Abstract
The purpose of this study was to investigate the influence of exercise training on intramyocellular lipid (IMCL) content and test the hypothesis that the effect of endurance-oriented exercise training on IMCL is dependent on characteristics of the population studied. Lean (N = 11, body mass index (BMI) = 22.2 ± 0.7 kg·m−2), obese (N = 14, BMI = 38.8 ± 1.7 kg·m−2), and type 2 diabetic (N = 9, BMI = 35.5 ± 2.5 kg·m−2) participants were examined before and after 10 consecutive days of endurance-oriented (60 min·day−1 at ~70% V̇ O2peak) exercise training. IMCL and muscle glycogen were measured by Oil-Red-O and periodic acid – Schiff staining, respectively. The results indicated that IMCL was elevated (p < 0.05) in the obese and diabetic groups compared with the lean subjects prior to training. After training, IMCL content decreased (−35%) in the participants with type 2 diabetes; there were no changes in IMCL in the lean or obese groups. Muscle glycogen content was lower in the diabetic subjects than in the lean subjects both before and after training. These data indicate that changes in IMCL with exercise training do not exhibit a universal response but rather depend on the metabolic status of the population studied.
Keywords: lipid metabolism, muscle glycogen, skeletal muscle, triglycerides
Introduction
In sedentary individuals with either type 2 diabetes (T2D) or obesity, a high concentration of intramyocellular lipid (IMCL) has been associated with insulin resistance and low skeletal muscle mitochondrial density and (or) function (Coen et al. 2010; Goodpaster et al. 2001; Krssak et al. 1999; Moro et al. 2009). Surprisingly, endurance athletes who are insulin-sensitive with a high mitochondrial mass also display IMCL levels that approximate those reported in sedentary obese and diabetic individuals (Goodpaster et al. 2001). This discordance has been referred to as the “athletes paradox,” in which an increase in IMCL with endurance training may represent a positive adaptation contributing to lipid utilization during exercise in contrast to the conditions of lipid oversupply and reduced utilization typically present with T2D and obesity (Goodpaster et al. 2001). However, the interaction between exercise training and IMCL content is confounded when comparing prospective exercise training studies as some demonstrate increases in IMCL (Goodpaster et al. 2001; Howald et al. 1985; Pruchnic et al. 2004; Schrauwen-Hinderling et al. 2003) and others report no change (Helge and Dela 2003; Kiens et al. 1993) or a decrease (Bruce et al. 2004; Kim et al. 2004).
These discrepancies may be due to several reasons. First, the relationship between IMCL and insulin action and (or) muscle oxidative capacity may not be linear but rather “U” shaped, with sedentary T2D participants (insulin-resistant and low muscle oxidative capacity) and endurance-trained athletes (insulin-sensitive and high muscle oxidative capacity) comprising the opposite ends of a U-shaped curve (Moro et al. 2008). It may therefore be impractical to predict a standard response of IMCL to exercise training in populations with initial differences in metabolic status. For example, if the U-shaped curve hypothesis is accurate, sedentary individuals with initially high IMCL levels who are insulin-resistant would be anticipated to decrease IMCL in response to exercise training as they move along the curve. Second, weight loss has been reported to independently reduce IMCL content (Goodpaster et al. 2000; Gray et al. 2003); therefore, differences in the degree of weight loss among exercise training studies could produce conflicting findings. Lastly, the methodology for determining IMCL content can vary in respect to the influence of possible contamination from extramyocellular lipid sources (Goodpaster et al. 2001; Gray et al. 2003; Schrauwen et al. 2003).
The purpose of the present study was to test the hypothesis that the effect of endurance-oriented exercise training on IMCL content is dependent on the initial metabolic characteristics of the population being studied. Sedentary lean, obese, and T2D participants were examined before and after exercise training. In an attempt to minimize possible confounding factors, we used a 10-day exercise training program previously demonstrated to improve lipid oxidation with minimal changes in body mass and improvement in mitochondrial enzyme activity (Berggren et al. 2008; Chesley et al. 1996; Cox et al. 1999; Hansen et al. 2005). In addition, IMCL content was determined using microscopy in an attempt to minimize possible contamination from other lipid pools (Goodpaster et al. 2001; Gray et al. 2003).
Research design and methods
Subjects and study design
Subjects were recruited through advertisements on the East Carolina University (ECU) campus and in Greenville, North Carolina. Individuals with cardiovascular disease, pregnant women, and mentally disabled subjects were excluded. All women were premenopausal. Participants with documented T2D were referred from the ECU Endocrinology Clinic; subjects being treated with insulin were not examined, and all subjects were being treated with metformin. Approval of methods was given by the ECU Institutional Review Board. Before participation, subjects completed prescreening measurements and questionaires that included anthropometric measures, physical activity level, and medical history and provided written informed consent.
The experimental design consisted of examining muscle lipid content before and after 10 consecutive days of supervised exercise training. Subjects performed a maximal exercise test in the 2–3 weeks preceding training. Following a 12 h overnight fast on days 1 and 11, subjects reported to the laboratory between 0600 and 0800 (6 and 8 am), and a muscle sample was obtained for the subsequent analyses of indices of intracellular fuel content (lipid, glycogen), oxidative capacity, and fiber type. Approximately 60 min after the biopsy, subjects performed 10 min of submaximal exercise at two exercise intensities (absolute workload of 15 W and relative workload at 65% of V̇ O2peak) with indirect calorimetry used to determine fuel utilization. On day 1, the 20 min of submaximal testing was considered as part of the 60 min training session. Subjects were instructed to maintain their body mass by consuming additional energy proportionate to their expenditure during exercise (~400 kcal) but otherwise consume their typical diet during training and testing.
Determination of peak aerobic capacity
V̇O2peak was measured during incremental exercise on an electrically braked cycle ergometer (Lode, Diversified, Brea, California). Oxygen consumption was measured with continuous gas exchange measurements using a ParvoMedics True-Max 2400 computerized metabolic system (Consentius Technology, Sandy, Utah), and a 12-lead EKG was obtained during exercise to verify absence of cardiovascular disease. V̇O2peak was determined to ensure that subjects were sedentary and had no evidence of heart disease and to estimate workloads during training and submaximal testing.
Exercise training
Subjects exercised for 60 min·day−1 on a cycle ergometer at ~70% V̇ O2peak for 10 consecutive days similar to previous studies in our laboratory (Berggren et al. 2008; Tanner et al. 2002a). Heart rate was continually monitored and V̇ O2 was measured periodically to validate exercise intensity. Energy expenditure during exercise was similar (lean, 367 ± 41 kcal·session−1; obese, 405 ± 13 kcal·session−1; type 2 diabetic, 422 ± 48 kcal·session−1; p = 0.64) for each of the experimental groups.
Submaximal exercise testing
Expired gases were continuously autoanalyzed (ParvoMedics TrueMax 2400) during 20 min of submaximal exercise. The first 10 min of exercise was performed at 15 W (~40% V̇O2peak) and the second 10 min was performed at a workload eliciting ~65% V̇ O2peak. A similar testing protocol was utilized to examine differences in substrate utilization in obese individuals during submaximal exercise at equivalent absolute (15 W) and relative (65% V̇ O2peak) workloads (Guesbeck et al. 2001). Fat oxidation rate was calculated using respiratory exchange ratio and O2 consumption (McArdle et al. 2009) data during the final 7–10 min of exercise.
Muscle biopsy and analysis
Muscle from the vastus lateralis was obtained via a Bergström needle biopsy (Bergström 1975). Biopsies were obtained in the morning on day 1 prior to the beginning of exercise training after an overnight fast and on day 11 at ~16 h after the last bout of exercise. Immediately after the biopsy, tissue was mounted and frozen in isopentane cooled over liquid nitrogen and subsequently sectioned (12 μm serial sections) using a cryostat at −20 °C. To minimize variability, samples from each subject (before and after training) were stained in the same solution at the same time. Muscle was histochemically analyzed for fiber type distribution (myosin ATPase), intramyocellular lipid content (Oil-Red-O, ORO), oxidative capacity (NADH tetrazolium reductase, NADH-TR), and glycogen content (periodic acid – Schiff stain, PAS) using previously described methods (Gray et al. 2003; Tanner et al. 2002b).
Intramyocellular lipid (IMCL) content
IMCL content was determined using the ORO staining procedure as previously described (Gray et al. 2003). A stock solution was prepared by mixing 300 mg of ORO in 100 mL of 2-propanol (99%) at room temperature, and a working solution was prepared by mixing 24 mL of the stock solution and 16 mL distilled water. After standing for 10 min, the working solution was filtered and used for staining. All sections were incubated for 12 min at room temperature. After incubation, slides were rinsed with distilled water, followed by a rinse in cold tap water for 10 min, and were allowed to dry in the dark at room temperature. A coverslip was applied using glycerol jelly.
Fiber typing
The myosin ATPase staining method was used for fiber type determination as previously described (Tanner et al. 2002b). An alkaline preincubation solution of pH 10.3 was used to delineate between type I and type II fibers.
Oxidative capacity
The oxidative capacity of individual muscle fibers was assessed using the NADH-TR stain (Gray et al. 2003). A working solution was prepared by mixing 6 mg Nitro Blue Tetrazolium (NBT) and 24 mg NADH in 30 mL of Trizma buffer solution (pH 7.4 at 25 °C). Sections were incubated in the working solution for 30 min at 37 °C, gently rinsed three times with distilled water, and allowed to air-dry at room temperature, and a coverslip applied. Fibers appeared as either dark blue, indicating a high oxidative capacity, or light blue, indicating a low oxidative capacity.
Glycogen content
Samples were fixed to enhance glycogen preservation and to help prevent streaming artifact in Carnoy’s fixative (6:3:1 ethanol, chloroform, and glacial acetic acid, respectively, by volume) for 10 min at 4 °C. Upon fixation, sections were washed in distilled water and incubated in 1% periodic acid solution for 5 min at room temperature. After washing with distilled water, Schiff’s reagent was added and the sections were incubated for 8 min. All slides were rinsed in running, cold tap water for 10 min and air-dried, and a coverslip applied.
Image analyses
Serial sections were viewed under a light microscope (10×), and images were captured using Spot Advanced 3.2.4 software (Diagnostic Instruments, Sterling Heights, Michigan). Sigma Scan Pro 5.0 (SPSS Science, Chicago, Illinois) software was used to perform image analysis. To determine muscle fiber type specific IMCL and oxidative capacity, two images representing each stain (myosin ATPase, ORO, or NADH-TR) were viewed simultaneously and fiber type was matched to the ORO images. To assess IMCL content per fiber, optical transmittance (arbitrary units, AU) was determined from the ORO image on a 0–1000 intensity scale, with 0 representing completely dark (i.e., maximum amount of lipid) and 1000 representing completely light (i.e., no visibly stained lipid). To simplify interpretation (i.e., higher AU values indicate increased lipid), the data are expressed as (1/transmittance) × 1000. Optical transmittance was determined using similar methods to quantify oxidative capacity and glycogen content.
Statistics
Statistical analyses were performed using JMP 9.0.0 (SAS Institute Inc.), and graphs were made using GraphPad Prism version 5.0 (GraphPad Software Inc., La Jolla, California). Two-way ANOVA with repeated measures was used to compare group (lean, obese, and T2D), time (before vs. after exercise training), and group × time effects. Statistical significance was denoted at p ≤ 0.05.
Results
Subject characteristics
Subject characteristics are presented in Table 1. The obese and T2D participants had significantly higher body mass (obese, 65%; T2D, 59%) and BMI (obese, 75%; T2D, 60%) and significantly lower relative V̇ O2peak (obese, −31%; T2D, −40%) compared with lean subjects (p < 0.05). Absolute (l/min) V̇ O2peak, however, did not differ between the groups (p = 0.98; Table 1). The T2D participants were older than the lean and obese groups (p < 0.05). Fasting glucose was elevated in T2D participants (38%), and fasting insulin was elevated in the obese (120%) and T2D (160%) individuals compared with the lean individuals (p < 0.05). The T2D participants averaged 5.2 ± 2.1 years (mean ± SE) with the disease. Ten days of exercise training did not change body mass (p > 0.05).
Table 1.
Descriptive characteristics of the lean, obese, and type 2 diabetic subjects.
| Parameter | Lean | Obese | Type 2 diabetic |
|---|---|---|---|
| No. of subjects | 11 (2M, 9F) | 14 (1 M, 13 F) | 9 (3 M, 6 F) |
| Age (years) | 27.7±2.7 | 33.6±1.9 | 48.1±2.60*,† |
| Height (m) | 1.70±0.12 | 1.66±0.02 | 1.69±0.04 |
| Mass (kg) | 64.3±2.8 | 106.0±4.1* | 102.1±8.3* |
| BMI (kg·m−2) | 22.2±0.7 | 38.8±1.7* | 35.5±2.3* |
| V̇O2peak (mL·kg−1·min−1) | 31.3±3.2 | 20.0±2.0* | 18.8±1.6* |
| V̇O2peak (L·min−1) | 2.0±0.2 | 2.1±0.2 | 1.9±0.6 |
| Glucose (mmol·L−1) | 4.8±0.2 | 5.2±0.2 | 6.6±0.9*,† |
| Insulin (µU·mL−1) | 5.3±1.2 | 11.7±2.5* | 13.8±3.2*,† |
Note: Glucose and insulin are fasting plasma values. Values are expressed as mean ± SE. M, male; F, female; BMI, body mass index; V̇ O2peak, peak oxygen consumption.
Significant difference compared with the lean group.
Significant difference compared with the obese group.
Fiber type
There were no significant differences in fiber type distribution between the groups (p = 0.67; lean, 36.6%; obese, 32.7%; and T2D, 37.5% type I fibers). No significant training effect on fiber type distribution was evident.
Intramyocellular lipid content
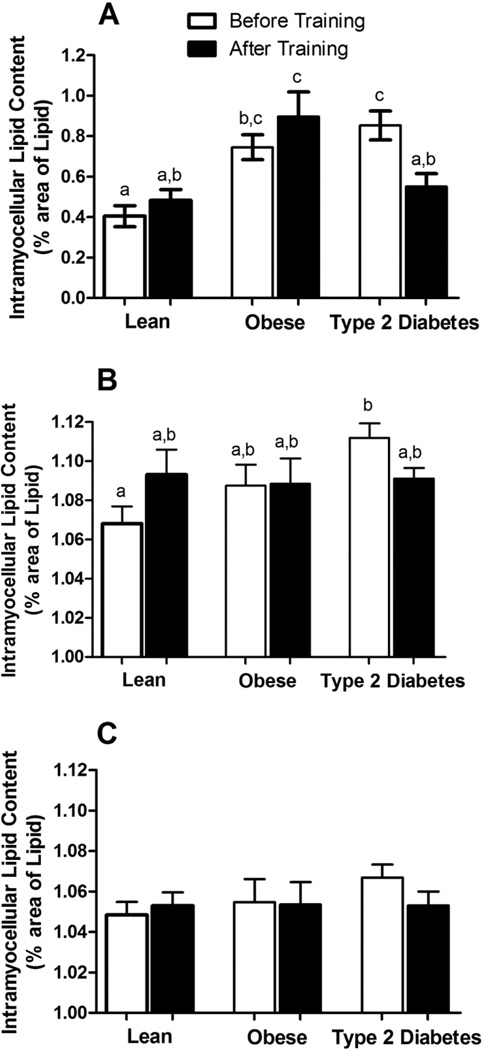
As presented in Fig. 1A, IMCL content in the obese and T2D subjects was about twofold higher than in the lean subjects (p < 0.001) prior to the initiation of exercise training. There was no difference in IMCL content between the obese and T2D groups. IMCL content was significantly higher in type I fibers than in type II fibers in all groups (p < 0.01). As presented in Fig. 1B, prior to training, T2D subjects had significantly elevated IMCL in the type I fibers (p < 0.01) compared with lean subjects, whereas there were no differences between groups in IMCL content in the type II fibers (Fig. 1C) (p = 0.72). After 10 days of training, IMCL content did not change in the lean and obese subjects (Fig. 1A) (p = 0.96 and p = 0.71, respectively); however, the T2D subjects showed a significant decrease (~32%) in IMCL and normalized content to levels similar to those observed in lean subjects (Fig. 1A). Change in total IMCL (%) was significantly different in T2D subjects compared with lean and obese subjects (lean, 29.5 ± 15.8%; obese, 26.1 ± 21.7%; T2D, −31.9 ± 10.1%). Exercise training had no effect on type I and type II fiber type specific IMCL content in lean and obese subjects (Fig. 1B), but similar to overall lipid content, IMCL tended (p = 0.09) to decrease in the type I fibers in the subjects with diabetes (Fig. 1B). There was no change in IMCL in the type II fibers with training in any of the three groups (Fig. 1C) (p = 0.46).
Fig. 1.
(A) Total and (B and C) fiber type specific (B, type I fibers; C, type II fibers) intramyocellular lipid (IMCL) content before and after 10 days of endurance-oriented exercise training in lean, obese, and type 2 diabetic subjects. Values are expressed as mean ± SE. Bars with different letters denote statistically significant differences (p < 0.05).
Muscle glycogen content
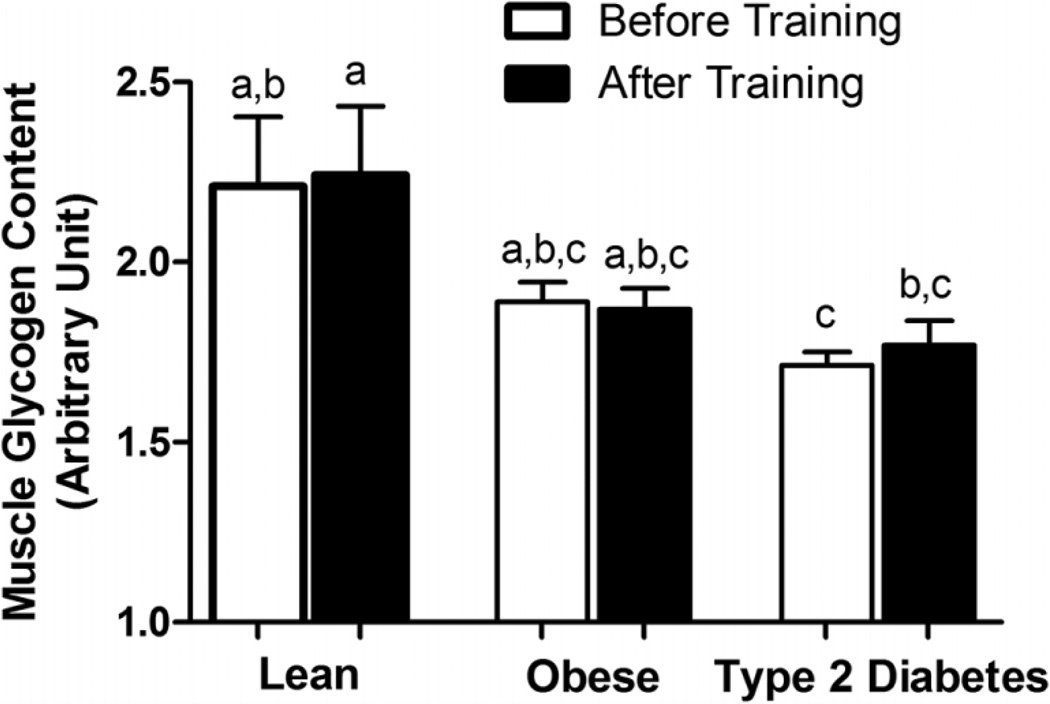
As presented in Fig. 2, pretraining muscle glycogen content was significantly lower in the T2D individuals than in the lean subjects (p < 0.05) but was similar to that in the obese subjects. Ten days of exercise training did not change muscle glycogen content in any of the three groups.
Fig. 2.
Skeletal muscle glycogen content determined by periodic acid – Schiff stain (PAS) in skeletal muscle of sedentary lean, obese, and type 2 diabetic patients. Values are expressed as mean ± SE. Bars with different letters denote statistically significant differences (p < 0.05).
Fuel utilization during submaximal exercise
The contribution of lipid to the fuel needs during submaximal exercise at both workloads increased significantly in all three groups with exercise training (p < 0.05) (data not shown). There were no differences in the relative improvement in lipid oxidation between subjects (p = 0.86). At 15 W, relative fuel utilization was ~45% fat and ~55% carbohydrate; after training, values changed (p < 0.05) to ~55% fat and ~45% carbohydrate. At 65% V̇ O2peak, relative fuel utilization was 5% fat and 95% carbohydrate; after training, values changed to 20% fat and 80% carbohydrate (p < 0.05). Accordingly, carbohydrate oxidation decreased after training in all groups (p < 0.05).
Muscle oxidative capacity
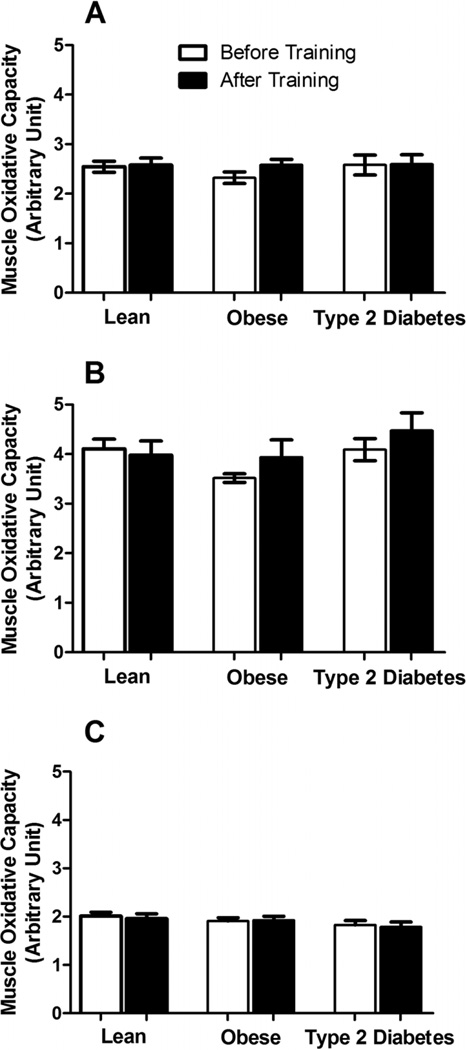
There were no significant differences in overall oxidative capacity, measured by NADH-TR staining, between the lean, obese, and T2D groups (Fig. 3A). Specific oxidative capacity in fiber type I and fiber type II was also not different (Figs. 3B and 3C). There were no significant changes in total fiber type I and fiber type II specific oxidative capacity after exercise training (p > 0.05). Type I fibers had higher oxidative capacity than type II fibers (p < 0.01).
Fig. 3.
(A) Total and (B and C) fiber type specific (B, type I fibers; C, type II fibers) skeletal muscle oxidative capacity measured by NADH tetrazoluum reductase stain. Values are expressed as mean ± SE.
Discussion
An elevated IMCL content has been associated with insulin resistance and a reduced capacity for oxidative metabolism in obese and T2D participants (Coen et al. 2010; Forouhi et al. 1999; Goodpaster et al. 1997; Krssak et al. 1999; Moro et al. 2009; Petersen et al. 1997; Phillips et al. 1996). Endurance-trained athletes who are highly insulin-sensitive and possess an elevated mitochondrial mass also display an elevated level of IMCL (Goodpaster et al. 2001). These observations have led to a proposal that the relationship between IMCL content and insulin sensitivity or muscle oxidative capacity is a U-shaped curve (Moro et al. 2008). If this hypothesis is correct, then sedentary, insulin-resistant individuals with initially elevated IMCL could theoretically reduce IMCL content with an intervention such as exercise training as they move along the curve. However, some studies suggest that an increase in IMCL is an integral aspect of the response to exercise training (Goodpaster et al. 2001; Howald et al. 1985; Petersen et al. 1997; Pruchnic et al. 2004). The main finding of the present study was that endurance-oriented exercise training does not appear to evoke a standard response in IMCL content, but rather appears to depend on the characteristics of the population being studied.
In agreement with others (Goodpaster et al. 2000, 2001), initial IMCL content was elevated in obese and obese T2D individuals (Fig. 1). However, 10 days of exercise training produced disparate responses, as there were no significant alterations in total IMCL in the lean and obese groups but a significant decrease in total IMCL in T2D subjects (Fig. 1). A systematic review (Wang et al. 2009) surmised that only two studies have examined the relationship between T2D, exercise training, and IMCL and that these experiments (Bruce et al. 2004; Kim et al. 2004) observed a decrease in IMCL with 8–12 weeks of endurance-oriented exercise training. Using electron microscopy, Nielsen et al. (2010) also reported a reduction in subsarcolemmal lipid content in T2D subjects with 10 weeks of endurance-oriented exercise. In support of a training specificity effect, the decrease in IMCL in the T2D participants in the present study appeared to be primarily driven by a reduction in IMCL in the type I fibers (Fig. 1A); the type I fibers would be preferentially recruited during the moderate intensity, endurance-oriented exercise training performed. These limited findings (Bruce et al. 2004; Kim et al. 2004; Nielsen et al. 2010) coupled with the current data (Fig. 1) suggest that T2D individuals adapt to endurance-oriented exercise by decreasing IMCL, which differs from the increase or no change reported in nondiabetic populations (Goodpaster et al. 2001; Helge and Dela 2003; Kiens et al. 1993; Pruchnic et al. 2004; Schrauwen-Hinderling et al. 2003).
Our observation of a reduction (Fig. 1) in IMCL with exercise training in T2D individuals is consistent with other longer term (8 to 12 weeks) studies in this population (Bruce et al. 2004; Kim et al. 2004). In contrast, after a 2-week training program, Schrauwen-Hinderling et al. (2003) reported a 42% increase in total IMCL using proton magnetic resonance spectroscopy in young, lean individuals with similar characteristics to those of the lean subjects in the present study (Fig. 1). It is not clear how the current (Fig. 1) and other work (Schrauwen-Hinderling et al. 2003) could obtain such disparate results in young, lean subjects. The present data, however, emphasize the notion that IMCL content is likely dependent on factors linked to the metabolic status of the individual and that the adoption of an exercise training program does not evoke a singular and consistent adaptation. This is supported by the disparate IMCL responses among the three groups of subjects to the same exercise stimulus in the current study.
The change in IMCL after training in the present study could be explained, at least in part, in the context of the U-shaped curve hypothesis (Moro et al. 2008). The T2D subjects were the most insulin-resistant (Table 1) and thus initially higher on the curve, with the obese and lean subjects initially being closer to the bottom of the curve. Using this model, it could be predicted that the obese and lean subjects would exhibit little change or possibly an increase in IMCL, whereas the T2D individuals would reduce IMCL with exercise intervention. Initial fuel stores alone does not dictate the response to exercise training, as the obese individuals entered exercise training with approximately the same IMCL and glycogen content as the T2D individuals (Fig. 1). Aging is associated with increased lipid utilization during and after exercise (Lanza et al. 2007; Melanson et al. 2007); however, fat oxidation was not elevated during submaximal exercise in the obese vs. T2D groups (see Results), who were similar in virtually every aspect other than age (Table 1). Metformin given to healthy, nondiabetic individuals has been shown to increase lipid oxidation (Malin et al. 2010) and thus cannot be overlooked as a possible cause of greater decrease in IMCL specific to the T2D group. A lack of dietary control during the study can also be considered a potential limitation, as diet also influences fuel storage and fuel use during exercise. A mechanistic explanation for why IMCL decreased in the T2D participants and not in the lean or obese groups with exercise training remains to be provided. Regardless, the present study provides the critical information that there is a differential response in IMCL to exercise training according to the metabolic phenotype and (or) the age of the subjects studied.
Muscle oxidative capacity determined by histochemical methods (NADH-TR staining) did not differ between the groups and was not altered with training (Fig. 3). This finding was surprising as we have previously reported that an identical training program increased lipid oxidation along with other markers of oxidative capacity in the skeletal muscle of lean and obese individuals (Berggren et al. 2008). These data suggest that this histochemical method, although able to distinguish the oxidative capacity between fiber types, was relatively insensitive to alterations occurring with exercise training and possibly to differences between our subject populations (Bajpeyi et al. 2011).
In summary, 10 days of exercise training did not alter IMCL content in sedentary lean and obese subjects, whereas IMCL content decreased in T2D participants. These data suggest that changes in IMCL with exercise training do not exhibit a universal response and, rather, depend on the metabolic status or age of the population studied.
Acknowledgements
We thank Jessica Van Meter for her help in recruitment of study participants, Holiday Durham for the help with statistics, and all study participants for their time and willingness to participate in our study. This study was supported by NIDDK RO1-56112 (JAH).
Footnotes
Authors have no conflict of interest.
Contributor Information
Sudip Bajpeyi, Department of Exercise and Sports Science, East Carolina University, Greenville, NC 27858, USA; Human Performance Laboratory, East Carolina University, Greenville, NC 27858, USA.
Melissa A. Reed, Department of Exercise and Sports Science, East Carolina University, Greenville, NC 27858, USA; Human Performance Laboratory, East Carolina University, Greenville, NC 27858, USA
Sara Molskness, Department of Exercise and Sports Science, East Carolina University, Greenville, NC 27858, USA; Human Performance Laboratory, East Carolina University, Greenville, NC 27858, USA.
Christopher Newton, Department of Internal Medicine, Emory University School of Medicine, Atlanta, GA 30303, USA.
Charles J. Tanner, Department of Exercise and Sports Science, East Carolina University, Greenville, NC 27858, USA; Human Performance Laboratory, East Carolina University, Greenville, NC 27858, USA
Jennifer S. McCartney, Department of Exercise and Sports Science, East Carolina University, Greenville, NC 27858, USA; Human Performance Laboratory, East Carolina University, Greenville, NC 27858, USA
Joseph A. Houmard, Department of Exercise and Sports Science, East Carolina University, Greenville, NC 27858, USA; Human Performance Laboratory, East Carolina University, Greenville, NC 27858, USA; East Carolina Diabetes and Obesity Institute, East Carolina University, Greenville, NC 27858, USA
References
- Bajpeyi S, Pasarica M, Moro C, Conley K, Jubrias S, Sereda O, et al. Skeletal muscle mitochondrial capacity and insulin resistance in type 2 diabetes. J. Clin. Endocrinol. Metab. 2011;96(4):1160–1168. doi: 10.1210/jc.2010-1621. PMID: 21307136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berggren JR, Boyle KE, Chapman WH, Houmard JA. Skeletal muscle lipid oxidation and obesity: influence of weight loss and exercise. Am. J. Physiol. Endocrinol. Metab. . 2008;294(4):E726–E732. doi: 10.1152/ajpendo.00354.2007. PMID: 18252891. [DOI] [PubMed] [Google Scholar]
- Bergström J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand. J. Clin. Lab. Invest. 1975;35(7):609–616. PMID: 1108172. [PubMed] [Google Scholar]
- Bruce CR, Kriketos AD, Cooney GJ, Hawley JA. Disassociation of muscle triglyceride content and insulin sensitivity after exercise training in patients with type 2 diabetes. Diabetologia. 2004;47(1):23–30. doi: 10.1007/s00125-003-1265-7. PMID: 14673522. [DOI] [PubMed] [Google Scholar]
- Chesley A, Heigenhauser GJ, Spriet LL. Regulation of muscle glycogen phosphorylase activity following short-term endurance training. Am. J. Physiol. . 1996;270(2 Pt 1):E328–E335. doi: 10.1152/ajpendo.1996.270.2.E328. PMID: 8779956. [DOI] [PubMed] [Google Scholar]
- Coen PM, Dube JJ, Amati F, Stefanovic-Racic M, Ferrell RE, Toledo FG, Goodpaster BH. Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes. 2010;59(1):80–88. doi: 10.2337/db09-0988. PMID: 19833891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JH, Cortright RN, Dohm GL, Houmard JA. Effect of aging on response to exercise training in humans: skeletal muscle glut-4 and insulin sensitivity. J. Appl. Physiol. 1999;86(6):2019–2025. doi: 10.1152/jappl.1999.86.6.2019. PMID: 10368369. [DOI] [PubMed] [Google Scholar]
- Forouhi NG, Jenkinson G, Thomas EL, Mullick S, Mierisova S, Bhonsle U, et al. Relation of triglyceride stores in skeletal muscle cells to central obesity and insulin sensitivity in European and south Asian men. Diabetologia. 1999;42(8):932–935. doi: 10.1007/s001250051250. PMID: 10491752. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46(10):1579–1585. doi: 10.2337/diacare.46.10.1579. PMID: 9313753. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism. 2000;49(4):467–472. doi: 10.1016/s0026-0495(00)80010-4. PMID: 10778870. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J. Clin. Endocrinol. Metab. 2001;86(12):5755–5761. doi: 10.1210/jcem.86.12.8075. PMID: 11739435. [DOI] [PubMed] [Google Scholar]
- Gray RE, Tanner CJ, Pories WJ, MacDonald KG, Houmard JA. Effect of weight loss on muscle lipid content in morbidly obese subjects. Am. J. Physiol. Endocrinol. Metab. . 2003;284(4):E726–E732. doi: 10.1152/ajpendo.00371.2002. PMID: 12488242. [DOI] [PubMed] [Google Scholar]
- Guesbeck NR, Hickey MS, MacDonald KG, Pories WJ, Harper I, Ravussin E, et al. Substrate utilization during exercise in formerly morbidly obese women. J. Appl. Physiol. 2001;90(3):1007–1012. doi: 10.1152/jappl.2001.90.3.1007. PMID: 11181612. [DOI] [PubMed] [Google Scholar]
- Hansen AK, Fischer CP, Plomgaard P, Andersen JL, Saltin B, Pedersen BK. Skeletal muscle adaptation: training twice every second day vs. training once daily. J. Appl. Physiol. 2005;98(1):93–99. doi: 10.1152/japplphysiol.00163.2004. PMID: 15361516. [DOI] [PubMed] [Google Scholar]
- Helge JW, Dela F. Effect of training on muscle triacylglycerol and structural lipids: a relation to insulin sensitivity? Diabetes. 2003;52(8):1881–1887. doi: 10.2337/diabetes.52.8.1881. PMID: 12882901. [DOI] [PubMed] [Google Scholar]
- Howald H, Hoppeler H, Claassen H, Mathieu O, Straub R. Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pflugers Arch. . 1985;403(4):369–376. doi: 10.1007/BF00589248. PMID: 4011389. [DOI] [PubMed] [Google Scholar]
- Kiens B, Essen-Gustavsson B, Christensen NJ, Saltin B. Skeletal muscle substrate utilization during submaximal exercise in man: effect of endurance training. J. Physiol. 1993;469:459–478. doi: 10.1113/jphysiol.1993.sp019823. PMID: 8271208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Lee JS, Kim CK. Effect of exercise training on muscle glucose transporter 4 protein and intramuscular lipid content in elderly men with impaired glucose tolerance. Eur. J. Appl. Physiol. 2004;93(3):353–358. doi: 10.1007/s00421-004-1214-2. PMID: 15480742. [DOI] [PubMed] [Google Scholar]
- Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42(1):113–116. doi: 10.1007/s001250051123. PMID: 10027589. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Larsen RG, Kent-Braun JA. Effects of old age on human skeletal muscle energetics during fatiguing contractions with and without blood flow. J. Physiol. . 2007;583(3):1093–1105. doi: 10.1113/jphysiol.2007.138362. PMID: 17673506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SK, Stephens BR, Sharoff CG, Hagobian TA, Chipkin SR, Braun B. Metformin’s effect on exercise and postexercise substrate oxidation. Int. J. Sport Nutr. Exerc. Metab. 2010;20(1):63–71. doi: 10.1123/ijsnem.20.1.63. PMID: 20190353. [DOI] [PubMed] [Google Scholar]
- McArdle WD, Katch F, Katch V. Exercise physiology energy, nutrition, and human performance. 7th ed. Lippincott Williams and Wilkins; 2009. [Google Scholar]
- Melanson EL, Donahoo WT, Grunwald GK, Schwartz R. Changes in 24-h substrate oxidation in older and younger men in response to exercise. J. Appl. Physiol. . 2007;103(5):1576–1582. doi: 10.1152/japplphysiol.01455.2006. PMID: 17717111. [DOI] [PubMed] [Google Scholar]
- Moro C, Bajpeyi S, Smith SR. Determinants of intramyocellular triglyceride turnover: implications for insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. . 2008;294(2):E203–E213. doi: 10.1152/ajpendo.00624.2007. PMID: 18003718. [DOI] [PubMed] [Google Scholar]
- Moro C, Galgani JE, Luu L, Pasarica M, Mairal A, Bajpeyi S, et al. Influence of gender, obesity, and muscle lipase activity on intramyocellular lipids in sedentary individuals. J. Clin. Endocrinol. Metab. 2009;94(9):3440–3447. doi: 10.1210/jc.2009-0053. PMID: 19531593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Mogensen M, Vind BF, Sahlin K, Hojlund K, Schroder HD, Ortenblad N. Increased subsarcolemmal lipids in type 2 diabetes: effect of training on localization of lipids, mitochondria, and glycogen in sedentary human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. . 2010;298(3):E706–E713. doi: 10.1152/ajpendo.00692.2009. PMID: 20028967. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Jacob R, West AB, Sherwin RS, Shulman GI. Effects of insulin-like growth factor I on glucose metabolism in rats with liver cirrhosis. Am. J. Physiol. . 1997;273(6 Pt 1):E1189–E1193. doi: 10.1152/ajpendo.1997.273.6.E1189. PMID: 9435535. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Han XX, Green HJ, Bonen A. Increments in skeletal muscle glut-1 and glut-4 after endurance training in humans. Am. J. Physiol. . 1996;270(3 Pt 1):E456–E462. doi: 10.1152/ajpendo.1996.270.3.E456. PMID: 8638693. [DOI] [PubMed] [Google Scholar]
- Pruchnic R, Katsiaras A, He J, Kelley DE, Winters C, Goodpaster BH. Exercise training increases intramyocellular lipid and oxidative capacity in older adults. Am. J. Physiol. Endocrinol. Metab. . 2004;287(5):E857–E862. doi: 10.1152/ajpendo.00459.2003. PMID: 15226098. [DOI] [PubMed] [Google Scholar]
- Schrauwen P, Hoeks J, Schaart G, Kornips E, Binas B, Van De Vusse GJ, et al. Uncoupling protein 3 as a mitochondrial fatty acid anion exporter. FASEB. J. 2003;17(15):2272–2274. doi: 10.1096/fj.03-0515fje. PMID: 14525936. [DOI] [PubMed] [Google Scholar]
- Schrauwen-Hinderling VB, Schrauwen P, Hesselink MK, van Engelshoven JM, Nicolay K, Saris WH, et al. The increase in intramyocellular lipid content is a very early response to training. J. Clin. Endocrinol. Metab. 2003;88(4):1610–1616. doi: 10.1210/jc.2002-021464. PMID: 12679446. [DOI] [PubMed] [Google Scholar]
- Tanner CJ, Koves TR, Cortright RL, Pories WJ, Kim YB, Kahn BB, et al. Effect of short-term exercise training on insulin-stimulated PI 3-kinase activity in middle-aged men. Am. J. Physiol. Endocrinol. Metab. . 2002a;282(1):E147–E153. doi: 10.1152/ajpendo.2002.282.1.E147. PMID: 11739095. [DOI] [PubMed] [Google Scholar]
- Tanner CJ, Barakat HA, Dohm GL, Pories WJ, MacDonald KG, Cunningham PR, et al. Muscle fiber type is associated with obesity and weight loss. Am. J. Physiol. Endocrinol. Metab. . 2002b;282(6):E1191–E1196. doi: 10.1152/ajpendo.00416.2001. PMID: 12006347. [DOI] [PubMed] [Google Scholar]
- Wang Y, Simar D, Fiatarone Singh MA. Adaptations to exercise training within skeletal muscle in adults with type 2 diabetes or impaired glucose tolerance: a systematic review. Diabetes Metab. Res. Rev. 2009;25(1):13. doi: 10.1002/dmrr.928. [DOI] [PubMed] [Google Scholar]