284–286. doi: 10.4161/cbt.82. [DOI] [PubMed] [Google Scholar]
- 46.Choi W, Cogdell D, Feng Y, et al. Transcriptional activation of the carboxylesterase 2 gene by the p53 pathway. Cancer Biol Ther. 2006;5:1450–1456. doi: 10.4161/cbt.5.11.3271. [DOI] [PubMed] [Google Scholar]
- 47.Ichwan SJ, Yamada S, Sumrejkanchanakij P, et al. Defect in serine 46 phosphorylation of p53 contributes to acquisition of p53 resistance in oral squamous cell carcinoma cells. Oncogene. 2006;25:1216–1224. doi: 10.1038/sj.onc.1209158. [DOI] [PubMed] [Google Scholar]
- 48.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 49.Khosravi R, Maya R, Gottlieb T, et al. Rapid ATM-dependent phosphorylation of MDM2 precedes p53 accumulation in response to DNA damage. Proc Natl Acad Sci USA. 1999;96:14973–14977. doi: 10.1073/pnas.96.26.14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maya R, Balass M, Kim ST, et al. ATM-dependent phosphorylation of Mdm2 on serine 395: role in p53 activation by DNA damage. Genes Dev. 2001;15:1067–1077. doi: 10.1101/gad.886901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng Q, Chen L, Li Z, et al. ATM activates p53 by regulating MDM2 oligomerization and E3 processivity. EMBO J. 2009;28:3857–3867. doi: 10.1038/emboj.2009.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oda K, Arakawa H, Tanaka T, et al. p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell. 2000;102:849–862. doi: 10.1016/s0092-8674(00)00073-8. [DOI] [PubMed] [Google Scholar]
- 53.Lee JH, Paull TT. Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science. 2004;304:93–96. doi: 10.1126/science.1091496. [DOI] [PubMed] [Google Scholar]
- 54.Lee JH, Paull TT. Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene. 2007;26:7741–7748. doi: 10.1038/sj.onc.1210872. [DOI] [PubMed] [Google Scholar]
- 55.Uziel T, et al. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J. 2003;22:5612–5621. doi: 10.1093/emboj/cdg541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horejsi Z, et al. Distinct functional domains of Nbs1 modulate the timing and magnitude of ATM activation after low doses of ionizing radiation. Oncogene. 2004;23:3122–3127. doi: 10.1038/sj.onc.1207447. [DOI] [PubMed] [Google Scholar]
- 57.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 58.Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308:551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- 59.Bartkova J, et al. DNA damage response as a candidate anti-cancer barrier in early human tumourigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 60.Andegeko Y, Moyal L, Mittelman L, et al. Nuclear retention of ATM at sites of DNA double strand breaks. J Biol Chem. 2001;276:38224–38230. doi: 10.1074/jbc.M102986200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.