Abstract
Hereditary pancreatitis shares a majority of clinical and morphologic features with chronic alcoholic pancreatitis, but may present at an earlier age. The term hereditary pancreatitis has primarily been associated with mutations in the serine protease 1 gene (PRSS1) which encodes for cationic trypsinogen. PRSS1 mutations account for approximately 68–81% of hereditary pancreatitis. Mutations in other genes, primarily serine protease inhibitor Kazal type 1 (SPINK1) and the cystic fibrosis transmembrane conductance regulator (CFTR) are also associated with hereditary pancreatitis. While chronic alcoholic pancreatitis may develop in the fourth or fifth decades, patients with hereditary pancreatitis may develop symptoms in the first or second decades of life. Hereditary pancreatitis is diagnosed either by detecting a causative gene mutation or by the presence of chronic pancreatitis in two first-degree or three second-degree relatives, in two or more generations, without precipitating factors and with a negative workup for known causes. Patients with hereditary pancreatitis may have recurrent acute pancreatitis and may develop pancreatic exocrine and endocrine insufficiency. Hereditary pancreatitis may involve premature trypsinogen activation or decreased control of trypsin. Recurrent inflammation can lead to acute pancreatitis and subsequently to chronic pancreatitis with parenchymal calcification. There is a markedly increased risk of pancreatic carcinoma compared with the general population. Patients are often referred for evaluation of pancreatitis, biliary or pancreatic ductal dilatation, jaundice, biliary obstruction, pancreatic duct stone or stricture, pancreatic pseudocysts, and for evaluation for malignancy. Medical treatment includes pancreatic enzyme supplementation, nutritional supplementation, diabetes management, and palliation of pain. Patients should avoid tobacco use and alcohol exposure. Hereditary pancreatitis is reviewed and recommendations for genetic testing are discussed.
Keywords: chronic pancreatitis, cystic fibrosis, endoscopic surgical procedures, familial pancreatitis, hereditary pancreatitis, idiopathic pancreatitis, pancreatitis
Introduction
Hereditary pancreatitis is a rare disorder which is sometimes considered in the evaluation of patients with pancreatitis who do not have a history of heavy alcohol exposure and have had negative testing for biliary disease such as gallstones. This is a heterogeneous group of patients for whom a careful evaluation will reveal multiple etiologies for pancreatitis, such as microlithiasis, surreptitious alcohol exposure, hypertriglyceridemia, malignancy, autoimmune pancreatitis, pancreas divisum, pancreatic and biliary sphincter dysfunction, and adverse medication reactions. A subset of patients may have a genetic predisposition to recurrent acute or chronic pancreatitis. The term hereditary pancreatitis is used to describe patients with genetic mutations which predispose them to pancreatitis. Cationic trypsinogen is encoded by the serine protease 1 gene (PRSS1), and PRSS1 mutations account for approximately 68–81% of patients with hereditary pancreatitis [Howes et al. 2004; Rebours et al. 2009b]. Mutations in the serine protease inhibitor Kazal type 1 (SPINK1) gene and cystic fibrosis transmembrane conductance regulator (CFTR) gene are also associated with pancreatitis and account for a subset of cases (Table 1). Compared with alcoholic chronic pancreatitis, patients with hereditary pancreatitis may develop manifestations earlier in life and may become symptomatic in their first or second decades [Comfort and Steinberg, 1952]. Frequent attacks of pancreatitis with increased or recurrent pancreatic inflammation may lead to chronic pancreatitis and pancreatic parenchymal calcifications. There is a markedly increased lifetime risk of pancreatic cancer. Hereditary pancreatitis is diagnosed either by detecting a causative gene mutation such as PRSS1 or by the presence of chronic pancreatitis in two first-degree or in three second-degree relatives, in two or more generations, without precipitating factors and with a negative workup for known causes of chronic pancreatitis.
Table 1.
Common mutations in patients with hereditary pancreatitis.
| Gene | Mechanism of action | Inheritance |
|---|---|---|
| PRSS1 | Mutations in the serine protease 1 gene (PRSS) which encodes cationic trypsinogen can lead to increased trypsin activity, increased trypsin stability, and increased autoactivation | Autosomal dominant |
| SPINK1 | SPINK1 encodes a pancreatic secretory trypsin inhibitor, mutations interfere with the protective function and predispose a person to pancreatitis possibly via increased intrapancreatic trypsin activity | Complex inheritance pattern |
| CFTR | Mutation causes a defect in the CFTR protein which causes abnormal sodium and chloride transport, leading to defective pancreatic secretion | Autosomal recessive |
SPINK1, serine protease inhibitor Kazal type 1; CFTR, cystic fibrosis transmembrane conductance regulator.
Clinical presentation
Patients with hereditary pancreatitis may present clinically with recurrent bouts of acute pancreatitis in the first two decades of life. Progression to chronic pancreatitis may occur in the late teenage years and early adult life. Symptoms are typical of acute pancreatitis from other etiologies, including abdominal pain, nausea and vomiting. As damage to the pancreas progresses, malabsorption may occur due to pancreatic insufficiency, and diabetes mellitus may develop due to pancreatic islet cell damage. Patients may ultimately require insulin for glucose homeostasis. Morphologic changes of chronic pancreatitis may develop and lead to presentations with biliary obstruction, pancreatic calcification, pancreatic duct stone formation, pancreatic duct stricture, and peri-pancreatic pseudocyst formation. The most common overall presentation of hereditary pancreatitis is abdominal pain and acute pancreatitis [Sibert, 1978; Sossenheimer et al. 1997; Howes et al. 2004; Rebours et al. 2009b]. Patients with hereditary pancreatitis may endure numerous hospitalizations with chronic pain and diagnostic uncertainty. They may have frequent contacts with the healthcare system, and may have long delays in time to diagnosis. In addition to their medical issues, patients may face emotional, psychological, and financial burdens.
Two large studies evaluated the clinical features in patients with hereditary pancreatitis. One study evaluated 418 patients meeting genetic or clinical criteria for hereditary pancreatitis from 112 different families in 14 countries [Howes et al. 2004] and another study examined 200 patients meeting clinical criteria for hereditary pancreatitis or with PRSS1 mutations in 78 families in France [Rebours et al. 2009b]. The median age at diagnosis was 10 and 19 years respectively. Patients with the R122H mutation of the PRSS1 gene may have an earlier onset and more severe presentation [Howes et al. 2004]. Pancreatic-type pain, acute pancreatitis, and psuedocysts were found in 83%, 69%, and 23% of hereditary pancreatitis patients respectively. By imaging, pancreatic calcifications were seen in 61% of patients [Rebours et al. 2009b]. Pancreatic exocrine insufficiency developed in 34–37% of patients with a median age of onset of 29 years. Diabetes mellitus was found in 26–32% of patients [Rebours et al. 2009b; Howes et al. 2004]. The median age of onset of diabetes was 38, and 60% of these patients required insulin [Rebours et al. 2009b]. Between acute episodes, patients may remain asymptomatic for extended periods of time before recurrence. Other patients may present with chronic pancreatitis without known prior episodes of recurrent acute pancreatitis.
History
Hereditary pancreatitis was first described in 1952 with early onset chronic pancreatitis found in three generations [Comfort and Steinberg, 1952]. A hereditary pancreatitis genealogy study with 249 members from one family over eight generations from 1800 to 1993 determined that 63 had definite and 17 had probable hereditary pancreatitis. There was an autosomal dominant inheritance pattern with a penetrance of 80% [Le Bodic et al. 1996b]. Genetic linkage analysis found an association between hereditary pancreatitis and the long arm of chromosome 7 (7q35) [Le Bodic et al. 1996a; Whitcomb et al. 1996b]. Mutational analysis of candidate genes identified a genetic defect in the cationic trypsinogen serine protease 1 gene known as PRSS1. The affected patients were found to carry a G to A transition in exon 3 of the PRSS1 gene, which led to a substitution of arginine to histidine in codon 122. This is known as the R122H mutation [Whitcomb et al. 1996a]. Many more mutations have subsequently been reported.
Definition
Hereditary pancreatitis is diagnosed either by detecting a causative gene mutation such as PRSS1 or by the presence of chronic pancreatitis in two first-degree or three second-degree relatives, in two or more generations, without precipitating factors and with a negative workup for known causes of chronic pancreatitis.
Genetics
PRSS1 gene
The most common cause of hereditary pancreatitis is a mutation in the PRSS1 gene, which encodes cationic trypsinogen (Figure 1). Studies of patients with hereditary pancreatitis detected mutations in the PRSS1 gene in 68–81% of patients [Howes et al. 2004; Rebours et al. 2009b].The most common of the PRSS1 gene mutations in those patients were R122H (78%) and N29I (12%) [Rebours et al. 2009b]. Multiple other mutations and nucleotide changes/genetic variants involving PRSS1 have been discovered and can be found at the following website: www.uni-leipzig.de/pancreasmutation
Figure 1.

An axial post contrast image from a magnetic resonance imaging (MRI) scan in a 59-year-old woman with a history of recurrent acute pancreatitis found to have a serine protease 1 gene (PRSS1) mutation on sequencing. Her MRI revealed a mildly atrophic pancreas without changes of acute pancreatic inflammation.
Cationic trypsin is the most abundant form of trypsin produced by the pancreas and acts as a catalyst for the conversion of pancreatic zymogens into pancreatic digestive enzymes. Acute pancreatitis may occur due to premature activation of digestive enzymes in the pancreas. Physiologic protective mechanisms include prevention of premature conversion of trypsinogen to trypsin and autodigestion or inhibition of trypsin within the pancreas. Autodigestion of trypsin is linked to a particular site of the protein in the R122 position. Thus, when mutated, there are increased levels of trypsin, increased trypsin stability, zymogen stability and increased autoactivation, predisposing patients to pancreatitis [Sahin-Toth, 2000; Sahin-Toth and Toth, 2000]. The N29I mutation is the second most common PRSS1 mutation and appears to result in increased levels of autoactivation [Sahin-Toth and Toth, 2000]. Other mutations effect trypsinogen in different ways; for example, the R122 mutation stabilizes trypsinogen, protecting against autocatalytic degradation [Sahin-Toth, 1999]. The R116C mutation causes misfolding leading to intracellular retention of cationic trypsinogen [Kereszturi et al. 2009], and both D22G and K23R increase trypsin release from trypsinogen [Teich et al. 2000].
Hereditary pancreatitis patients with PRSS1 gene mutations have an autosomal dominant pattern of inheritance with incomplete penetrance. Clinical manifestations appear in 80% of family members known to carry the PRSS1 gene mutation [Comfort and Steinberg, 1952; Sibert, 1978; Le Bodic et al. 1996a]. Genetic analysis reveals an estimated penetrance of 77–80% in patients with PRSS1 gene mutations [Keim et al. 2001; Joergensen et al. 2010]. When including symptomatic and asymptomatic patients with radiologic evidence of disease, the penetrance may be 93% [Rebours et al. 2009b]. The penetrance of the R122H and N29I mutations are thought to be 80% and 93% [Sossenheimer et al. 1997; Rebours et al. 2009b]. It remains unclear why some patients with these mutations do not develop chronic pancreatitis. There is likely synergy with other exposures and other risk factors for pancreatitis. It is also possible that other mutations may protect against chronic pancreatitis. For example, the G191R variant in the PRSS2 gene mutation in the anionic trypsinogen gene results in the loss of the activity of trypsin and appears to be protective against chronic pancreatitis [Witt et al. 2006].
The estimated prevalence of hereditary pancreatitis was 1 per 800,000 in Germany and 0.3 per 100,000 in France [Rebours et al. 2009b]. Another study examined 977 patients from the Danish National Registry from 1977 to 2004 with pancreatitis of unknown origin. Genetic testing was performed and found a prevalence rate of 0.57 per 100,000 for symptomatic hereditary pancreatitis and a rate of 0.13 per 100,000 for carriers of PRSS1 [Joergensen et al. 2010]. Genetic testing was performed on a subgroup of patients with idiopathic recurrent acute pancreatitis who were younger than 30 years old and found a genetic cause of pancreatitis in 32% of the group [Joergensen et al. 2010].
SPINK1 gene
Mutations in the SPINK1 gene have been shown to increase the risk of pancreatitis and have been associated with familial and idiopathic pancreatitis. This gene is also referred to as the pancreatic secretory trypsin inhibitor gene. Mutations in SPINK1 cause decreased functioning levels of the inhibitor of trypsin, which lead to elevated levels of trypsin. Therefore patients are more prone to pancreatitis and chronic pancreatic inflammation (Figure 2). SPINK1 is an acute phase protein and is a specific trypsin inhibitor which becomes more clinically important in the setting of recurrent trypsin activation. In these cases, SPINK1 mutations may act as a disease modifier, lowering the overall threshold to develop clinical pancreatitis [Aoun et al. 2008].
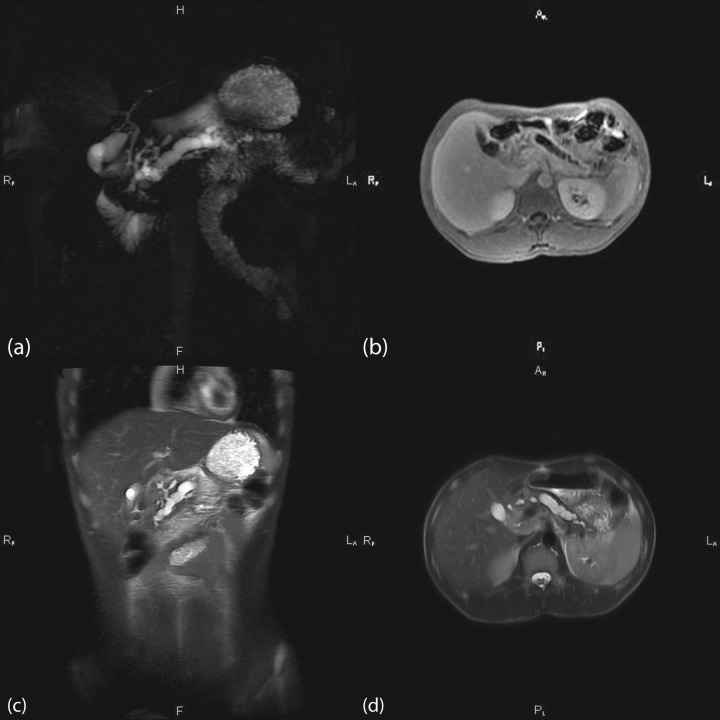
Figure 2.
Multipanel images from magnetic resonance imaging (MRI) and magnetic resonance cholangiopancreatography (MRCP) in a 28-year-old patient who presented with chronic pancreatitis and was found to have an N34S mutation on serine protease inhibitor Kazal type 1 (SPINK1) sequencing. (a) MRCP revealing advanced changes of chronic pancreatitis with an ectatic and markedly dilated pancreatic duct. (b) Axial MRI scan revealing parenchymal atrophy consistent with chronic pancreatitis. (c) Coronal image showing an ectatic pancreatic duct with dilatation. (d) Axial image of the atrophic pancreas most pronounced in the head with a dilatated pancreatic duct without signs of acute inflammatory changes.
SPINK1 is thought to be a candidate gene for pancreatitis. Mutations can lead to the loss of function of the SPINK1 gene, increasing the overall risk of developing chronic pancreatitis. The inheritance pattern does not clearly fit autosomal recessive or dominant patterns, and is likely a complex inheritance pattern which is still being examined [Aoun et al. 2008; Reddy and Prasad, 2011; Shimosegawa et al. 2006; Whitcomb, 2002]. Overall, the high-risk SPINK1 N34S heterozygous haplotype has been observed in 1–3% of most populations. The SPINK1 gene has been more clinically relevant in patients with idiopathic chronic pancreatitis [Pfutzer et al. 2000].
CFTR gene
Cystic fibrosis (CF) is caused by mutations in the CFTR gene. CF is a multisystem disease affecting the digestive system, sweat glands, reproductive tract, and respiratory system. Patients with CF have abnormal chloride and sodium transport channels. Specifically CFTR mutations cause thickened biliary and pancreatic secretions with impaired flow leading to progressive liver and pancreatic disease (Figure 3). A total of 85% of patients with CF develop pancreatic insufficiency leading to chronic malabsorption and fat-soluble vitamin deficiency [Nousia-Arvanitakis, 1999]. About 1–4% of the overall CF population will have an episode of pancreatitis. The incidence of pancreatitis is increased in patients with milder forms of CF [De Boeck et al. 2005; Dray et al. 2004; Durno et al. 2002; Gaskin et al. 1982; Parkins et al 2011]. About 25% develop CF-related diabetes by age 20 and the prevalence increases with age [Lanng, 2001]. Patients with CF are also prone to intestinal intussusception (1%) [Holmes et al. 1991] and distal intestinal obstruction syndrome (10–47%) [Dray et al. 2004; Rowe et al. 2005].
Figure 3.

Coronal image from a computed tomography scan in a 30-year-old patient who presented with recurrent acute pancreatitis and was found to carry a causative mutation on cystic fibrosis transmembrane conductance regulator (CFTR) sequencing. There is global pancreatic atrophy with mild peripancreatic inflammatory soft tissue stranding.
CF is inherited in an autosomal recessive pattern. There are multiple different mutations in the CFTR gene. Some mutations lead to mild clinical manifestations and some lead to severe forms of the disease. Approximately 10% of patients with CF may have incomplete penetrance with only one organ system involved [Boyle, 2003]. CF mutations occur in approximately 1:3000 white people, 1:9200 Hispanics, and 1:15,000 African Americans. CF is also seen in parts of Asia, Africa, and Latin America. Prevalence estimates have increased with the spread of newborn screening and recognition of patients with milder forms of disease [Hamosh et al. 1998].
Pancreatic cancer
There is a markedly increased risk of pancreatic cancer in patients with hereditary pancreatitis, though hereditary pancreatitis accounts for a small percentage of all patients with pancreatic cancer [Lowenfels et al. 1997; Howes et al. 2004; Rebours et al. 2009a]. An international study of 246 patients with hereditary pancreatitis from the United States, Europe and Japan reported a cumulative risk of pancreatic cancer by age 70 of 40%, with a standardized incidence ratio of 53 [Lowenfels et al. 1997]. In the French study, the cumulative risk of pancreatic cancer was 10 % by age 50, 19% by age 60, and 54% by age 75. The highest risk was seen in smokers and patients with diabetes mellitus [Rebours et al. 2009a].
Smoking increases the risk of pancreatic cancer twofold in patients with hereditary pancreatitis. In a cohort study of patients with hereditary pancreatitis, pancreatic cancer presented 20 years earlier in smokers compared with nonsmokers with a risk ratio of 57 (95% confidence interval 35–90) [Lowenfels et al. 2001]. There is a lack of data regarding screening for pancreatic cancer in patients with hereditary pancreatitis, and no data from prospective trials. An expert symposium recommended that screening should be performed on a 1–3-year basis in individuals with a more than 10-fold risk of pancreatic cancer, including patients with hereditary pancreatitis [Brand et al. 2007]. Pancreatic cancer screening can be considered for hereditary pancreatitis patients starting at age 40–45, or 10–15 years before the earliest age of onset of pancreatic cancer in the family [Brand et al. 2007; Rebours et al. 2012]. Modalities such as computed tomography, magnetic resonance imaging (MRI), or endoscopic ultrasound (EUS) have been suggested, with many centers with expertise in hereditary pancreatitis performing EUS or MRI/magnetic resonance cholangiopancreatography due to decreased radiation exposure and improved imaging quality.
Genetic testing
The diagnosis of hereditary pancreatitis can be confirmed by genetic testing, however not all mutations causing hereditary pancreatitis have been detected. A list of laboratories that perform this hereditary pancreatitis testing can be found at www.genetests.org. Genetic testing should be considered in patients who fulfill one or more of the criteria listed in Table 2 [Ellis et al. 2001; Fink et al. 2007]. Patients who undergo genetic testing should have genetic counseling [Fink et al. 2007]. Asymptomatic testing can be considered in patients with first-degree relatives with known causative gene mutations, but should be performed in the context of genetic counseling.
Table 2.
Criteria for performing genetic testing for hereditary pancreatitis.
| Criteria (one or more) |
|---|
| Unexplained documented pancreatitis in a child |
| A family history of idiopathic chronic pancreatitis, recurrent acute pancreatitis, or pancreatitis in childhood without a known etiology |
| Recurrent acute pancreatitis where no cause can be determined |
| Relatives with known mutations associated with hereditary pancreatitis |
| Idiopathic chronic pancreatitis in patients who are younger than age 25 |
| Patients who meet criteria for participation in approved research projects |
Treatment
Medical management
The treatment for hereditary pancreatitis is similar to the treatment for pancreatitis due to other etiologies. Mainstays of medical management involve pain control, nutritional support, treatment for diabetes mellitus, and pancreatic enzyme supplementation for exocrine insufficiency. Patients with hereditary pancreatitis should avoid environmental triggers that can cause pancreatitis. Screening should be performed for diabetes mellitus and exocrine insufficiency. Dietary modification includes small, low-fat meals along with increased hydration. There is no known amount of alcohol that is safe for patients, therefore it is recommended that patients abstain from alcohol [Rebours et al. 2009b]. Smoking is associated with a twofold increased risk of pancreatic cancer and cessation is strongly recommended.
Medical management should follow a stepwise progression, with avoidance of triggers and dietary modification, followed by exogenous enzymes and finally by pain medication. Pancreatic enzyme supplementation may help relieve pain in some patients [Halgreen et al. 1986]. There is increasing evidence that patients with painful chronic pancreatitis have abnormal pain processing in the central nervous system similar to patients with chronic pain syndromes [Frøkjær et al. 2011]. Tricyclic antidepressants or gabapentin may have a role in the treatment of chronic pancreatitis. Nonsteroidal anti-inflammatory drugs may be preferable to opioid analgesics but are associated with gastritis and peptic ulcers. Consider coadministration with a proton-pump inhibitor. Chronic opioid analgesics may be required to control pain symptoms, with long-acting agents preferred over shorter-acting agents. In an open-label pilot study with three young related hereditary pancreatitis patients, antioxidant treatment led to a significant decrease in days with abdominal pain and lower use of analgesics; however, the optimum regimen of antioxidants, dosage, and frequency have not been studied [Uomo et al. 2001]. Amlodipine was studied in nine patients with hereditary pancreatitis with chronic abdominal pain; however, their daily pain scores did not significantly differ when on therapy [Morinville et al. 2007]. Octreotide and somatostatin have not shown benefit in symptom relief in patients with chronic pancreatitis [Malfertheiner et al. 1995; Uhl et al. 1999].
Endoscopic treatment
Therapeutic endoscopy has key roles in both diagnosis and management. EUS is often indicated in the evaluation of patients with pancreatic disease. EUS is the most sensitive modality for the detection of pancreatic mass lesions. In the setting of pancreatitis, EUS may be helpful in evaluating and managing cystic lesions, in detecting and confirming pancreas divisum and ductal abnormalities, in the detection of biliary stone disease, and in establishing criteria for a diagnosis of chronic pancreatitis. EUS and evaluation of the major and minor papillas may reveal ampullary and periampullary pathology. EUS with fine needle aspiration may be indicated for the evaluation of focal and mass type pancreatic lesions. EUS is also one of the primary modalities used in screening for pancreatic cancer in increased risk populations. In terms of diagnosis, endoscopic retrograde cholangiopancreatography (ERCP) may be indicated for acute recurrent or smoldering type pancreatitis that has been considered idiopathic. In this setting it may reveal pancreas divisum or other ductal anomalies, missed stones or sludge, mass lesions, chronic pancreatitis, or Sphincter of Oddi dysfunction. For this indication, ERCP should typically be performed in an expert center with experience in techniques such as minor papilla cannulation and papillotomy and Sphincter of Oddi manometry.
In terms of therapeutics, ERCP may be indicated for complications such as biliary obstruction, pancreatic duct strictures, pancreatic duct stones, and pseudocyst formation. In these cases, endoscopic treatment in hereditary pancreatitis is similar to that for other forms of chronic pancreatitis. Endoscopic therapy aimed at decompression of an obstructed pancreatic duct may be associated with pain relief in some patients [Okolo et al. 2000]. The largest study of endoscopic treatment in patients with chronic pancreatitis consisted of 1018 patients at eight centers with average follow up of 4.9 years (ranging from 2 to 12 years). Obstruction of the pancreatic duct was found to be secondary to strictures (47%), stones (18%), or both (32%). A total of 60% of the patients had completed endoscopic therapy, 16% were still undergoing endoscopic therapy, and 24% underwent surgery. The long-term success of endotherapy determined by a significant reduction in pain (no pain or mild pain) was 86% in the entire group and 65% in an intention-to-treat analysis. There were no significant differences between patients with strictures, stones, or both [Rosch et al. 2002]. A study of therapeutic ERCP in children and adolescents with chronic pancreatitis revealed that 64.9% had complete pain resolution and 81% had decreased abdominal pain, with 17% having ERCP-related complications [Li et al. 2010]. In another study, in 21 patients with hereditary pancreatitis, ERCP resulted in significantly reduced mean pain scores and decreased daily oxycodone use, hospitalizations, and emergency room visits [Dever et al. 2010]. A case report of a young girl with hereditary pancreatitis who had pancreatic ductal dilation and chronic abdominal pain reported significant improvement of symptoms after the placement of a pancreatic duct stent. Early intervention may also help slow the progression of disease symptoms [Vaughan et al. 1999]. Extracorporeal shock wave lithotripsy (ESWL) is another method to treat pancreatic duct stones. Partial (86%) to complete (62%) pain relief has been reported in patients who underwent ESWL of obstructive pancreatic duct stones after a mean length of follow up of 20 months [Parsi et al. 2010]. Endoscopic or percutaneous celiac nerve blocks have limited success in chronic pancreatitis, and even when successful, patients often have recurrence of symptoms within 2–6 months. The procedure can be repeated as needed but is often disappointing in this population. Transgastric pseudocyst gastrostomy can be indicated for the management of mature pancreatic pseudocysts and endoscopic approaches may be preferred as a first-line approach [Johnson et al. 2009].
Surgical treatment
Surgery may be indicated for pain, for pancreatectomy with islet cell transplant, for management of acute complications such as necrosis, or for chronic complications such as pseudocyst formation, and for patients with resectable or worrisome mass lesions, surgery may be first-line therapy. In younger patients with hereditary pancreatitis who still have functional islet cells, pancreactectomy with islet cell autotransplantation can preserve islet cell function as well as reduce chronic abdominal pain. In a case series of 18 pediatric patients with chronic pancreatitis not from hereditary pancreatitis undergoing pancreatectomy with islet autotransplantation, islet cell function was maintained in 78% of the group and 56% of patients were able to remain insulin independent after 1-year follow up. A total of 61% of patients were able to be weaned off of narcotics. Patients who were younger than 13 years old had better graft function and required fewer narcotics postoperatively [Bellin et al. 2008]. Pancreatectomy with islet cell autotransplantation should be considered when a total pancreatectomy is felt to be the best option in young patients with hereditary pancreatitis. Older patients with hereditary pancreatitis who are suffering from chronic pancreatitis may be candidates for a total pancreatectomy but not typically for pancreatectomy with islet cell autotransplantation due to an increased risk of transplanting malignant cells. Older patients may also already have diabetes or be insulin dependent. More commonly, surgery may be indicated for the management of acute and chronic complications of pancreatitis. Debridement for pancreatic necrosis and drainage of peri-pancreatic fluid collections are often performed surgically. Decompression of a distended and obstructed pancreatic duct with procedures such as a Frye and Puestow may be considered. A Puestow is a lateral pancreaticjejunostomy in which the pancreas with a dilated duct is incised longitudinally and a loop of jejunum is anastomosed to provide decompression. A Frye procedure combines a coring out of diseased tissue in the pancreatic head with a Puestow-type decompression of the pancreatic duct.
Prognosis
Providers should have a multifaceted approach in treating patients with hereditary pancreatitis. Yearly screening for pancreatic exocrine and endocrine insufficiency is recommended. A multidisciplinary approach including psychiatry, pain management, social work, gastroenterology, and close follow up with an established primary care provider can optimize treatment for patients with hereditary pancreatitis [Rebours et al. 2012]. The overall mortality is not increased in patients with hereditary pancreatitis who do not develop pancreatic cancer compared with the general population. The median survival age is 74 [Rebours et al. 2009a].
Conclusion
Although hereditary pancreatitis is a rare disorder, the diagnosis is often considered when patients present with pancreatitis without a history of heavy alcohol exposure and with some negative testing for biliary disease. In this group, a careful evaluation may reveal multiple etiologies, including microlithiasis, autoimmune pancreatitis, pancreas divisum, surreptitious alcohol exposure, pancreatic and biliary sphincter dysfunction, and adverse medication reactions. ERCP is typically indicated for acute recurrent or smoldering-type pancreatitis that has been considered idiopathic, often revealing conditions such as pancreas divisum, missed stones or sludge, or Sphincter of Oddi dysfunction. A subset of patients will have a genetic predisposition to recurrent acute or chronic pancreatitis. This should be considered in patients with early onset pancreatitis, multiple family members with chronic pancreatitis, or after a thorough negative evaluation for other etiologies. As genetic testing is increasingly used, the prevalence of hereditary pancreatitis may be greater than has been assumed previously. The treatment of hereditary pancreatitis is in many ways similar to treatment for pancreatitis due to other etiologies. Patients should avoid alcohol and tobacco exposure. Therapeutic endoscopy may be used for biliary obstruction, pancreatic duct strictures, pancreatic duct stones, pseudocyst formation, and for exclusion of malignancy with mass lesions. Surgery may be indicated for pain, for pancreatectomy with islet cell transplant, or for management of complications. Overall mortality if the patient is followed closely is similar to that of the general population, unless pancreatic cancer develops. Screening for pancreatic cancer is recommended but has not been evaluated in a large series. Testing for the common mutations, PRSS1, SPINK1, and CFTR, is available in multiple laboratories and should be accompanied by genetic counseling. Patients with hereditary pancreatitis should be followed by a multidisciplinary team, which may include primary care, psychiatry, pain management, genetics, social work, gastroenterology, and surgery.
Acknowledgments
The authors would like to thank Sarah Zingarelli for help with preparing the images for the manuscript.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Milan R. Patel, Division of Digestive Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, GA, USA
Amanda L. Eppolito, Department of Human Genetics, Emory University School of Medicine, Atlanta, GA, USA
Field F. Willingham, Director of Endoscopy, Assistant Professor of Medicine, Division of Digestive Diseases, Department of Medicine, 1365 Clifton Road, NE, Atlanta, GA 30322, USA
References
- Aoun E., Chang C., Greer J., Papachristou G., Barmada M., Whitcomb D. (2008) Pathways to injury in chronic pancreatitis: decoding the role of the high-risk SPINK1 N34s haplotype using meta-analysis. PLoS One 3: e2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellin M., Carlson A., Kobayashi T., Gruessner A., Hering B., Moran A., et al. (2008) Outcome after pancreatectomy and islet autotransplantation in a pediatric population. J Pediatr Gastroenterol Nutr 47: 37–44 [DOI] [PubMed] [Google Scholar]
- Boyle M. (2003) Nonclassic cystic fibrosis and CTFR-related diseases. Curr Opin Pulm Med 9: 498–503 [DOI] [PubMed] [Google Scholar]
- Brand R., Lerch M., Rubinstein W., Neoptolemos J., Whitcomb D., Hruban R., et al. (2007) Advances in counselling and surveillance of patients at risk for pancreatic cancer. Gut 56: 1460–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comfort M., Steinberg A. (1952) Pedigree of a family with hereditary chronic relapsing pancreatitis. Gastroenterology 21: 54–63 [PubMed] [Google Scholar]
- De Boeck K., Weren M., Proesmans M., Kerem E. (2005) Pancreatitis among patients with cystic fibrosis: correlation with pancreatic status and genotype. Pediatrics 115: e463–e469 [DOI] [PubMed] [Google Scholar]
- Dever J., Irani S., Brandabur J., Traverso L., Kozarek R. (2010) Outcomes of interventional ERCP in hereditary pancreatitis. J Clin Gastroenterol 44: 46–51 [DOI] [PubMed] [Google Scholar]
- Dray X., Bienvenu T., Desmazes-Dufeu N., Dusser D., Marteau P., Hubert D. (2004) Distal intestinal obstruction syndrome in adults with cystic fibrosis. Clin Gastroenterol Hepatol 2: 498–503 [DOI] [PubMed] [Google Scholar]
- Durno C., Corey M., Zielenski J., Tullis E., Tsui L., Durie P. (2002) Genotype and phenotype correlations in patients with cystic fibrosis and pancreatitis. Gastroenterology 123: 1857–1864 [DOI] [PubMed] [Google Scholar]
- Ellis I., Lerch M., Whitcomb D. (2001) Genetic testing for hereditary pancreatitis: guidelines for indications, counselling, consent and privacy issues. Pancreatology 1: 405–415 [DOI] [PubMed] [Google Scholar]
- Fink E., Kant J., Whitcomb D. (2007) Genetic counseling for nonsyndromic pancreatitis. Gastroenterol Clin North Am 36: 325–333, ix [DOI] [PubMed] [Google Scholar]
- Frøkjær J., Olesen S., Gram M., Yavarian Y., Bouwense S., Wilder-Smith O., et al. (2011) Altered brain microstructure assessed by diffusion tensor imaging in patients with chronic pancreatitis. Gut 60: 1554–1562 [DOI] [PubMed] [Google Scholar]
- Gaskin K., Gurwitz D., Durie P., Corey M., Levison H., Forstner G. (1982) Improved respiratory prognosis in patients with cystic fibrosis with normal fat absorption. J Pediatr 100: 857–862 [DOI] [PubMed] [Google Scholar]
- Halgreen H., Pedersen N., Worning H. (1986) Symptomatic effect of pancreatic enzyme therapy in patients with chronic pancreatitis. Scand J Gastroenterol 21: 104–108 [DOI] [PubMed] [Google Scholar]
- Hamosh A., Fitzsimmons S., Macek M., Jr, Knowles M., Rosenstein B., Cutting G. (1998) Comparison of the clinical manifestations of cystic fibrosis in black and white patients. J Pediatr 132: 255–259 [DOI] [PubMed] [Google Scholar]
- Holmes M., Murphy V., Taylor M., Denham B. (1991) Intussusception in cystic fibrosis. Arch Dis Child 66: 726–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes N., Lerch M., Greenhalf W., Stocken D., Ellis I., Simon P., et al. (2004) Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol 2: 252–261 [DOI] [PubMed] [Google Scholar]
- Joergensen M., Brusgaard K., Cruger D., Gerdes A., De Muckadell O. (2010) Incidence, prevalence, etiology, and prognosis of first-time chronic pancreatitis in young patients: a nationwide cohort study. Dig Dis Sci 55: 2988–2998 [DOI] [PubMed] [Google Scholar]
- Joergensen M., Brusgaard K., Cruger D., Gerdes A., Schaffalitzky De Muckadell O. (2010) Genetic, epidemiological, and clinical aspects of hereditary pancreatitis: a population-based cohort study in Denmark. Am J Gastroenterol 105: 1876–1883 [DOI] [PubMed] [Google Scholar]
- Johnson M., Walsh R., Henderson J., Brown N., Ponsky J., Dumot J., et al. (2009) Surgical versus nonsurgical management of pancreatic pseudocysts. J Clin Gastroenterol 43: 586–590 [DOI] [PubMed] [Google Scholar]
- Keim V., Bauer N., Teich N., Simon P., Lerch M., Mossner J. (2001) Clinical characterization of patients with hereditary pancreatitis and mutations in the cationic trypsinogen gene. Am J Med 111: 622–626 [DOI] [PubMed] [Google Scholar]
- Kereszturi E., Szmola R., Kukor Z., Simon P., Weiss F., Lerch M., et al. (2009) Hereditary pancreatitis caused by mutation-induced misfolding of human cationic trypsinogen: a novel disease mechanism. Hum Mutat 30: 575–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanng S. (2001) Glucose intolerance in cystic fibrosis patients. Paediatr Respir Rev 2: 253–259 [DOI] [PubMed] [Google Scholar]
- Le Bodic L., Bignon J., Raguenes O., Mercier B., Georgelin T., Schnee M., et al. (1996a) The hereditary pancreatitis gene maps to long arm of chromosome 7. Hum Mol Genet 5: 549–554 [DOI] [PubMed] [Google Scholar]
- Le Bodic L., Schnee M., Georgelin T., Soulard F., Ferec C., Bignon J., et al. (1996b) An exceptional genealogy for hereditary chronic pancreatitis. Dig Dis Sci 41: 1504–1510 [DOI] [PubMed] [Google Scholar]
- Li Z., Wang W., Liao Z., Zou D., Jin Z., Chen J., et al. (2010) A long-term follow-up study on endoscopic management of children and adolescents with chronic pancreatitis. Am J Gastroenterol 105: 1884–1892 [DOI] [PubMed] [Google Scholar]
- Lowenfels A., Maisonneuve P., Dimagno E., Elitsur Y., Gates L., Jr, Perrault J., et al. (1997) Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst 89: 442–446 [DOI] [PubMed] [Google Scholar]
- Lowenfels A., Maisonneuve P., Whitcomb D., Lerch M., Dimagno E. (2001) Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA 286: 169–170 [DOI] [PubMed] [Google Scholar]
- Malfertheiner P., Mayer D., Buchler M., Dominguez-Munoz J., Schiefer B., Ditschuneit H. (1995) Treatment of pain in chronic pancreatitis by inhibition of pancreatic secretion with octreotide. Gut 36: 450–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinville V., Lowe M., Elinoff B., Whitcomb D. (2007) Hereditary pancreatitis amlodipine trial: a pilot study of a calcium-channel blocker in hereditary pancreatitis. Pancreas 35: 308–312 [DOI] [PubMed] [Google Scholar]
- Nousia-Arvanitakis S. (1999) Cystic fibrosis and the pancreas: recent scientific advances. J Clin Gastroenterol 29: 138–142 [DOI] [PubMed] [Google Scholar]
- Okolo P., Pasricha P., Kalloo A. (2000) What are the long-term results of endoscopic pancreatic sphincterotomy? Gastrointest Endosc 52: 15–19 [DOI] [PubMed] [Google Scholar]
- Parkins M., Parkins V., Rendall J., Elborn S. (2011) Changing epidemiology and clinical issues arising in an ageing cystic fibrosis population. Ther Adv Respir Dis 5: 105–119 [DOI] [PubMed] [Google Scholar]
- Parsi M., Stevens T., Lopez R., Vargo J. (2010) Extracorporeal shock wave lithotripsy for prevention of recurrent pancreatitis caused by obstructive pancreatic stones. Pancreas 39: 153–155 [DOI] [PubMed] [Google Scholar]
- Pfutzer R., Barmada M., Brunskill A., Finch R., Hart P., Neoptolemos J., et al. (2000) SPINK1/PSTI polymorphisms act as disease modifiers in familial and idiopathic chronic pancreatitis. Gastroenterology 119: 615–623 [DOI] [PubMed] [Google Scholar]
- Rebours V., Boutron-Ruault M., Jooste V., Bouvier A., Hammel P., Ruszniewski P., et al. (2009a) Mortality rate and risk factors in patients with hereditary pancreatitis: uni- and multidimensional analyses. Am J Gastroenterol 104: 2312–2317 [DOI] [PubMed] [Google Scholar]
- Rebours V., Boutron-Ruault M., Schnee M., Ferec C., Le Marechal C., Hentic O., et al. (2009b) The natural history of hereditary pancreatitis: a national series. Gut 58: 97–103 [DOI] [PubMed] [Google Scholar]
- Rebours V., Levy P., Mosnier J., Scoazec J., Soubeyrand M., Flejou J., et al. (2010) Pathology analysis reveals that dysplastic pancreatic ductal lesions are frequent in patients with hereditary pancreatitis. Clin Gastroenterol Hepatol 8: 206–212 [DOI] [PubMed] [Google Scholar]
- Rebours V., Levy P., Ruszniewski P. (2012) An overview of hereditary pancreatitis. Dig Liver Dis 44: 8-15 [DOI] [PubMed] [Google Scholar]
- Reddy D., Prasad S. (2011) Genetic basis of chronic pancreatitis in Asia Pacific region. J Gastroenterol Hepatol 26(Suppl. 2): 2–5 [DOI] [PubMed] [Google Scholar]
- Rosch T., Daniel S., Scholz M., Huibregtse K., Smits M., Schneider T., et al. (2002) Endoscopic treatment of chronic pancreatitis: a multicenter study of 1000 patients with long-term follow-up. Endoscopy 34: 765–771 [DOI] [PubMed] [Google Scholar]
- Rowe S., Miller S., Sorscher E. (2005) Cystic fibrosis. N Engl J Med 352: 1992–2001 [DOI] [PubMed] [Google Scholar]
- Sahin-Toth M. (1999) Hereditary pancreatitis-associated mutation Asn(21) → Ile stabilizes rat trypsinogen in vitro. J Biol Chem 274: 29699–29704 [DOI] [PubMed] [Google Scholar]
- Sahin-Toth M. (2000) Human cationic trypsinogen. Role of Asn-21 in zymogen activation and implications in hereditary pancreatitis. J Biol Chem 275: 22750–22755 [DOI] [PubMed] [Google Scholar]
- Sahin-Toth M., Toth M. (2000) Gain-of-function mutations associated with hereditary pancreatitis enhance autoactivation of human cationic trypsinogen. Biochem Biophys Res Commun 278: 286–289 [DOI] [PubMed] [Google Scholar]
- Shimosegawa T., Kume K., Masamune A. (2006) SPINK1 gene mutations and pancreatitis in Japan. J Gastroenterol Hepatol 21(Suppl. 3): S47–S51 [DOI] [PubMed] [Google Scholar]
- Sibert J. (1978) Hereditary pancreatitis in England and Wales. J Med Genet 15: 189–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossenheimer M., Aston C., Preston R., Gates L., Jr, Ulrich C., Martin S., et al. (1997) Clinical characteristics of hereditary pancreatitis in a large family, based on high-risk haplotype. The Midwest Multicenter Pancreatic Study Group (MMPSG). Am J Gastroenterol 92: 1113–1116 [PubMed] [Google Scholar]
- Teich N., Ockenga J., Hoffmeister A., Manns M., Mossner J., Keim V. (2000) Chronic pancreatitis associated with an activation peptide mutation that facilitates trypsin activation. Gastroenterology 119: 461–465 [DOI] [PubMed] [Google Scholar]
- Uhl W., Anghelacopoulos S., Friess H., Buchler M. (1999) The role of octreotide and somatostatin in acute and chronic pancreatitis. Digestion 60(Suppl. 2): 23–31 [DOI] [PubMed] [Google Scholar]
- Uomo G., Talamini G., Rabitti P. (2001) Antioxidant treatment in hereditary pancreatitis. A pilot study on three young patients. Dig Liver Dis 33: 58–62 [DOI] [PubMed] [Google Scholar]
- Vaughan D., Imrie C., Kelleher J., Drumm B., Osborne H. (1999) Pancreatic duct stenting as a treatment for hereditary pancreatitis. Pediatrics 104: 1129–1133 [DOI] [PubMed] [Google Scholar]
- Whitcomb D. (2002) How to think about SPINK and pancreatitis. Am J Gastroenterol 97: 1085–1088 [DOI] [PubMed] [Google Scholar]
- Whitcomb D., Gorry M., Preston R., Furey W., Sossenheimer M., Ulrich C., et al. (1996a) Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet 14: 141–145 [DOI] [PubMed] [Google Scholar]
- Whitcomb D., Preston R., Aston C., Sossenheimer M., Barua P., Zhang Y., et al. (1996b) A gene for hereditary pancreatitis maps to chromosome 7q35. Gastroenterology 110: 1975–1980 [DOI] [PubMed] [Google Scholar]
- Witt H., Sahin-Toth M., Landt O., Chen J., Kahne T., Drenth J., et al. (2006) A degradation-sensitive anionic trypsinogen (PRSS2) variant protects against chronic pancreatitis. Nat Genet 38: 668–673 [DOI] [PMC free article] [PubMed] [Google Scholar]