Abstract
Stem cells have a number of properties, which make them excellent candidates for the treatment of various neurologic disorders, the most important of which being their ability to migrate to and differentiate predictably at sites of pathology in the brain. The disease-directed migration and well-characterized differentiation patterns of stem cells may eventually provide a powerful tool for the treatment of both localized and diffuse disease processes within the human brain. A thorough understanding of the molecular mechanisms governing their migratory properties and their choice between different differentiation programs is essential if these cells are to be used therapeutically in humans. This review focuses on summarizing the migration and differentiation of therapeutic neural and mesenchymal stem cells in different disease models in the brain and also discusses the promise of these cells to eventually treat various forms of neurologic disease.
Keywords: stem cell, pathotropic migration, differentiation, therapeutics
Stem cells are defined by their ability to continuously renew themselves by symmetric division and to give rise to more mature progenitors of multiple lineages through asymmetric division (Fig. 1). They can be isolated from both embryonic and adult human tissues, expanded and manipulated in vitro, and subsequently regrafted at which point they can differentiate into terminal cell types and integrate into living tissues (Gage, 2000; Barker and Wagner, 2003). These characteristics give stem cells enormous therapeutic potential, and because of this, stem cell-mediated therapies for a variety of diseases are actively being sought in both basic and clinical research. Many of these ongoing studies concern the use of stem cells for the treatment of neurologic disorders including but not limited to multiple sclerosis (Pluchino et al., 2009; Carbajal et al., 2010), brain tumors (Shah et al., 2008; Sasportas et al., 2009), stroke (Chu et al., 2003; Kim et al., 2004), and lysosomal storage diseases (Snyder et al., 1995). Of great importance to all of these efforts is the ability of therapeutic stem cells to successfully migrate to and function at targeted regions of pathology.
Fig. 1.
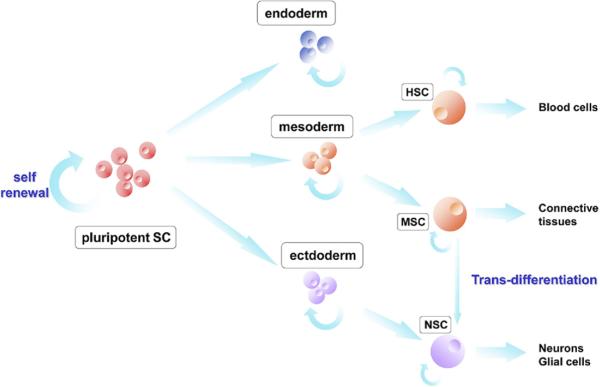
Model of stem cell fate. From a pluripotent state, stem cells gradually lose breadth of differentiation potential. Note the potential for “trans-differentiation” of MSCs to NSCs. HSC, hematopoietic stem cell; MSC, mesenchymal stem cell; NSC, neural stem cell. Adapted from Corsten and Shah (2008).
The efficient migration of stem cells toward regions of pathology in the brain followed by their successful integration, differentiation, and long-term survival at these sites is required for effective stem cell-mediated treatment of neurological disease. Recent studies in which therapeutic stem cells have been deployed in vivo into the brains of disease-bearing mice have shown that neural and mesenchymal stem cells (NSCs and MSCs) have these capabilities. Specifically, NSCs and MSCs have been observed to travel to regions of neuropathology, differentiate predictably, and survive while stably expressing transgenes that they had been engineered to carry (Chu et al., 2003; Jeong et al., 2003; Ryu et al., 2004; Lee et al., 2007, 2009; Nagai et al., 2007; Shah et al., 2008; Pluchino et al., 2009; Sasportas et al., 2009; Carbajal et al., 2010). These findings, taken together with those of other studies concerning the migration and fate of therapeutic stem cells in vivo, demonstrate the great promise of this developing paradigm. However, before this potential can be realized in a clinical setting an in depth understanding of the physical and molecular mechanisms controlling the migratory properties and fates of therapeutic stem cells is necessary. In this review we summarize our current knowledge of therapeutic stem cell migration and fate in the mammalian brain. Furthermore, a brief overview of how these cells have been modified in vitro, imaged in vivo, and used to treat various animal models of neurologic disease will be discussed.
STEM CELL MIGRATION
A number of studies have shown that therapeutic stem cells transplanted into the mammalian brain preferentially migrate toward the regions of pathology. This was first demonstrated by Snyder and colleagues, who transplanted NSCs into a mouse model of mucopolysaccharidosis VII, a lysosomal storage disorder characterized by the fatal accumulation of glycosaminoglycans due to the absence of the enzyme β-glucuronidase. The NSCs, which were engineered to express β-glucuronidase and engrafted into the cerebral ventricles of newborn mice, were found to migrate extensively throughout the diseased brain and restore β-glucuronidase activity (Snyder et al., 1995). Stem cell pathotropism has also been observed toward ischemic (Veizovic et al., 2001; Chu et al., 2003; Jeong et al., 2003; Kim et al., 2004; Xu et al., 2007; Daadi et al., 2009), neoplastic (Shah et al., 2008; Lee et al., 2009; Frank et al., 2009; Sasportas et al., 2009), and demyelinating lesions (Pluchino et al., 2009; Carbajal et al., 2010). The migration of stem cells to pathologies such as these is highly efficient, so much so that murine NSCs injected into the tail vein (Aboody et al., 2000) or implanted into the contralateral hemisphere (Tang et al., 2003; Shah et al., 2005) of glioma-bearing mice are able to travel to and distribute throughout distant tumor masses (Fig. 2). This high efficiency and the pathotropism of transplanted stem cells are in large part due to cell signaling pathways that are activated during acute and chronic neurological dysfunction.
Fig. 2.

Neural stem cells migrate specifically to tumors. (A) Schematic representation showing tropism of transplanted stem cells showing for malignant cells in mouse models of brain tumors. (B–G) NSC-LUC cells were implanted into the right hemisphere of glioma-bearing (B–D) or control mice that did not have tumors (E–G). A time series of the same animal from the first group imaged on day 0 (B), at 1 wk (C), and at 2 wk (D). Migration toward the tumor (dotted circle) was first noted after 1 wk (C; see faint bioluminescence signal along arrow) and migration across the midline was evident at 2 wk. (E–G) Time series of another animal representative of the non-tumor-bearing group, in which no migration toward the contralateral side was observed. (H–J) X-Gal staining of coronal sections of the brain, showing β-Gal-expressing NSCs (blue). β-Gal-expressing cells evident at the injection site (H), the corpus callosum (I), and inside the tumor (J). (K) Intravital microscopy of Fluc-DsRed2 hNSCs implanted in mice with established GFP-Rluc gliomas. (L) Fluorescent image of showing hNSCs (red) infiltrating a tumor (green) at 40× magnification. Adapted from Tang et al. (2003) and Shah et al. (2008).
While stem cells have been shown to travel toward various neuropathologies in vivo, the molecular mechanisms governing this migration are at present incompletely understood. Studies of stem cell pathotropism have shown that a number of cytokine/receptor pairs are associated with the homing of stem/progenitor cells to diseased areas of the brain (Table 1), including stem cell factor (SCF)/c-Kit (Sun et al., 2004; Bantubungi et al., 2008), monocyte chemoattractant protein-1 (MCP-1)/chemokine receptor 2 (CCR2) (Widera et al., 2004; Magge et al., 2009), vascular endothelial growth factor/receptor (VEGF/VEGFR) (Zhao et al., 2008; Schmidt et al., 2009), hepatocyte growth factor (HGF)/c-Met (Heese et al., 2005; Garzotto et al., 2008), and stromal cell-derived factor 1 (SDF-1)/CXCR4 (Zhou et al., 2002; Imitola et al., 2004; Robin et al., 2006; Son et al., 2006; Carbajal et al., 2010). The first of these cytokines, SCF, is a membrane-bound protein that, when cleaved by proteases such as matrix metalloproteinase-9 (MMP-9), gives rise to a soluble peptide that binds to the tyrosine kinase receptor c-kit that is found on the surface of several stem/progenitor cell lines (Fazel et al., 2008; Huang et al., 2009; Souyima et al., 2009). The interaction of SCF with c-kit results in the activation of signaling pathways that have been implicated in the survival, proliferation, and chemotaxis of stem cells participating in hematopoiesis, gametogenesis, and melanogenesis (Ueda et al., 2002; Dentelli et al., 2007; Huang et al., 2009). SCF/c-kit signaling is also involved in the migration of exogenous NSCs toward pathology within the brain, as demonstrated in mice subjected to “freeze” brain injury (Sun et al., 2004) and in a rat model of Huntington's disease (Bantubungi et al., 2008). In both of these studies, SCF was shown to be strongly upregulated by cells residing in and around lesioned areas. Furthermore, the blockade of stem cell c-kit was observed to significantly impair the migration of transplanted NSCs to the diseased striatum (Bantubungi et al., 2008). These studies provide convincing evidence that the interaction of SCF with its receptor c-kit plays an important role in the migration of stem cells toward regions of pathology within the brain.
Table 1.
Receptor/ligand pairs implicated in NSC pathotropism
| Receptor/ligand | Disease model | References |
|---|---|---|
| SCF/c-Kit | "Freeze" brain injury | Sun et al. (2004) |
| SCF/c-Kit | Huntington's disease | Bantubungi et al. (2008) |
| MCP-1/CCR2 | Inflammation | Widera et al. (2004) |
| MCP-1/CCR2 | Tumor | Magge et al. (2009) |
| VEGF/VEGFR | Ischemia | Xu et al. (2007) |
| Zhao et al. (2008) | ||
| Schmidt et al. (2009) | ||
| HGF/c-Met | Tumor | Heese et al. (2005) |
| SDF-1/CXCR4 | Demyelination | Carbajal et al. (2010) |
| SDF-1/CXCR4 | Ischemia | Imitola et al. (2004) |
| SDF-1/CXCR4 | Tumor | Zhou et al. (2002) |
| Robin et al. (2006) |
MCP-1 is a chemotactic cytokine that promotes the migration of macrophages and monocytes through binding to CCR2 (Fuentes et al., 1995; Gunn et al., 1997). In addition to macrophages and monocytes, CCR2 is expressed by several other cell types, including memory T cells, regulatory T cells (Tregs), natural killer cells, and NSCs (Charo et al., 1994; Bartoli et al., 2001; Widera et al., 2004). Because of the expression of CCR2 by NSCs, it is not surprising that they migrate in the direction of an MCP-1 gradient, as shown by Magge and colleagues who demonstrated that NSCs travel toward MCP-1 infusion sites in the brains of healthy rats (Magge et al., 2009). MCP-1/CCR2 signaling has also been implicated in mediating the pathology-directed tropism of NSCs given that it has been found to induce the migration of stem cells toward inflammatory (Widera et al., 2004) and neoplastic (Magge et al., 2009) lesions within the mammalian brain. These studies reveal that, similar to SCF-1/c-kit, MCP-1/CCR2 signaling is a probable regulator of stem cell pathotropism.
VEGF is a protein mitogen that acts through the VEGFR to promote angiogenic endothelial cell proliferation and sprouting (Ferrara et al., 1992). VEGF is important in a number of physiological contexts, including development (Ferrara et al., 1996) and wound healing (Bates and Pritchard Jones, 2003). In addition to these roles in normal physiology, VEGF is also a major player in tumor angiogenesis and the hypoxic response, which is known to be activated in neuropathologies, such as ischemia (Bergeron et al., 1999) and cancer (Jensen et al., 2006). Hypoxia is known to promote NSC tropism in vitro (Xu et al., 2007) and in vivo (Zhao et al., 2008), mainly due to the upregulation of VEGF by hypoxic cells, which was observed to result in the increased expression of chemotactic factors Ang2 and GROα (Schmidt et al., 2009). These two proteins, a growth factor and a chemokine respectively, promote the migration of NSCs toward regions of hypoxia within the brain and thus form the basis of the mechanism by which elevated levels of VEGF cause enhanced stem cell pathotropism.
Another cytokine/receptor pair involved in the migration of neural stem/progenitor cells in the brain is HGF, which acts through the cell surface c-Met receptor. HGF was initially characterized as a hepatocyte mitogen (Lundberg and Mollgard, 1979; Nakamura et al., 1984) and later studies established it as an important neurotrophic factor in the developing and adult central nervous system (Hobara et al., 2008; Nicoleau et al., 2009). Recent work has shown that the interaction of HGF with glioma cell c-Met results in the upregulation of chemokine receptor 4 (CXCR4) by tumor cells (Esencay et al., 2010), which is known to enhance their capacity for migration (Ehtesham et al., 2006; Stevenson et al., 2008). While the mechanism by which HGF/c-Met signaling mediates stem cell migration is at present unclear, it is likely that it involves the upregulation of CXCR4 as well (Son et al., 2006).
One of the best studied mediators of stem cell tropism is the G-protein coupled receptor CXCR4 and its only known ligand SDF-1. CXCR4 is among the most highly expressed chemokine receptors during both pre- and postnatal development (Jazin et al., 1997; Lavi et al., 1997). During development it is crucial for the proper formation of neural structures given that the deletion of either CXCR4 (Lu et al., 2002) or SDF-1 (Ara et al., 2003) has been shown to result in embryonic lethality. In the adult brain SDF-1 and CXCR4 continue to be expressed at high levels, particularly in the dentate gyrus and subventricular zone where they mediate the tropism of endogenous NSCs (Tran et al., 2007). The SDF-1/CXCR4 axis therefore has a significant role in neural development and subsequent functioning, and recent studies have displayed that it is also important in directing the migration of therapeutic stem cells toward regions of pathology in the diseased brain. SDF-1/CXCR4 signaling has been reported to promote the migration of stem cells to diseased areas within the brain by a number of studies (Zhou et al., 2002; Imitola et al., 2004; Robin et al., 2006; Carbajal et al., 2010). Ischemic, neoplastic, and demyelinating lesions have been observed to induce the secretion of SDF-1 by reactive astrocytes, microglia, and endothelial cells (Zhou et al., 2002; Imitola et al., 2004; Thored et al., 2006; Carbajal et al., 2010), and our laboratory has recently collected data suggesting that CXCR4 is expressed by NSCs and MSCs at high levels (Duebgen et al., unpublished observation). The importance of the interaction between secreted SDF-1 and cell surface CXCR4 for stem cell migration has been displayed by experiments in which their activity has been inhibited. The blockade of both CXCR4 (Imitola et al., 2004) and SDF-1 (Carbajal et al., 2010) in vivo in diseased mice has been found to markedly reduce the migration of transplanted NSCs toward tumor foci and regions of demyelination, indicating that SDF-1/CXCR4 signaling is essential for effective pathotropism of therapeutic stem cells.
The SDF-1/CXCR4 system mediates the tropism of both endogenous and therapeutic stem cells by promoting chain migration, the principal mode of stem cell movement in the mature brain (Imitola et al., 2004). During early embryonic development, primitive neural structures are formed through the migration of NSCs along scaffolds created by specialized radial glia (Noctor et al., 2001). Because the adult brain lacks such scaffolding, endogenous stem cells in the dentate gyrus and subventricular zone must therefore rely on chain migration, a process characterized by α and β integrin-mediated homotypic interactions between migrating NSCs and tube-like structures formed by specialized astrocytes (Lois and Alvarez-Buylla, 1994; Jacques et al., 1998). The migration of therapeutic stem cells proceeds in a similar fashion to that of endogenous NSCs in that the transplanted cells employ chain migration to reach regions of neuropathology (Aboody et al., 2000; Tang et al., 2003; Shah et al., 2005). Through signaling pathways such as the SDF-1/CXCR4 axis and the chain migration that they mediate, therapeutic stem cells are able to undergo pathology-directed tropism within the brain, a phenomenon that has been observed in several animal models of neurologic disease.
A number of studies have shown that therapeutic stem cells transplanted into the brains of tumor-bearing mice migrate specifically toward areas of neoplastic growth. In addition to homing to main tumor burdens, engrafted NSCs and MSCs have been observed to track and efficiently clear malignant deposits located outside of the principal tumor mass (Aboody et al., 2000; Tang et al., 2003; Shah et al., 2005, 2008; Lee et al., 2009; Sasportas et al., 2009). This makes therapeutic stem cells particularly attractive candidates for the treatment of glioblastoma multiforme, a highly aggressive form of brain cancer characterized by the deposition of microscopic clusters of cancer cells outside of the main tumor burden that preclude surgical re-section and limit the effectiveness of conventionally delivered chemotherapies. In order to treat glioblastoma multiforme and other neurologic malignancies stem cells have been genetically modified to express transgenes encoding proapoptotic (Shah et al., 2004, 2005, 2008; Sasportas et al., 2009), antiangiogenic (van Eekelen et al., 2010), immunostimulatory (Yang et al., 2004; Dickson et al., 2007; Frank et al., 2009), and prodrug-converting (Aboody et al., 2000) protein products. Our laboratory has shown that stem cells manipulated in such a manner retain their migratory capabilities (Shah et al., 2008; Sasportas et al., 2009; Hingtgen et al., 2010; van Eekelen et al., 2010) and are therefore able to deliver therapeutics directly to tumors. We have displayed this in a number of contexts, most recently with NSCs engineered to produce a novel variant of the antiangiogenic glycoprotein thrombospondin-1 (TSP-1). Transplantation of NSCs expressing TSP-1 into the brains of mice in the setting of malignant brain tumors resulted in markedly reduced tumor vessel density and the inhibition of tumor progression (van Eekelen et al., 2010). Our laboratory has also had great success with stem cells expressing a secretable form of tumor necrosis factor-related apoptosis-inducing ligand (S-TRAIL), a protein ligand that has been shown to preferentially target malignant cells while sparing normal brain tissue (Shah et al., 2005). We have found that both NSCs (Shah et al., 2008) and MSCs (Sasportas et al., 2009) engineered to secrete S-TRAIL are capable of tracking and efficiently clearing disseminated gliomas in mice. These results, taken together with those of the studies cited above, demonstrate the great promise of stem cell-mediated therapies for neoplastic lesions within the brain.
Stem cells engrafted into the brains of animal models of stroke and demyelinating disease have also been observed to migrate toward regions of ischemia and myelin depletion, respectively (Veizovic et al., 2001; Chu et al., 2003; Kurozumi et al., 2004). Many of the factors described above have been implicated in mediating this pathotropic migration, most notably SDF-1/CXCR4, which is known to have a role in the stem cell tropism that occurs in ischemia (Kelly et al., 2004). Recent work by Carbajal and colleagues has shown that the SDF-1/CXCR4 axis is also crucial to the pathotropic migration that takes place in demyelination. Through an elegant set of experiments involving SDF-1 and CXCR4 antagonism in the setting of myelin depletion, this group demonstrated that inhibition of CXCR4/SDF-1 signaling significantly reduced the migration and proliferation of engrafted stem cells as compared with unantagonized controls (Carbajal et al., 2010). Pathotropic migration, while essential for the effective treatment of these and other disorders via stem cells, is not sufficient to achieve a therapeutic outcome in ischemia and demyelinating disease. This is because, unlike in tumors, stem cells employed in these contexts must differentiate at sites of pathology in order to have an appreciable therapeutic effect.
STEM CELL DIFFERENTIATION
A number of studies investigating the fate of NSCs transplanted into the mammalian brain have shown that the extrinsic factors mainly drive their differentiation into either neurons or glia (Gage, 2000). The diseased adult brain appears to create an environment that stimulates endogenous and exogenous stem cells to differentiate into specific cell types. For example, both endogenous NSCs in the dentate gyrus and subventricular zone and those engrafted into the brain have been observed to preferentially undergo neurogenesis when subjected to ischemia (Zhang et al., 2001; Chu et al., 2003), thus replacing the cells most susceptible to ischemic insult. Similar differentiation to correct for the effects of pathology is evident in demyelinating lesions, where the majority of engrafted stem cells differentiate into oligodendrocytes resulting in the extensive remyelination of CNS axons (Pluchino et al., 2009; Carbajal et al., 2010). Contrary to what has been observed in ischemic and demyelinating lesions, therapeutic stem cells deployed into the brains of tumor-bearing mice remain in a quiescent and undifferentiated state (Aboody et al., 2000; Miletic et al., 2007; Shah et al., 2008; Sasportas et al., 2009; van Eekelen et al., 2010; Fig. 3). This failure of stem cells to differentiate when transplanted into the vicinity of neoplastic lesions has not been studied over long periods of time postengraftment; however, the results of the studies cited above strongly suggest that the tumor microenvironment simply lacks the factors necessary to promote stem cell differentiation into either neurons or glia.
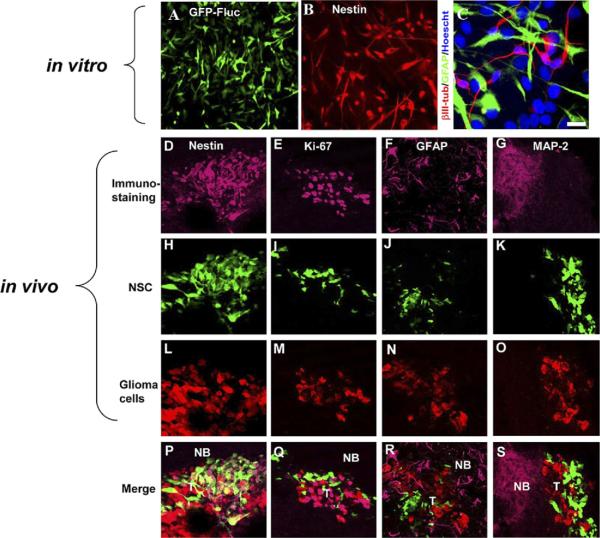
Fig. 3.
Engineered human (h) NSCs do not differentiate in vitro or in the mouse glioma model in vivo. (A–C) hNSCs were differentiated for 7 d, and immunocytochemistry was performed with nestin (B) and βIII-tubulin, and GFAP antibodies (C) and detected with CY3- or CY5-conjugated antibody. (D–S) hNSC-aaTSP-1 or control hNSC-GFP-Rluc were implanted in the close vicinity of established Gli36-EGFRvIII-FD gliomas. Representative images of brain sections of hNSC-aaTSP-1 mice killed on day 12 and immunostained with nestin, Ki67, GFAP, and mitogen-activated protein (MAP)-2 antibodies. Different panels showing the expression of tumor cells (red), hNSC (green), and nestin (D–E), Ki67 (H–J), GFAP (L–O), or MAP-2 (P–S) immunostaining (purple). Abbreviations: NB, normal brain; T, tumor. Adapted from Shah et al. (2008) and van Eekelen et al. (2010).
A number of growth and transcription factors have been implicated as mediators of endogenous NSC differentiation. Research into the neurogenesis that takes place during early brain development has shown that multiple basic helix-loop-helix (bHLH) genes play a critical role in regulating the differentiation of NSCs into neurons (Ross et al., 2003). The transcription factor products of these genes promote a neuronal fate by several mechanisms, including the activation of neuron-specific genes, the induction of exit from the cell cycle, and the inhibition of gliogenesis (Morrison, 2001; Shuurmans and Guillemot, 2002; Ross et al., 2003). There are two types of bHLH genes, the repressor type and the activator type. Repressor-type bHLH genes include the Hes genes which, in response to Notch signaling, antagonize activator-type bHLH genes resulting in the suppression of neuronal differentiation (Yoon and Gaiano, 2005). Activator-type bHLH genes include the Mash and Ngn families, which play an important role in promoting developmental neurogenesis. Activator-type and repressor-type bHLH genes regulate each other during brain development, allowing only subsets of cells to undergo differentiation and thus ensuring that others remain as NSCs. This precise regulation is essential for generation of complex brain structures of appropriate size, shape, and cellular arrangement.
During early brain development, astrocytes are produced in a second wave of differentiation, once most neurons have differentiated and migrated to their correct destinations within the developing brain (Gangemi et al., 2001). Effective astrocyte differentiation is dependent on Notch activation, which results in the downstream stimulation of Hes1 and Hes5. As discussed above, these bHLH transcription factors suppress neurogenesis, and in doing so, they indirectly promote the astroglial differentiation program (Hirabayashi and Gotoh, 2005). Signal transducer and activator of transcription (STAT3) signaling, which is responsible for the expression of GFAP (a biomarker for astrocytes) has also been implicated in astrogenesis although in a more direct manner. When stimulated by cytokine grown factors, ciliary neurotrophic factor (CNTF), and leukemia inhibitory factor (LIF), STAT3 has been shown to trans-locate to the nucleus of neural stem/progenitor cells where it mediates the expression of genes known to promote astrocyte differentiation including but not limited to GFAP (Moon et al., 2002; Hirabayashi and Gotoh, 2005). The generation of astrocytes therefore requires both the inhibition of proneural transcription factors and the direct stimulation of proastrocytic genes by downstream effectors of STAT3.
Another condition that is of vital importance to the early generation of astrocytes is the inhibition of neural stem/progenitor cell differentiation into oligodendrocyte progenitors that are committed to an oligodendral lineage. This suppression is accomplished through the activity of the TGF-β family growth factor, bone morphogenetic protein (BMP). In addition to its induction of transcription factors that promote the expression of astrocyte-specific genes (Fukuda and Taga, 2006), BMP is able to indirectly facilitate the differentiation of astrocytes by inhibiting that of NSCs into oligodendrocyte progenitors via a mechanism that is at present unclear (Kasai et al., 2005; See et al., 2007). Although not resulting in increased oligodendrogenesis, the loss of BMP signaling in bmpr double knockout mice has been observed to significantly reduce the differentiation of NSCs into astrocytes both in vitro and in vivo (See et al., 2007). This indicates that while BMP activity is essential for the effective development of astrocytes, its downregulation is not sufficient for oligodendrocyte differentiation.
Oligodendrocytes are among the latest cells to differentiate in the nervous system, although oligodendrocyte precursor (OPL) cells are specified very early during development (Thomas et al., 2000). The process of oligodendral differentiation is mediated primarily by sonic hedgehog (Shh) signaling, which promotes the expression of the Olig1 and Olig2 genes. The bHLH transcription factor products of these genes, Olig1 and Olig2, serve to commit NSCs to the oligodendrocyte developmental program (Lu et al., 2000; Zhou et al., 2002; Danesin et al., 2006). While Shh is the major promoter of developmental oligodendrogenesis, there is evidence that suggests that a Shh-independent pathway mediated by fibroblast growth factor 2 (FGF2) also plays a significant role. This was first reported by Chandran and colleagues, who found that treatment with FGF2 was able to induce the differentiation of NSCs into OPL cells despite cyclopamine-mediated inhibition of Shh signaling (Chandran et al., 2003). Through both the Shh-dependent and -independent differentiation pathways neural stem/progenitor cells are thus committed to an oligodendral lineage; however, other factors are required for the further development and maturation of these cells.
After the specification of an oligodendral fate by either Shh or FGF2 signaling, other factors are required to mediate the further maturation and differentiation of OPL cells. One family of genes that has been implicated in this are the Sox genes of the E class, which includes Sox8, Sox9, and Sox10. Loss-of-function experiments have shown that mice without functional copies of either Sox8 or Sox9 generate markedly reduced numbers of astrocytes and oligodendrocytes (Cheung and Briscoe, 2003; Stolt et al., 2004a). This suggests that the protein products of these genes are involved in specifying a glial fate. Sox10, which is expressed exclusively in the oligodendrocyte lineage, is essential for the maturation of committed OPL cells. This was also established through loss-of-function studies, in which Sox10 double knockout mice were found to have normal numbers of oligodendrocyte precursors that nevertheless were unable to mature into functioning oligodendrocytes (Stolt et al., 2004b). Through promoting gliogenesis and facilitating the maturation of OPL cells, genes of the Sox family are thus essential for the generation of oligodendrocytes and the development of the brain as a whole.
In order to differentiate into neurons and glia, endogenous stem cells in the developing brain rely on the factors discussed above as well as many others (reviewed by Wen et al., 2009). Exogenous stem cells transplanted into the mature brain have been found to respond to stroke and demyelination via some of the same mechanisms. Yandava and colleagues were among the first to demonstrate that that the engraftment of NSCs into the brains of myelin-depleted shiverer mice results in the significant remyelination and CNS axons (Yandava et al., 1999). More recent work has revealed that the use of stem cells genetically modified to express Shh (Liu et al., 2007) and Olig2 (Copray et al., 2006; Hwang et al., 2009) for this purpose allow for more efficient remyelination, presumably through inducing transplanted NSCs to undergo the oligodendral differentiation program. It is thus apparent that therapeutic stem cells engrafted into animal models of multiple sclerosis differentiate into oligodendrocytes by mechanisms similar to those employed by endogenous NSCs.
While stem cells transplanted into the brains of animal models of stroke are known to correct for the effects of ischemia and hemorrhage, the molecular mechanisms by which this occurs are not as clear as in demyelinating disease. NSCs and MSCs engrafted in the brains of rats subjected to middle cerebral artery (MCA) occlusion have been observed to improve neurological function versus untreated controls (Veizovic et al., 2001; Chu et al., 2003; Kurozumi et al., 2004). Similar restoration of function is evident in postintracerebral hemorrhage rats treated with immortalized NSCs (Lee et al., 2007) and bone marrow-derived MSCs (Nagai et al., 2007). In all of these studies functional restoration was attributed to the differentiation of the engrafted stem cells into neurons and astrocytes; however, the molecular mediators responsible for this differentiation are at present poorly understood.
The use of MSCs for the treatment of neural lesions has not been studied as extensively as that of NSCs. Despite this relative paucity of research into therapeutic MSCs, there is evidence suggesting that they have the ability to play a significant role in therapies for neurologic diseases both alone and in combination with NSCs. Recent work by Croft and colleagues has demonstrated that genetically engineered MSCs are able to influence differentiation patterns of NSCs in coculture. Specifically, MSCs expressing the neural antigens Tuj-1 and GFAP were observed to induce the differentiation of NSCs into neuronal and astrocytic lineages, respectively (Croft and Przyborski, 2009). These results suggest that similarly engineered MSCs may be used to fine-tune the differentiation of both endogenous and exogenous NSCs in the setting of neurologic dysfunction.
MSCs have definite potential to be used in an adjunctive capacity to NSCs; however, a number of studies have also shown that they also have the capability to be used therapeutically in the absence of cotransplanted NSCs. As cited above, MSCs have been observed to efficiently migrate to and effectively treat ischemic (Kurozumi et al., 2004), hemorrhagic (Nagai et al., 2007), and neoplastic (Lee et al., 2009; Sasportas et al., 2009) lesions in the mammalian brain. It is likely that this extensive therapeutic capability is at least in part due to the ability of exogenous MSCs to differentiate into neural stem-like cells, a phenomenon that has been reported by several groups (Deng et al., 2006; Kim et al., 2006; Yuan et al., 2006). The mechanisms underlying this “trans-differentiation” are at present poorly understood; however, recent work by Egea and colleagues has shown that tumor necrosis factor-α (TNF-α) is a powerful mediator of MSC differentiation into cells of a neural phenotype. In an elegant set of experiments this group demonstrated that long-term incubation of unaltered MSCs in low concentrations of TNF-α resulted in the development of morphologically astrocytic cells (Egea et al., 2011). Microarray analyses displayed that TNF-α induces the expression of many genes specifying a neural lineage, including GFAP, MAP2, LIF, BMP2, and SOX2 (Egea et al., 2011). Interestingly, markers of mature oligodendrocytes and neurons, such as galactocerebro-side and βIII-tubulin, respectively, were not expressed by treated cells, indicating that TNF-α specifically drives MSCs toward an astrocytic phenotype (Egea et al., 2011). The reason for this preference toward astrocytes is unclear, and while these results do represent a major step forward in terms of our understanding of exogenous stem cell differentiation, it is evident that a great deal of research is still needed before neural and mesenchymal stem cells can be used to their full therapeutic potential.
VISUALIZING MULTIPLE ASPECTS OF STEM CELL-BASED THERAPIES IN REAL TIME IN VIVO
It is quite obvious that before any potential human application of NSC-based therapies can be envisaged, knowledge of the location, temporospatial migration, and differentiating fate of transplanted modified NSCs will be of the utmost importance in analyzing mechanisms of correction and cell distribution. Initial studies from our laboratory have shown that NSC migration can be noninvasively followed in real time along their migratory path toward tumors after transplantation of NSC engineered with firefly luciferase (Fluc) in mouse models of glioma (Tang et al., 2003). In order to follow therapeutic NSC and changes in glioma burden in real time, we designed a study in which NSCs were genetically modified to express both S-TRAIL and Fluc and tumor cells were engineered to express Renilla luciferase (Rluc). The entire process of tumor formation, NSC migration, NSC dispersion throughout the tumor, and NSC killing of glioma cells was monitored noninvasively by dual bioluminescence imaging (Shah et al., 2005). In recent studies, we have shown tumor tracking of human NSCs and MSCs at a cellular resolution using hybrid (fluorescence and bioluminescence) reporter constructs and intravital imaging in vivo (Shah et al., 2008; Sasportas et al., 2009). This allows not only the imaging in real time of gross stem cell migration, but moreover to visualize tumor penetration by therapeutic stem cells at the single cell level. The above-mentioned capacities for tracking and monitoring of grafted NSC behavior, both longitudinally in a quantitative manner and qualitatively with high resolution, add greatly to our tool kit in the studying of stem cell biology and developing therapeutic strategy. Although stem cells are promising therapeutic delivery vehicles, pre-clinical and clinical applications of stem cell-based therapy would benefit significantly from the ability to simultaneously determine therapeutic efficacy and pharmacokinetics of therapies delivered by engineered stem cells. In a recent study, we have engineered and screened numerous fusion variants that contained therapeutic (TRAIL) and diagnostic (luciferase) domains designed to allow simultaneous investigation of multiple events in stem cell-based therapy in vivo. When various stem cell lines were engineered with the optimized molecule, SRLOL2TR, diagnostic imaging showed marked differences in the levels and duration of secretion between stem cell lines, while the therapeutic activity of the molecule showed the different secretion levels translated to significant variability in tumor cell killing (Hingtgen et al., 2010). In vivo, simultaneous diagnostic and therapeutic monitoring revealed that stem cell-based delivery significantly improved the pharmacokinetics and antitumor effectiveness of the therapy compared with i.v. or intratumoral delivery (Hingtgen et al., 2010).
Given the need for stem cell imaging modalities in larger animals or humans (where bioluminescent and fluorescent imaging is precluded by limited depth of tissue penetration), magnetic resonance imaging (MRI) and positron emission tomography (PET) have been evaluated for feasibility of stem cell tracking. NSCs tagged with ferromagnetic material or with gadolinium rhodamine dextran have been tracked using MRI (Brekke et al., 2007). In addition to the creation of new molecularly based imaging tools, the development of novel image analytical methods have also helped to improve understanding of the dynamics of stem cell-based brain tumor therapy.
CONCLUSIONS AND CLINICAL PERSPECTIVES
Stem cells have a tremendous potential to be used in the treatment of a variety of neurologic disorders. Transplantation experiments have shown that NSCs and MSCs are capable of migrating efficiently toward neurological lesions, where they have been observed to deliver disease-specific therapeutics and spontaneously repair damaged tissue. A detailed understanding of these mechanisms would be of great value in designing strategies to boost endogenous repair and engineer exogenous stem cells to express therapeutic transgenes. However, there are many questions that must be answered before they can be used in this capacity. The ability of NSCs to differentiate into neurons and oligodendrocytes appears to be an avenue by which stroke and multiple sclerosis may eventually be treated, but before this is possible a reliable source of NSCs must be found given that at present they cannot be derived from adult tissues. The in vitro manipulation of mature somatic cells to induce regression toward pluripotentiality and exploitation of the plasticity of MSCs have both been proposed as alternatives to using NSCs derived from human tissues. While reasonable, further research into the feasibility of these techniques is needed. Much more pressing questions involve the safety of transplanting genetically engineered stem cells into the human brain. As with other stem cell-mediated therapies for human disease, de novo tumor formation by transplanted stem cells is of definite concern. Historically, nonimmortalized stem cells have been used safely and effectively to treat various forms of leukemia through bone marrow transplantation, and many studies concerning the use of nonimmortalized stem cells in the treatment of neuropathology in mammals have found no evidence of de novo tumorigenesis. While these results are promising, more work is needed to ensure the safety of these novel therapies before they can be extensively studied in humans.
Abbreviations
- bHLH
basic helix-loop-helix
- BMP
bone morphogenetic protein
- CCR2
chemokine receptor 2
- CXCR4
chemokine receptor 4
- FGF2
fibroblast growth factor 2
- HGF
hepatocyte growth factor
- MCP-1
monocyte chemoattractant protein-1
- MSC
mesenchymal stem cell
- NSC
neural stem cell
- OPL
oligodendrocyte precursor
- SCF
stem cell factor
- SDF-1
stromal cell-derived factor 1
- Shh
sonic hedgehog
- S-TRAIL
secretable form of tumor necrosis factor-related apoptosis-inducing ligand
- TNF-α
tumor necrosis factor-α
- TSP-1
thrombospondin-1
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
REFERENCES
- Aboody KS, Brown A, Rainov NG, Bower KA, Shaoxiong L, Yang W, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ara T, Nakamura Y, Egawa T, Sugiyama T, Abe K, Kishimoto T, et al. Impaired colonization of the gonads by primordial germ cells in mice lacking a chemokine, stromal cell-derived factor-1 (SDF-1) Proc Natl Acad Sci U S A. 2003;100:5319–5323. doi: 10.1073/pnas.0730719100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantubungi K, Blum D, Cuvelier L, Wislet-Gendebien S, Rogister B, Brouillet B, et al. Stem cell factor and mesenchymal and neural stem cell transplantation in a rat model of Huntington's disease. Mol Cell Neurosci. 2008;37:454–470. doi: 10.1016/j.mcn.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Barker JN, Wagner JE. Umbilical-cord blood transplantation for the treatment of cancer. Nat Rev Cancer. 2003;3:526–532. doi: 10.1038/nrc1125. [DOI] [PubMed] [Google Scholar]
- Bartoli C, Civatte M, Pellissier JF, Figarella-Branger D. CCR2A and CCR2B, the two isoforms of the monocyte chemoattractant protein-1 receptor are up-regulated and expressed by different cell subsets in idiopathic inflammatory myopathies. Acta Neuropathol. 2001;102:385–392. doi: 10.1007/s004010100394. [DOI] [PubMed] [Google Scholar]
- Bates DO, Pritchard Jones RO. The role of vascular endothelial growth factor in wound healing. Int J Low Extrem Wounds. 2003;2:107–120. doi: 10.1177/1534734603256626. [DOI] [PubMed] [Google Scholar]
- Bergeron M, Yu AY, Solway KE, Semenza GL, Sharp FR. Induction of hypoxia-inducible factor-1 (HIF-1) and its target genes following focal ischemia in rat brain. Eur J Neurosci. 1999;11:4159–4170. doi: 10.1046/j.1460-9568.1999.00845.x. [DOI] [PubMed] [Google Scholar]
- Brekke C, Williams SC, Price J, Thorsen F, Modo M. Cellular multiparametric MRI of neural stem cell therapy in a rat glioma model. Neuroimage. 2007;37:769–782. doi: 10.1016/j.neuroimage.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Carbajal KS, Schaumburg C, Strieter R, Kane J, Lane TE. Migration of engrafted neural stem cells is mediated by CXCL2 signaling through CXCR4 in a viral model of multiple sclerosis. Proc Natl Acad Sci U S A. 2010;107:11068–11073. doi: 10.1073/pnas.1006375107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran S, Kato H, Gerreli D, Compston A, Svendsen CN, Allen ND. FGF-dependent generation of oligodendrocytes by a hedgehog-independent pathway. Development. 2003;130:6599–6609. doi: 10.1242/dev.00871. [DOI] [PubMed] [Google Scholar]
- Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci U S A. 1994;91:2752–2756. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung M, Briscoe J. Neural crest development is regulated by the transcription factor Sox9. Development. 2003;130:5681–5693. doi: 10.1242/dev.00808. [DOI] [PubMed] [Google Scholar]
- Chu K, Kim M, Jeong SW, Kim SU, Yoon BW. Human neural stem cells can migrate, differentiate and integrate after intravenous transplantation in adult rats with transient forbrain ischemia. Neurosci Lett. 2003;343:637–643. doi: 10.1016/s0304-3940(03)00174-5. [DOI] [PubMed] [Google Scholar]
- Copray S, Balasubramaniyan V, Levenga J, de Bruijn J, Liem R, Boddeke E. Olig2 overexpression induces the in vitro differentiation of neural stem cells into mature oligodendrocytes. Stem Cells. 2006;24:1001–1010. doi: 10.1634/stemcells.2005-0239. [DOI] [PubMed] [Google Scholar]
- Corsten MF, Shah K. Therapeutic stem-cells for cancer treatment: hopes and hurdles in tactical warfare. Lancet Oncol. 2008;9:376–384. doi: 10.1016/S1470-2045(08)70099-8. [DOI] [PubMed] [Google Scholar]
- Croft AP, Przyborski SA. Mesenchymal stem cells expressing neural antigens instruct a neurogenic cell fate on neural stem cells. Exp Neurol. 2009;216:329–341. doi: 10.1016/j.expneurol.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Daadi MM, Li Z, Arac A, Grueter BA, Sofilos M, Malenka RC, et al. Molecular and magnetic resonance imaging of human embryonic stem cell-derived neural stem cell grafts in ischemic rat brain. Mol Ther. 2009;17:1282–1291. doi: 10.1038/mt.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesin C, Agius E, Escalas N, Ai XB, Emerson C, Cochard P, et al. Ventral neural progenitors switch toward an oligodendroglial fate in response to increased Sonic hedgehog (Shh) activity: involvement of sulfatase 1 in modulating Shh signaling in the ventral spinal cord. J Neurosci. 2006;26:5037–5048. doi: 10.1523/JNEUROSCI.0715-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Petersen BE, Steindler DA, Jorgensen ML, Laywell ED. Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells. 2006;24:1054–1064. doi: 10.1634/stemcells.2005-0370. [DOI] [PubMed] [Google Scholar]
- Dentelli P, Rosso A, Balsamo A, Benedetto SC, Zeoli A, Pegoraro M, et al. c-kit, by interacting with the membrane-bound ligand, recruits endothelial progenitor cells to inflamed endothelium. Blood. 2007;109:4264–4271. doi: 10.1182/blood-2006-06-029603. [DOI] [PubMed] [Google Scholar]
- Dickson PV, Hamner JB, Burger RA, Garcia E, Ouma AA, Kim SU, et al. Intravascular administration of tumor tropic neural progenitor cells permits targeted delivery of interferon-beta and restricts tumor growth in murine model of disseminated neuroblastoma. J Pediatr Surg. 2007;42:48–53. doi: 10.1016/j.jpedsurg.2006.09.050. [DOI] [PubMed] [Google Scholar]
- Egea V, von Baumgarten L, Schichor C, Berninger B, Popp T, Neth P, et al. TNF-α respecifies human mesenchymal stem cells to a neural fate and promotes migration toward experimental glioma. Cell Death Differ. 2011;18:853–863. doi: 10.1038/cdd.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehtesham M, Winston JA, Kabos P, Thompson RC. CXCR4 expression mediates glioma cell invasiveness. Oncogene. 2006;25:2801–2806. doi: 10.1038/sj.onc.1209302. [DOI] [PubMed] [Google Scholar]
- Esencay M, Newcomb EW, Zagzag D. HGF upregulates CXCR4 expression in gliomas via NF-kappaB: implications for glioma cell migration. J Neurooncol. 2010;99:33–40. doi: 10.1007/s11060-010-0111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazel SS, Chen L, Angoulvant D, Li SH, Weisel RD, Keating A, et al. Activation of c-kit is necessary for mobilization of reparative bone marrow progenitor cells in response to cardiac injury. FASEB J. 2008;22:930–940. doi: 10.1096/fj.07-8636com. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carvermoore K, Chen H, Dowd M, Lu L, Oshea KS, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Houck KA, Jakeman LB, Leung DW. Molecular and biological properties of vascular endothelial growth factor family of proteins. Endocr Rev. 1992;13:18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- Frank RT, Edmiston M, Kendal SE, Najbauer J, Cheung CW, Kassa T, et al. Neural stem cells as a novel platform for tumor-specific delivery of therapeutic antibodies. PLoS One. 2009;15:e8314. doi: 10.1371/journal.pone.0008314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes ME, Durham SK, Swerdel MR, Lewin AC, Barton DS, Megill JR, et al. Controlled recruitment of monocytes and macrophages to specific organs through transgenic expression of monocyte chemoattractant protein-1. J Immunol. 1995;155:5769–5776. [PubMed] [Google Scholar]
- Fukuda S, Taga T. Roles of BMP in the development of the central nervous system. Clin Calcium. 2006;16:781–785. [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gangemi RM, Daga A, Marubbi D, Rosatto N, Capra MC, Corte G. Emx2 in adult neural precursor cells. Mech Dev. 2001;109:323–329. doi: 10.1016/s0925-4773(01)00546-9. [DOI] [PubMed] [Google Scholar]
- Garzotto D, Giacobini P, Crepaldi T, Fasolo A, De Marchis S. Hepatocyte growth factor regulates migration of olfactory interneuron precursors in the rostral migratory stream through Met-Grb2 coupling. J Neurosci. 2008;28:5901–5909. doi: 10.1523/JNEUROSCI.1083-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn MD, Nelken NA, Liao X, Williams LT. MCP-1 is sufficient for the chemotaxis of monocytes and lymphocytes in transgenic mice but requires an additional stimulus for inflammatory activation. J Immunol. 1997;158:376–383. [PubMed] [Google Scholar]
- Heese O, Disko A, Zirkel D, Westphal M, Lamszus K. Neural stem cell migration toward gliomas in vitro. Neuro Oncol. 2005;7:476–484. doi: 10.1215/S1152851704000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingtgen SD, Kasmieh R, van de Water J, Weissleder R, Shah K. A novel molecule integrating therapeutic and diagnostic activities reveals multiple aspects of stem cell-based therapy. Stem Cells. 2010;28:832–841. doi: 10.1002/stem.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi Y, Gotoh Y. Stage-dependent fate determination of neural precursor cells in mouse forebrain. Neurosci Res. 2005;51:331–344. doi: 10.1016/j.neures.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Hobara N, Yoshida N, Goda M, Yokomizo A, Kitamura Y, Sendou T, et al. Neurotrophic effect of hepatic growth factor (HGF) on reinnervation of perivascular calcitonin gene-related peptide (CGRP)-containing nerves following phenol-induced nerve injury. J Pharmacol Sci. 2008;108:495–504. doi: 10.1254/jphs.08225fp. [DOI] [PubMed] [Google Scholar]
- Huang PH, Chen YH, Wang CH, Chen JS, Tsai HY, Lin FY, et al. Matrix metalloproteinase-9 is essential for ischemia-induced neovascularization by modulating bone marrow-derived endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2009;29:1179–1184. doi: 10.1161/ATVBAHA.109.189175. [DOI] [PubMed] [Google Scholar]
- Hwang DH, Kim BG, Kim EJ, Lee SI, Joo IS, Suh-Kim H, et al. Transplantation of human neural stem cells transduced with Olig2 transcription factor improves locomotor recovery and enhances myelination in the white matter of rat spinal cord following contusive injury. BMC Neurosci. 2009;19:117. doi: 10.1186/1471-2202-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1α/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques TS, Relvas JB, Nishimura S, Pytela R, Edwards GM, Streuli CH, et al. Neural precursor cell chain migration and division are regulated through different β1 integrins. Development. 1998;125:3167–3177. doi: 10.1242/dev.125.16.3167. [DOI] [PubMed] [Google Scholar]
- Jazin EE, Soderstrom S, Ebendal T, Larhammar D. Embryonic expression of the mRNA for the rat homologue of the fusion/CXCR-4 HIV-1 co-receptor. J Neuroimmunol. 1997;79:148–154. doi: 10.1016/s0165-5728(97)00117-3. [DOI] [PubMed] [Google Scholar]
- Jensen RL, Ragel B, Whang K, Gillepsie D. Inhibition of hypoxia inducible factor-1a decreases vascular endothelial growth factor (VEGF) secretion and tumor growth in malignant gliomas. J Neurooncol. 2006;78:233–247. doi: 10.1007/s11060-005-9103-z. [DOI] [PubMed] [Google Scholar]
- Jeong SW, Chu K, Kim MH, Kim SU, Roh JK. Human neural stem cell transplantation in experimental intracerebral hemorrhage. Stroke. 2003;34:2258–2263. doi: 10.1161/01.STR.0000083698.20199.1F. [DOI] [PubMed] [Google Scholar]
- Kasai M, Satoh K, Akiyama T. Wnt signaling regulates the sequential onset of neurogenesis and gliogenesis via induction of BMPs. Genes Cells. 2005;10:777–783. doi: 10.1111/j.1365-2443.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci U S A. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DE, Schellingerhout D, Ishii K, Shah K, Weissleder R. Imaging of stem cell recruitment to ischemic infarcts in a murine model. Stroke. 2004;35:952–957. doi: 10.1161/01.STR.0000120308.21946.5D. [DOI] [PubMed] [Google Scholar]
- Kim S, Honmou O, Kato K, Nonaka T, Houkin K, Hamada H, et al. Neural differentiation potential of peripheral blood- and bone-marrow-derived precursor cells. Brain Res. 2006;1123:27–33. doi: 10.1016/j.brainres.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Kobune M, Hirai S, et al. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther. 2004;9:189–197. doi: 10.1016/j.ymthe.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Lavi E, Strizki JM, Ulrich AM, Zhang W, Fu L, Wang Q, et al. CXCR-4 (Fusin), a co-receptor for the type 1 human immunodeficiency virus (HIV-1), is expressed in the human brain in a variety of cell types, including microglia and neurons. Am J Pathol. 1997;151:1035–1042. [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Ahn Y, Kim SU, Wang KC, Cho BY, Phi JH, et al. Targeting rat brainstem glioma using human neural stem cells and human mesenchymal stem cells. Clin Cancer Res. 2009;15:4925–4934. doi: 10.1158/1078-0432.CCR-08-3076. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kim KS, Kim EJ, Choi HB, Lee KH, Park IH, et al. Brain transplantation of human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells. 2007;25:1204–1212. doi: 10.1634/stemcells.2006-0409. [DOI] [PubMed] [Google Scholar]
- Liu Z, Hu X, Cai J, Liu B, Peng X, Wegner M, et al. Induction of oligodendrocyte differentiation by Olig2 and Sox10: evidence for reciprocal interactions and dosage-dependent mechanisms. Dev Biol. 2007;302:683–693. doi: 10.1016/j.ydbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc Natl Acad Sci U S A. 2002;99:7090–7095. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu QR, Yuk D, Alberta JA, Zhu Z, Pawlitzky I, Chan J, et al. Sonic hedgehog-regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- Lundberg JJ, Mollgard K. Mitotic activity in adult rat brain induced by implantation of pieces of fetal rat brain and liver. Neurosci Lett. 1979;13:265–270. doi: 10.1016/0304-3940(79)91505-2. [DOI] [PubMed] [Google Scholar]
- Magge SN, Malik SZ, Royo NC, Chen HI, Yu L, Snyder EY, et al. Role of monocyte chemoattractant protein-1 (MCP-1/CCL2) in migration of neural progenitor cells toward gliomas. J Neurosci Res. 2009;87:1547–1555. doi: 10.1002/jnr.21983. [DOI] [PubMed] [Google Scholar]
- Miletic H, Fischer Y, Litwak S, Giroglou T, Waerzeggers Y, Winkeler A, et al. Bystander killing of malignant glioma by bone marrow-derived tumor-infiltrating progenitor cells expressing a suicide gene. Mol Ther. 2007;15:1373–1381. doi: 10.1038/sj.mt.6300155. [DOI] [PubMed] [Google Scholar]
- Moon C, Yoo JY, Matarazzo V, Sung YK, Kim EJ, Ronnett GV. Leukemia inhibitory factor inhibits neuronal terminal differentiation through STAT3 activation. Proc Natl Acad Sci U S A. 2002;99:9015–9020. doi: 10.1073/pnas.132131699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ. Neuronal potential and lineage determination by neural stem cells. Curr Opin Cell Biol. 2001;13:666–672. doi: 10.1016/s0955-0674(00)00269-6. [DOI] [PubMed] [Google Scholar]
- Nagai A, Kim WK, Lee J, Jeong HS, Kim KS, Hong SH, et al. Multilineage potential of stable human mesenchymal stem cell line derived from fetal marrow. PLoS One. 2007;2:e1272. doi: 10.1371/journal.pone.0001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Nawa K, Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984;122:1450–1459. doi: 10.1016/0006-291x(84)91253-1. [DOI] [PubMed] [Google Scholar]
- Nicoleau C, Benzakour O, Agasse F, Thiriet N, Petit J, Prestoz L, et al. Endogenous hepatocyte growth factor is a niche signal for subventricular zone neural stem cell amplification and self-renewal. Stem Cells. 2009;27:408–419. doi: 10.1634/stemcells.2008-0226. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Brini E, Ferrari S, Martino G. Regeneration and repair in multiple sclerosis: the role of cell transplantation. Curr Opin Neurol. 2009;21:607–614. doi: 10.1016/j.neulet.2008.03.097. [DOI] [PubMed] [Google Scholar]
- Robin AM, Zhang ZG, Wang L, Zhang RL, Katakowski M, Zhang L, et al. Stromal cell-derived factor-1 alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:125–134. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- Ross SE, Greenberg ME, Stiles CD. Basic helix-loop-helix factors in cortical development. Neuron. 2003;39:13–25. doi: 10.1016/s0896-6273(03)00365-9. [DOI] [PubMed] [Google Scholar]
- Ryu JK, Kim J, Cho SJ, Hatori K, Nagai A, Choi HB, et al. Proactive transplantation of human neural stem cells blocks neuronal cell death in rat model of Huntington disease. Neurobiol Dis. 2004;16:68–77. doi: 10.1016/j.nbd.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Sasportas LS, Kasmieh R, Wakimoto H, Hingtgen S, van de Water JA, Mohapatra G, et al. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci U S A. 2009;106:4822–4827. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt NO, Koeder D, Messing M, Mueller FJ, Aboody KS, Kim SU, et al. Vascular endothelial growth factor-stimulated cerebral microvascular endothelial cells mediate the recruitment of neural stem cells to the neurovascular niche. Brain Res. 2009;1268:24–37. doi: 10.1016/j.brainres.2009.02.065. [DOI] [PubMed] [Google Scholar]
- Shah K, Bureau E, Kim DE, Yang K, Tang Y, Weissleder R, et al. Glioma therapy using a novel TRAIL secreting neural stem cells (NSCs) and in vivo tracking of NSC migration and tumor regression. Ann Neurol. 2005;57:34–41. doi: 10.1002/ana.20306. [DOI] [PubMed] [Google Scholar]
- See J, Mamontov P, Ahn K, Crenshaw EB, Grinspan JB. BMP signaling mutant mice exhibit glial cell maturation defects. Mol Cell Neurosci. 2007;35:171–182. doi: 10.1016/j.mcn.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K, Hingtgen S, Kasmieh R, Figueiredo JL, Garcia-Garcia E, Martinez-Serrano A, et al. Bimodal viral vectors and in vivo imaging reveal the fate of human neural stem cells in experimental glioma model. J Neurosci. 2008;28:4406–4413. doi: 10.1523/JNEUROSCI.0296-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K, Tung CH, Yang K, Weissleder R, Breakefield XO. Inducible release of TRAIL fusion proteins from proapoptotic form for tumor therapy. Cancer Res. 2004;64:3236–3242. doi: 10.1158/0008-5472.can-03-3516. [DOI] [PubMed] [Google Scholar]
- Shuurmans C, Guillemot F. Molecular mechanisms underlying cell fate specification in the developing telencephalon. Curr Opin Neurobiol. 2002;12:26–34. doi: 10.1016/s0959-4388(02)00286-6. [DOI] [PubMed] [Google Scholar]
- Snyder EY, Taylor RM, Wolfe JH. Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature. 1995;374:367–370. doi: 10.1038/374367a0. [DOI] [PubMed] [Google Scholar]
- Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–1264. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- Souyima H, Fukumitsu H, Furukawa S. Stem cell factor induces heterotopic accumulation of cells in the mouse cerebral cortex. Biomed Res. 2009;30:121–128. doi: 10.2220/biomedres.30.121. [DOI] [PubMed] [Google Scholar]
- Stevenson CB, Ehtesham M, McMillan KM, Valadez JG, Edgeworth ML, Price RR, et al. CXCR4 expression is elevated in glioblastoma multiforme and correlates with an increase in intensity and extent of peritumoral T2-weighted magnetic resonance imaging signal abnormalities. Neurosurgery. 2008;63:560–569. doi: 10.1227/01.NEU.0000324896.26088.EF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt CC, Lommes P, Friedrich RP, Wegner M. Transcription factors Sox8 and Sox10 perform non-equivalent roles during oligodendrocyte development despite functional redundancy. Development. 2004a;131:2349–2358. doi: 10.1242/dev.01114. [DOI] [PubMed] [Google Scholar]
- Stolt CC, Rehberg S, Ader M, Lommes P, Riethmacher D, Schachner M, et al. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 2004b;16:165–170. doi: 10.1101/gad.215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Lee J, Fine HA. Neuronally expressed stem cell factor induces neural stem cell migration to areas of brain injury. J Clin Invest. 2004;113:1364–1374. doi: 10.1172/JCI20001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Shah K, Messerili SM, Snyder E, Breakefield X, Weissleder R. In vivo tracking of neural progenitor cell migration in glioblastomas. Hum Gene Ther. 2003;14:1247–1254. doi: 10.1089/104303403767740786. [DOI] [PubMed] [Google Scholar]
- Thomas JL, Spassky N, Perez Z, Olivier C, Cobos I, Goujet-Zalc C, et al. Spatiotemporal development of oligodendrocytes in the embryonic brain. J Neurosci. 2000;59:471–476. doi: 10.1002/(SICI)1097-4547(20000215)59:4<471::AID-JNR1>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J Comp Neurol. 2007;500:1007–1033. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S, Mizuki M, Ikeda H, Tsujimura T, Matsumura I, Nakano K, et al. Critical roles of c-kit tyrosine residues 567 and 719 in stem cell factor-induced chemotaxis; contribution of src family kinase and PI3-kinase on calcium mobilization and cell migration. Blood. 2002;99:3342–3349. doi: 10.1182/blood.v99.9.3342. [DOI] [PubMed] [Google Scholar]
- van Eekelen M, Sasportas LS, Kasmieh R, Yip S, Figueiredo JL, Louis DN, et al. Human stem cells expressing novel TSP-1 variant have anti-angiogenic effect on brain tumors. Oncogene. 2010;29:3185–3195. doi: 10.1038/onc.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veizovic T, Beech JS, Stroemer RP, Watson WP, Hodges H. Resolution of stroke deficits following contralateral grafts of conditionally immortal neuroepithelial stem cells. Stroke. 2001;32:1012–1019. doi: 10.1161/01.str.32.4.1012. [DOI] [PubMed] [Google Scholar]
- Wen S, Hong L, Liu J. Dynamic signaling for neural stem cell fate determination. Cell Adh Migr. 2009;3:107–117. doi: 10.4161/cam.3.1.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widera D, Holtkamp W, Entschladen F, Niggemann B, Zänker K, Kaltschmidt B, et al. MCP-1 induces migration of adult neural stem cells. Eur J Cell Biol. 2004;83:381–387. doi: 10.1078/0171-9335-00403. [DOI] [PubMed] [Google Scholar]
- Xu Q, Wang S, Jiang X, Zhao Y, Gao M, Zhang Y, et al. Hypoxia-induced astrocytes promote the migration of neural progenitor cells via vascular endothelial factor, stem cell factor, stromal-derived factor-1a and monocyte chemoattractant protein-1 up-regulation in vitro. Clin Exp Pharmacol Physiol. 2007;34:624–631. doi: 10.1111/j.1440-1681.2007.04619.x. [DOI] [PubMed] [Google Scholar]
- Yandava B, Billinghurst L, Snyder EY. Global cell replacement is feasible via neural stem cell transplantation: evidence from the dysmyelinated shiverer mouse brain. Proc Natl Acad Sci U S A. 1999;96:7029–7034. doi: 10.1073/pnas.96.12.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SY, Liu H, Zhang JN. Gene therapy of rat malignant gliomas using neural stem cells expressing IL-12. DNA Cell Biol. 2004;23:381–389. doi: 10.1089/104454904323145263. [DOI] [PubMed] [Google Scholar]
- Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat Neurosci. 2005;8:709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- Yuan X, Hu J, Belladonna ML, Black KL, Yu JS. Interleukin-23-expressing bone marrow-derived neural stem-like cells exhibit antitumor activity against intracranial glioma. Cancer Res. 2006;66:2630–2638. doi: 10.1158/0008-5472.CAN-05-1682. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Zhao D, Najbauer J, Garcia E, Metz MZ, Gutova M, Glackin CA, et al. Neural stem cell tropism to glioma: critical role of tumor hypoxia. Mol Cancer Res. 2008;6:1819–1829. doi: 10.1158/1541-7786.MCR-08-0146. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Larsen PH, Hao C, Yong VW. CXCR4 is a major chemokine receptor on glioma cells and mediates their survival. J Biol Chem. 2002;227:49481–49487. doi: 10.1074/jbc.M206222200. [DOI] [PubMed] [Google Scholar]