Abstract
Endoscopic therapy for achalasia is directed at disrupting or weakening the lower esophageal sphincter (LES). The two most commonly utilized endoscopic interventions are large balloon pneumatic dilation (PD) and botulinum toxin injection (BTI). These interventions have been extensively scrutinized and compared with each other as well as with surgical disruption (myotomy) of the LES. PD is generally more effective in improving dysphagia in achalasia than BTI, with the latter reserved for infirm older people, and PD may approach treatment results attained with myotomy. However, PD may need to be repeated. Small balloon dilation and endoscopic stent placement for achalasia have only been used in select centers. Per oral endoscopic myotomy is a newer endoscopic modality that will likely change the treatment paradigm for achalasia. It arose from the field of natural orifice transluminal endoscopic surgery and represents a scarless endoscopic approach to Heller myotomy. This is a technique that requires extensive training and preparation and thus there should be rigorous accreditation and monitoring of outcomes to ensure safety and efficacy.
Keywords: achalasia, botulinum toxin injection, per oral endoscopic myotomy, pneumatic dilation
Introduction
Achalasia is an uncommon esophageal motility disorder with an incidence of approximately 1/200,000 and a prevalence of approximately 1/10,000, characterized by dysphagia and, on occasion, chest pain, regurgitation, aspiration pneumonia and weight loss [Mayberry, 2001]. The term is derived from Greek (α = non, χαλασια = relaxation). Achalasia has no epidemiologic predilection for any demographic group and affects men and women equally and may occur at any age, from children to older people [Mayberry, 2001]. Diagnosis is often suggested by esophageal imaging demonstrating a dilated esophagus with a smooth distal narrowing resembling a ‘bird’s beak’. The validation of the diagnosis of achalasia requires esophageal manometry. The sine qua non is a lower esophageal sphincter (LES) that does not relax completely, but other common manometry features include a hypertensive LES and lack of peristalsis in the distal esophagus. Recent advances in diagnostic methods including high-resolution manometry (HRM) may allow more precise classification into manometric subtypes that can predict response to treatment. Drug therapy for achalasia is not effective. The traditional options for treatment include surgical myotomy and endoscopic methods that disrupt or weaken the LES [predominantly pneumatic dilation (PD) and botulinum toxin injection (BTI)]. Per oral endoscopic myotomy (POEM) is a novel endoscopic therapy that allows performance of myotomy via an endoscopic approach. We will proceed with discussion of the traditional endoscopic treatments followed by a detailed discussion of POEM. Table 1 compares salient features of the three traditional treatments side by side. It should be emphasized that, independent of the treatment selected, all patients with achalasia should have long-term follow up for symptom recurrence, acid reflux and the rare possibility of esophageal cancer.
Table 1.
Comparing traditional treatments of achalasia.
| Pneumatic dilation | Surgical myotomy | Botulinum toxin | |
|---|---|---|---|
| Ease of technique | ⋆⋆ | ⋆ | ⋆⋆⋆ |
| Duration of effect | ⋆⋆ | ⋆⋆⋆ | ⋆ |
| Safety | ⋆⋆ | ⋆⋆ | ⋆⋆⋆ |
| Cost of treatment | ⋆⋆ | ⋆ | ⋆⋆ |
| Easy return to work | ⋆⋆⋆ | ⋆ | ⋆⋆⋆ |
| Success in vigorous achalasia | ⋆ | ⋆⋆ | ⋆⋆⋆ |
| Success in pediatric patients | ⋆⋆ | ⋆⋆⋆ | ⋆ |
| Success in elderly patients | ⋆⋆⋆ | ⋆⋆⋆ | ⋆⋆ |
Pneumatic dilation
Forceful dilation of the LES dates back to the seventeenth century when a whalebone was employed [Spiess and Kahrilas, 1998] as a primitive bougie to achieve disruption of the sphincter. The main goal of therapy in achalasia is to reduce the LES basal pressure. Improvement of dysphagia is considered the prime clinical endpoint of treatment. PD has a long track record in achalasia therapy. Objective improvement in terms of LES pressure and esophageal diameter has been noted together with improvement in dysphagia [Hulselmans et al. 2010; Ghoshal et al. 2012].
There is no single, accented standard method for balloon dilation in patients with achalasia, and there are many variations based largely on anecdotal and personal experience. Most authorities start with the 30 mm balloon and progress serially to the 35 mm and 40 mm balloons if necessary. There is a suggestion that the risk of perforation may be less with the smallest balloon [Mikaeli et al. 2004; Boeckxstaens et al. 2011]. Balloon dilation is typically performed under fluoroscopic guidance utilizing a stiff guidewire with a soft distal tip that is placed at endoscopy. However, dilation without fluoroscopy has been described [Rai et al. 2005]. An antecedent endoscopy excludes intraluminal malignancy, ensures esophageal clearance and gauges the anatomy in terms of esophageal angulation and possible hiatus hernia and epiphrenic diverticula. The balloon is passed with fluoroscopic monitoring and positioned in the esophagus. We employ a pediatric endoscope to ensure the balloon is positioned in the LES area and then remove the endoscope.
The dilating balloon is typically partially inflated to optimize position within the LES by looking for a waist in the balloon center. The catheter is fixed in place by a firm grasp with the realization that inflation may alter the position of the balloon (usually involving distal migration). The balloon is inflated slowly to 7–10 psi (measured by a sphygmomanometer) with monitoring of the waist within the LES. The goal is to maintain dilation until the waist is obliterated, and some deflate the balloon immediately thereafter while others use more prolonged timed dilation [Khan et al. 1998].The dilator and guidewire are removed. Generally, the amount of blood on the dilator is not a useful criterion of successful dilation. A gastrograffin swallow is performed upon patient recovery from anesthesia and is used to assess for perforation and perhaps treatment efficacy. Currently, the Rigiflex pneumatic dilator (Boston Scientific, Boston, MA, USA) is the most commonly used balloon for achalasia therapy (Figure 1), though similar devices are available by other manufacturers. (Cook Medical, Bloomington, IN, USA and Hobbs Medical, Strattford Springs, CN, USA). These polyethylene balloons are more reliable than their latex balloon predecessors in inflating to a fixed diameter (available in 30, 35, 40 mm sizes).
Figure 1.
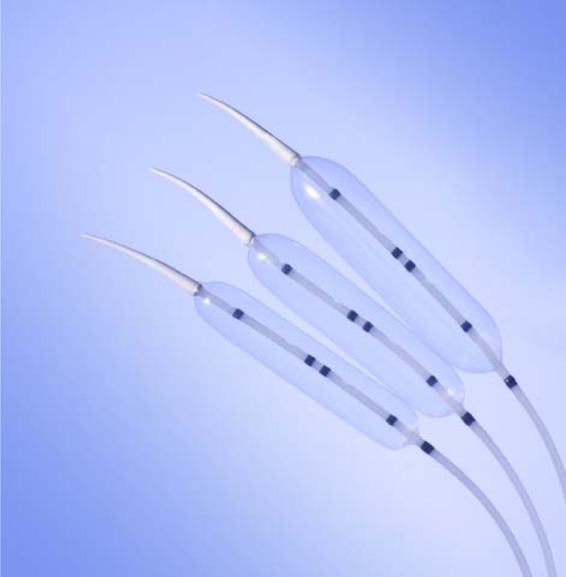
Rigiflex pneumatic dilation balloons (Boston Scientific).
Over 20 retrospective and prospective studies have examined the efficacy of PD for achalasia using the Rigiflex balloon dilator [Walzer and Hirano, 2008]. There is general consensus that a single successful dilation may have an efficacy duration of several years. However, patients typically require serial dilations to remain in remission. One group noted a 5-year response of 40% with a single dilation, and patients whose symptoms were well controlled at 5 years were likely to continue to do well [Eckardt et al. 2004]. In another study, three dilations maintained remission in 90% of patients over 5 years [Parkman et al. 1993]. Another group reported 97% and 93% efficacy over 5 and 10 years respectively with PD, but usually with serial dilations [Zerbid et al. 2006]. Close scrutiny of patients following PD allows identification of those requiring repeat PD, and usually myotomy can be avoided [Wong, 2006]. Most studies describe beginning dilation with the 30 mm balloon and progressing to the 35 mm balloon for refractory or recurrent dysphagia. A number of studies found that in expert hands use of a larger balloon if appropriate is associated with greater efficacy without a significant increase in perforation rate [Chan et al. 2004; Dobrucali et al. 2004; Wong, 2006]. PD is repeated on demand for symptomatic patients in these studies, but one group repeated PD immediately with the bigger balloon if manometry and barium esophagram did not meet treatment thresholds [Hulselmans et al. 2010]. Achalasia is a chronic disease, and only a few studies have followed patients over a decade after PD to confirm long-term efficacy [Katz and Gilbert, 1998]. The conclusion is that PD for achalasia yields good to excellent results in terms of swallowing capability and quality of life if performed by experienced operators; but few are ‘cured’ with the first dilation, and follow-up dilations are often required [Richter and Boeckxstaens, 2011].
The effects of PD or any intervention for achalasia have been assessed clinically by a simple quantitative measurement known as the Eckardt score, which assesses the symptoms of dysphagia, chest pain, regurgitation and weight loss (Table 2). Other post-treatment parameters of success are a decrease in esophageal LES pressure via manometry, and a decrease in esophageal diameter on a barium esophagram [Gockel et al. 2005]. Emptying of the esophagus can also be measured via a ‘timed’ barium esophagram. The height of the barium column at 1, 2 and 5 min is measured after ingestion of a predetermined amount of barium (usually 250 cm3) [de Oliveira et al. 1997]. The interobserver variability and the predictive value of various static and dynamic parameters of the timed barium esophagram with regards to response to achalasia treatment have been investigated and found to be satisfactory [Kostic et al. 2005; Andersson et al. 2009]. The height of the barium column at 1 min on timed barium esophagram after PD or Heller myotomy has been found to be a reliable predictor of long-term response [Andersson et al. 2009].The LES pressure decrease has been considered the best parameter of treatment success, but recent measurements of esophogastric distensibility via the EndoFLIP device may be more reliable [Kwiatek et al. 2010; Rohof et al. 2012].
Table 2.
Eckardt score.
| Eckardt score | Symptoms |
|||
|---|---|---|---|---|
| Weight loss (kg) | Dysphagia | Chest pain | Regurgitation | |
| 0 | None | None | None | None |
| 1 | <5 | Occasional | Occasional | Occasional |
| 2 | 5–10 | Daily | Daily | Daily |
| 3 | >10 | Each meal | Each meal | Each meal |
Several patient- and technique-associated factors have been noted to predict response to PD. Response rates in younger patients are relatively lower, though the exact cutoff in terms of response has been variably reported from 40 to 60 years of age [Eckhardt et al. 2004; Howard et al. 2010]. One study reported a PD failure rate in those younger than 21 years of 65% and 72% at 5 and 10 years respectively [Alderliesten et al. 2011]. Use of the 30 mm balloon as opposed to the larger sizes was associated with PD failure for younger patients (especially male) in some studies [Dobrucali et al. 2004; Wong, 2006]. Young men seem to be especially prone to failure with PD, and consideration should be given to starting with larger balloons [Farhoomand et al. 2004]. Those patients with achalasia with ‘end-stage’ disease signified by a dilated sigmoid-shaped esophagus usually do not respond well to PD (or other treatments for that matter) [Duranceau et al. 2012]. Recent use of HRM has suggested that those with classic achalasia (predominant apersistalsis of distal esophagus – Chicago classification type I) and those with esophageal smooth muscle compression pattern on manometry (Chicago classification type II) respond much better to PD than those with so-called ‘vigorous’ achalasia or achalasia with high-pressure waves in the distal esophagus on HRM (Chicago classification type III) [Pretap et al. 2011]. The absolute value of baseline LES pressure is not a predictive factor, but failure to lower the LES pressure significantly (usually to <10 mmHg) is associated with a poor outcome with PD [Hulselmans et al. 2010; Ghoshal et al. 2012].
PD has been compared with surgical myotomy for achalasia in terms of clinical response and need for repeat intervention. Both options result in excellent initial relief of dysphagia, though surgery is superior at longer follow up in older studies [Spiess and Kahrilas, 1998; Richter, 2008]. PD fares poorly in diminishing pretherapy chest pain [Hulselmans et al. 2010]. One comparison study found similar clinical results with PD and myotomy, but better LES pressure reduction with surgery [Gockel et al. 2005]. However, overall, there have been few prospective randomized controlled trials comparing these interventions. In addition, older trials had PD performed with outmoded dilation balloons and surgical techniques were not as refined as now. The need for subsequent intervention is greater among people treated with PD than with surgical myotomy mainly due to follow-up dilations for patients who were treated with PD [Lopushinski and Urbach, 2006]. Cost analysis conclusions vary depending on the study year(s), locale and cost assumptions, but generally PD is considered more cost effective in the short term, with myotomy being cost effective in the long term [Ahmed, 2008; Parkman et al. 1993].
A sentinel study by Boeckxstaens and colleagues compared PD with laparoscopic Heller myotomy (LHM) and Dor’s fundoplication using a rigorous prospective randomized study design [Boeckxstaens et al. 2011].The mean follow-up duration was 43 months and there was no difference between the groups at 1 year in terms of dysphagia and overall Eckardt score. At 2 years, there was no significant difference in terms of LES pressure, esophageal emptying as assessed by timed barium esophagram, or quality of life as assessed by standard questionnaire. Thus, PD remains a time tested effective modality in the achalasia treatment armamentarium. It has been suggested that PD may even be the first-line approach in preference to LHM for older patients who have amenable manometric and clinical criteria (predominant dysphagia as opposed to chest pain) and who are fit for the anticipated PD and possible follow-up dilation procedures. PD may also have a role as a ‘salvage’ alternative treatment; PD has been used to dilate patients following an unsuccessful surgical myotomy, but efficacy rate is less than for those who had no prior intervention [Guardino et al. 2004].
Esophageal perforation is a dreaded complication of PD but, fortunately, this occurred in less than 5% of dilations in most recent studies (it was more common with older balloons and less refined techniques). Furthermore, in general, perforations tend to be small, allowing possible conservative or laparoscopic management [Bell, 1997]. It is prudent to assure surgical backup prior to PD. There are no well validated risk factors for PD-associated perforation; and there is no increased perforation rate with the larger balloons in most studies [Borotto et al.1996]. Delayed perforation has been described, but we feel comfortable discharging patients after 4 h of observation if the patient is fairly comfortable and the gastrograffin esophagram does not demonstrate concerning findings. The overall complication rate from PD is less than 10% and, in addition to perforation, includes chest pain (usually transient), fever, hemorrhage, hematoma, esophagogastric lacerations and diverticula [Walzer and Hirano, 2008]. Reflux symptoms can be noted acutely after PD and later on as patients gain weight [Novais and Lemme, 2010]. Despite the apparent safety of PD, this modality is performed less commonly in the United States than in Europe, perhaps because of less experience and medicolegal concerns [Richter, 2012].
Botulinum toxin injection
Botulinum toxin is a paralytic agent that exerts its action by rapidly and strongly binding to presynaptic cholinergic nerve terminals. The toxin is then internalized and ultimately inhibits the exocytosis of acetylcholine by decreasing the frequency of acetylcholine release. The treatment of muscle with botulinum toxin results in an accelerated loss of junctional acetylcholine receptors. Chemical denervation after an injection of botulinum toxin reduces the contractility of the LES and therefore reduces obstruction [Jankovic and Brin, 1991].
BTI into the LES has been a mainstay of achalasia therapy. In an initial validating study, 82% of subjects reported symptomatic improvement after injection compared with just 10% in the placebo group [Pasricha et al.1995]. Subsequent studies have shown that most patients do have a clinical response in terms of dysphagia relief; and the duration of response is often over a year and response to a second injection is comparable to the one after the first injection [Pasricha et al. 1996]. These studies also demonstrated a better therapeutic response in ‘vigorous’ achalasia than in the other subtypes. One group had a 75% remission rate at 2 years after initial injection, but most required at least one repeat injection because of recurrent symptoms [Annese et al. 1998]. Improvements in LES pressure at manometry and esophageal emptying via radiology after therapy have also been documented [Neubrand et al. 2002; Richter, 2008]. The consensus is that BTI yields good short-term results in most patients with achalasia but the effect wanes within 2 years of the last injection and eventually the benefit of injection will be minimal even if repeated [Richter, 2008; Walzer and Hirano, 2008]. The waning effect may be due to regional fibrosis or antibodies to the toxin [Dughera et al. 2008]. Thus, BTI is best reserved for infirm older people, patients with significant comorbid conditions or patients refusing other forms of treatment.
As with PD, there are only general guidelines concerning the technique of BTI. An injection needle (Figure 2) is used to make injections at the squamocolumnar junction or up to 1 cm proximally, and usually 100 units in total are injected in four to five equal volume aliquots. An attempt is made to equally space the injections in a circumferential manner and at the same level. Attention should be made to maintaining a perpendicular relationship to the esophageal wall and avoiding submucosal injection (a visible bleb) or injection outside the esophageal wall. The assistant can usually give feedback regarding the degree of resistance to injection which should be consistent. Other variations such as injecting in retroflexion, utilizing endoscopic ultrasound or using different types of botulinum toxin have not gained popularity [Walzer and Hirano, 2008]. Antibiotics usually are not given and patients can be discharged immediately if stable.
Figure 2.

Injection needle.
Older age, moderate LES pressure and ‘vigorous’ achalasia have been identified by some as factors predicting a favorable response to BTI [Pasricha et al. 1996]. BTI has been clearly shown to be inferior in terms of therapeutic efficacy to both PD and myotomy [Leyden et al. 2006; Wang et al. 2009]. The most compelling aspects of BTI for achalasia are ease of technique and the paucity of and mild degree of complications. An American survey found that BTI is the most common initial therapy despite its known inferior efficacy [Enestvedt et al. 2011]. Transient chest pain is occasionally noted after BTI and reflux symptoms are seen in those who have a therapeutic response to the toxin. Case reports have noted mediastinitis, gastroparesis and fatal arrhythmia with BTI, but these may relate to suboptimal technique [Eaker et al. 1997]. BTI has been used as salvage therapy after unsuccessful PD and surgical myotomy [Annese et al. 1996]. However, BTI adds marginally to a technically successful PD [Kroupa et al. 2010].There is added risk for PD perforation after BTI [Srinivasan et al. 2000].
Endoscopic therapy prior to Heller myotomy
Multiple different endoscopic modalities are commonly employed. For example, 40% of a recent achalasia cohort required at least two different modalities [Vela et al. 2004]. There is a concern that endoscopic therapy may diminish the potential efficacy of subsequent surgical myotomy and possibly increase complications, although there are conflicting data regarding this issue [Bonavina et al. 1999; Snyder et al. 2009]. One group showed the incidence of surgical failure was higher in the prior intervention group than the naïve group (28% versus 7%, p < 0.01). In addition, the naive group reported greater symptom improvement compared with the prior intervention group [Synder et al. 2009]. Another group showed that having prior endoscopic treatment was more strongly associated with intraoperative complications, especially esophageal perforation and persistent and recurrent symptoms requiring additional therapy after myotomy [Smith et al. 2006]. It is thought that BTI at the gastroesophageal junction causes fibrosis which obliterates the surgical planes, thereby increasing the difficulty of the myotomy [Patti et al. 1999; Horgan et al. 1999]. However, there are other studies that show no difference in intraoperative complications, degree of surgical difficulty or symptom improvement with prior endoscopic treatment [Deb et al. 2005; Patti et al. 1999; Rosemurgy et al. 2005].
Nonvalidated alternative therapies and investigational therapies with limited data
Patients often receive therapy for achalasia that is not as well validated as the three traditional options of PD, BTI and myotomy. A retrospective analysis of a large achalasia treatment cohort identified patients receiving nontraditional endoscopic therapies such as Savary dilation (20%), Maloney dilation (10%) and small caliber balloon dilation similar to that used for esophageal strictures (4%) [Enesvedt et al. 2011]. Small caliber balloon dilation (<30 mm) is generally ineffective, but double small balloon dilation is more effective than using a single balloon [Yi et al. 2008].
There are also some recent single center studies reporting success with novel endoscopic approaches, which have not been validated by others so far. Examples of such approaches include the use of stents and ethanolamine injection into the LES. Achalasia has been treated successfully via the insertion of specially designed covered metallic stents (usually 30 mm); but most of the data are from a single center and there are no guidelines as to duration of stent placement and long-term follow up [Li et al. 2010]. Another small single center study from Iran reported successful treatment of 13 patients with achalasia with ethanolamine injection unto the LES [Niknam et al. 2011].
Per oral endoscopic myotomy
A recent comprehensive meta-analysis and systematic review of PD, BTI, laparoscopic and thoracoscopic Heller myotomy (THM) found LHM to offer the most durable and successful treatment outcome [Campos et al. 2009]. LHM has become a first-line approach in the USA for good surgical candidates. A first attempt to develop an endoscopic approach to LES myotomy was reported by Ortega more than 30 years ago [Ortega et al. 1980]. These investigators used a modified needle knife to dissect the LES directly through the mucosal layer and demonstrated good short-term clinical, radiologic and manometric results in 17 patients. However, no further data on their technique were reported subsequently by them or any other investigators, which may be due to the high risk of esophageal perforation, leak and mediastinitis with their direct transmural myotomy approach. It took three more decades after this early attempt at endoscopic myotomy for POEM, a safer technique for endoscopic LES myotomy, to be developed.
With the advent of natural orifice transluminal endoscopic surgery (NOTES) in 2004, novel techniques were being developed in the animal laboratory in an attempt to deconstruct laparoscopic surgical procedures and develop less invasive natural orifice ‘scarless’ endoscopic versions of these procedures. A primary focus of NOTES research has been the development of techniques that can ensure secure closure of the transluminal access track that is used to enter the mediastinum or peritoneal cavity. In 2007, Sumiyama and colleagues reported a technique of transluminal access via a submucosal tunnel approach which offsets the mucosotomy and myotomy sites and thus allows rapid secure closure with clips placed at the mucosotomy site [Sumiyama et al. 2007].This technique was utilized in a survival animal study by Pasricha to perform LES myotomy. This study demonstrated remarkably short procedure times and significant decrease in mean LES pressures in the four study pigs at manometry performed 2 weeks after the endoscopic myotomy [Pasricha et al. 2007]. Utilizing this submucosal tunnel approach, Inoue performed the first human endoscopic LES myotomy for achalasia in 2008 in Yokohama, Japan. He presented this procedure as a video at the video forum of Digestive Disease Week (DDW) 2009 [Inoue et al. 2009] and coined the elegant acronym POEM which has been widely adopted. We performed the first human POEM outside of Japan in 2009 at Winthrop University Hospital [Stavropoulos et al. 2010]. We have now performed POEM in 43 patients as part of an International Review Board (IRB) approved prospective study and our data on the first consecutive 29 patients with a mean age of 51 years and nearly 60% male predominance that have reached minimum follow up of 3 months and maximum follow up of 34 months were presented at DDW 2012 [Stavropoulos et al. 2012a]. We will present selected relevant data here as they may represent the largest POEM series with the longest follow up in the USA at present.
Over 20 centers are currently performing POEM worldwide and this number is expanding rapidly. Due to the now exponential growth of POEM, the scant peer-reviewed publications are poorly reflective of the rapidly expanding experience with this procedure. Therefore, in this review, we depend more than is customary on the data from our center presented in abstract form and awaiting publication as well as other data yet to be published, such as those of a comprehensive international POEM survey that we recently completed in July 2012. The purpose of this international POEM survey [Stavropoulos and Savides, 2012] was to obtain a very detailed up-to-date ‘snapshot’ of the status of POEM worldwide as of July 2012 and provide these data to a Natural Orifice Surgery Consortium for Assessment and Research panel tasked with the development of a white paper on POEM. Every effort was made to identify and invite all major pioneering POEM centers worldwide. A total of 19 centers were invited, 3 centers declined participation and 16 completed the survey (84% response rate) including all high-volume centers with more than 30 patients per center at the time of the survey. Although the quality of survey data is not as high as that of a published prospective series, the latter are currently in short supply. The survey has allowed us to rapidly accumulate extensive detailed data from five Asian, seven North American and four European expert centers with a combined volume of 841 POEMs. These data span every aspect of POEM. We will present selected relevant data from the survey as needed in areas where the scant published literature may provide limited or no data or data that may no longer be current (Table 3).
Table 3.
International per oral endoscopic myotomy (POEM) survey data.
| Total number of expert centers participating | 16 |
| Total number of POEMs | 841 |
| POEM operators | |
| Gastroenterologists | 11 |
| Surgeons | 14 |
| POEM setting | |
| Endoscopy room | 4 centers |
| Operating room | 11 centers |
| POEM procedural data | |
| Per center mean procedure time, min (range) | 107.8 (22–240) |
| Per center mean myotomy length, cm (range) | 10.1 (2–26) |
| Per center mean percent of patients requiring narcotics after POEM | 41.8% |
| Per center mean length of hospitalization, days (range) | 3.3 (1–18) |
| Manometry diagnoses | |
| Type I and II achalasia, n (%) | 607 (72.1%) |
| Type III (spastic) achalasia, n (%) | 45 (5.4%) |
| Disorders other than achalasia (DES, hypertensive LES, nutcracker), n (%) | 189 (22.5%) |
| POEM efficacy | |
| Per center pre-POEM mean Eckardt score (range) | 7.4 (6–9) |
| Per center post-POEM mean Eckardt score (range) | 1.17 (0.5–2) |
| Per center pre-POEM mean LES, mmHg (range) | 44.9 (19.3–82.7) |
| Per center, post-POEM mean LES, mmHg (range) | 16.1 (10.5–23.4) |
| Total overall POEM success, n (%) | 811 (96.4%) |
| Per center maximal patient follow up, mean, days (range) | 575 (56–1367) |
| POEM adverse events | |
| Total number (%) of severe nonfatal adverse events* | 27 (3.2%) |
| Minor technical adverse events with minimal clinical impact, n (%) | |
| Capnoperitoneum requiring intraprocedural venting | 70 (8.3%) |
| Inadvertent mucosal perforation of mucosal flap | 56 (6.7%) |
| Premature perforation of muscle layer at time of submucosal tunnel creation | 20 (2.4%) |
| Mortality | 0% |
Per center statistics not weighted by POEM volume per center.
Defined as follows: adverse events resulting in intensive care unit stay, readmission within 30 days, surgical conversion, surgical/IR/other intervention, prolongation of hospitalization to more than 5 days, intravenous antibiotics for more than 5 days, blood transfusions or disability requiring a higher level of care after discharge than prior to POEM.
DES, diffuse esophageal spasm; LES, lower esophageal sphincter; IR, Interventional Radiology.
Technique
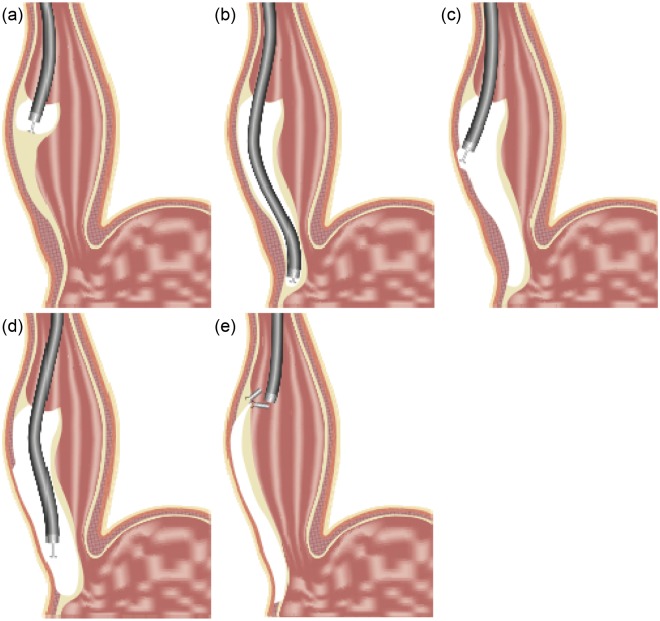
A simplified illustration of the POEM technique is shown in Figure 3. It consists of the following steps:
Figure 3.
Per oral endoscopic myotomy technique. (a) After submucosal (SM) saline injection, a mucosotomy is performed and dissection of the SM tunnel is initiated. (b) Dissection of SM tunnel is extended to the gastric cardia. (c) Myotomy initiation. Dissection of the circular layer. (d) Extension of the myotomy to the muscle of the cardia with approximately 2 cm long cardiomyotomy. (e) Closure of the mucosotomy (entrance to the SM tunnel) using endoscopic clips (reproduced with permission of Winthrop University Hospital, 2012).
A submucosal injection is used to expand the submucosal space at a point 10–15 cm proximal to the LES followed by a 2 cm long mucosal incision using an electrical knife.
The endoscope is inserted into the submucosal space and with sequential submucosal injection and submucosal dissection using an electrical knife, a dilation balloon or a combination thereof. A long submucosal tunnel is created along the right wall of the esophagus and is extended beyond the LES approximately 2–4 cm into the submucosa of the cardia along the lesser curvature.
The endoscope is then withdrawn to approximately 2–3 cm distal to the mucosal incision site where the start of the myotomy will take place, thus offsetting the mucosal defect and the muscle defect, which is the ingenious and critical feature of this technique that allows secure closure. At the starting point of the myotomy, the muscle is dissected until the plane between the inner circular and outer longitudinal layer is exposed. At that point, the circular muscle myotomy is initiated by hooking the circular fibers with the knife and cutting them proceeding distally until the myotomy is extended about 2 cm into the cardia (cardiomyotomy). Extension of the myotomy to the muscle of the cardia is based largely on LHM literature demonstrating higher efficacy of Heller myotomy when it includes cardiomyotomy [Oelschlager et al. 2003]. A small recent study in a porcine survival model suggested that this may also be the case with POEM [Bonin et al. 2012].
It should be emphasized that POEM is a technique in evolution. For example, at the beginning of our experience in 2009 the triangular tip (TT) Olympus Endoscopic Submucosal Dissection (ESD) knife (Tokyo, Japan) used by Inoue for POEM (Figure 4) was not available in the USA. In fact, no specialized Olympus ESD knife was available for purchase in the USA until September 2011. Furthermore, our experience with ESD, the technique encompassing the critical skills required for POEM, although extensive by Western standards, was limited by Asian standards (a common handicap of Western endoscopists). For these reasons, we used a variant technique (similar to the one described by Pasricha in the animal lab) [Pasricha et al. 2007] with complete tunnel dissection using a dilation balloon (rather than dissection with a dedicated ESD knife as described by Inoue) [Inoue et al. 2009] and we performed the myotomy using a standard needle knife. This balloon dilation approach resulted in faster tunnel formation but required care during blunt insertion of the dilation balloon in the submucosa to avoid injury of the mucosa or muscularis propria. The technique of balloon dilation to form the submucosal tunnel was recently also used by Gostout and colleagues successfully to perform the first POEM at the Mayo Clinic [Gostout, 2012, personal communication]. Once dedicated ESD knives became available in the USA, our team continued to use balloon dilation to initiate the submucosal tunnel formation in the esophageal body but then used a TT knife as described by Inoue to extend the submucosal tunnel dissection into the cardia as well as perform the myotomy. For the last 25 cases, however, we have used the T-type hybrid knife (ERBE Elektromedizin GmbH, Tübingen, Germany) (Figure 5), for submucosal dissection and myotomy without recourse to balloon dilation. Based on our initial experience, the ability of the hybrid knife to fluidly perform submucosal injection at any point during the submucosal dissection without change of accessories may increase safety and speed. In fact, the group from Shanghai with the largest number of completed POEMs to date (over 300) compared the hybrid knife with the TT knife in a head-to-head prospective randomized study of 43 POEMs. The results were reported at DDW 2012 and revealed shorter procedure time, lower bleeding rate and lower frequency of usage of coagulation forceps when the hybrid knife was used [Zhou et al. 2012a].
Figure 4.

Triangular tip knife (Olympus).
Figure 5.

T-type hybrid knife (ERBE Elektromedizin GmbH). Note the tiny injection port at the tip of the knife which allows saline injection during dissection.
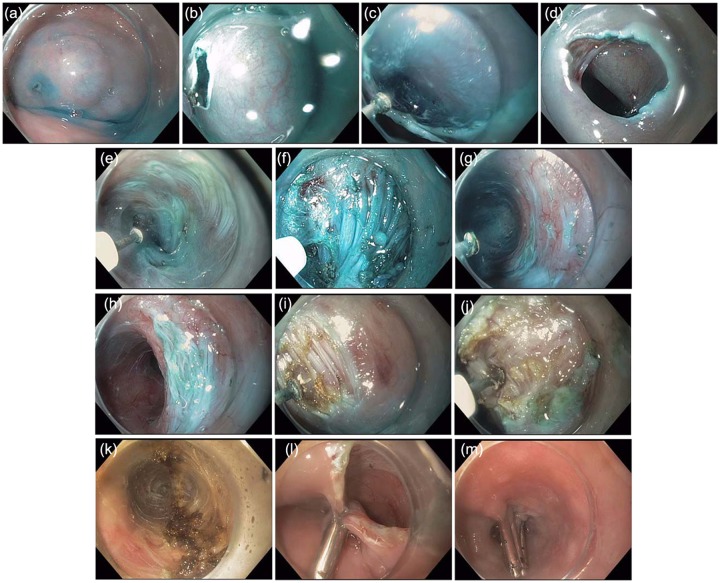
Endoscopic images illustrating the steps of the POEM technique are shown in Figure 6. A detailed review of POEM equipment and technique is not part of the scope of this review. For the interested reader we have reviewed these in detail in a 10 min instructional video submitted to Video Journal and Encyclopedia of GI Endoscopy [Stavropoulos et al. 2012b].
Figure 6.
(a) Submucosal (SM) injection (saline stained with methylene blue or indigo carmine) performed using the hybrid knife at the site of planned mucosotomy about 10–15 cm proximal to the lower esophageal sphincter (LES). (b) Mucosal incision. (c) Extension of mucosal incision. (d) Completed dissection of initial portion of SM tunnel seen through the mucosotomy orifice. (e) Dissection of the SM tunnel along the body of the esophagus (muscularis to the right, mucosa to the left). (f) Dissection of the SM tunnel over the LES seen as a thick bundle of circular fibers resisting passage of the endoscope into the cardia. (g) SM tunnel successfully extended into the cardia recognized by spacious SM space and increased vascularity (spindle-like veins seen in the muscle on the right side of the image). (h) Endoscope withdrawn from the cardia and demonstrating the LES protruding into the lumen of the SM tunnel. (i) Myotomy is initiated proximally at a site approximately 3 cm distal to the mucosotomy site (the entry site of the SM tunnel). (j) Circular muscle fibers are dissected at the same site and increasing depth until the plane between the circular and longitudinal layers is encountered as shown here. When this plane is encountered, dissection in a proximal to distal direction is performed with hooking and cutting of the circular fibers to approximately 2 cm into the cardia of the stomach. (k) Here the completed myotomy across the LES and into the cardia is demonstrated. The coagulated edges of the cut muscle are seen at 12 and 6 o’clock positions on the picture. (l) and (m) The mucoscotomy site (the entrance into the SM tunnel) is closed using endoclips (in this case Resolution Clips, Boston Scientific).
Setting, operator
Published data on setting and operator characteristics are extremely limited. However, the international POEM survey yielded important information (Table 3). In the 16 centers participating in the survey, POEM is performed by 11 gastroenterologists and 14 surgeons. POEM is performed in an operating room (OR) at most centers but notably 4/16 centers participating in the survey perform POEM in the endoscopy unit. The consensus that appears to be developing is that the setting itself is less important than ensuring that the following conditions are met:
Expert anesthesiologist and specialized monitoring and anesthesia equipment that can allow safe delivery of general anesthesia with anesthetic gas and a paralytic agent. Positive pressure ventilation is considered essential to minimize the risk of massive pneumothorax and pneumomediastinum, including tension pneumothorax [Inoue et al. 2011].
Advanced endoscopy expertise, including advanced ESD skills such as expert control of bleeding, endoscopic closure of perforations and needle decompression of tense pneumoperitoneum. Appropriate equipment and devices for advanced endoscopic interventions, including high-frequency electrosurgical generators with sophisticated current controls, carbon dioxide insufflation, high-definition endoscopes with accessory irrigation channel, ESD knives and distal cap attachments, and a variety of endoscopic clips and other closure devices.
Availability of surgical ‘back up’ and ability to expeditiously perform surgery should an emergent surgical intervention be required. POEM experts have suggested that this requirement can be fulfilled if transfer to an OR and surgery can be accomplished within about 30 min or so as is customary, for example, in cases of complex duodenal or colonic polypectomies if large perforations occur that cannot be adequately closed endoscopically or that are associated with gross peritoneal soiling. At our center, a surgeon with expertise in minimally invasive/robotic surgery is available should emergent surgical intervention be required. Although such an intervention has not been required on any of the 43 POEMs we have performed over the past 3 years, we believe that it would be prudent to wait for more extensive safety data before this requirement can be relaxed.
Efficacy
Even with the caveats of small numbers, early experience and short-term follow up, initial POEM data suggest POEM efficacy at least similar to that of LHM. For example, in our series of 43 patients, all attempted POEMs were successfully and safely completed, including in patients as old as 93 years old and patients with severe anatomic abnormalities such as megaesophagus (as large as 11. 5 cm), large 5 cm esophageal epihrenic diverticulum, sigmoid type 2 severe sigmoid achalasia, prior BTI, PD or failed Heller myotomy, and significant comorbidities, including emphysema and heart failure. In our series, the mean Eckardt score improved from 7.41 before to 1.0 after POEM (p < 0.0001). For the 29 patients for whom minimum follow up of at least 3 months is available, there have been only two failures (Eckardt score >3). PD was performed for these patients after POEM and both patients had an excellent response to PD and are currently asymptomatic (Eckardt score 0). It should be noted that both patients with treatment failure were early in our experience when we were performing shorter myotomies as initially described by Inoue (3–5 cm). Our results are similar to those published in full or abstract form and summarized in detail in Table 4 and to those reported by centers participating in the international POEM survey (Table 3). The universally reported POEM treatment success of over 90% is similar to the initial treatment success of LHM as reported, for example, in the recent meta-analysis by Campos and colleagues [Campos et al. 2009].
Table 4.
Comparing selected published per oral endoscopic myotomy (POEM) experiences.
| Lead author [year], site(s) |
|||||||
|---|---|---|---|---|---|---|---|
| Inoue [2010] Yokohama, Japan | Costamagna [2012] Rome, Italy | Swanström [2012] Portland, OR, USA | von Renteln [2012] Hamburg and Frankfurt, Germany; Amsterdam, The Netherlands; Zürich, Switzerland; Montreal, QC, Canada | von Renteln [2012] Hamburg and Frankfurt, Germany | Eleftheriadis [2012] Yokohama, Japan | Stavropoulos [2012] Mineola, NY, USA | |
| Number of centers | 1 | 1 | 1 | 5 (MCT) | 2 (MCT) | 1 | 1 |
| Number of patients | 17 | 11 | 5 | 51 | 16 | 236 | 12 |
| Completed POEMs | 17 | 10 | 5 | 51 | 16 | 236§ | 12 |
| Age (years), mean (range) | 41.4 (18–62) | 32 (24–58) | 67* (49–78) | 43 (20–76) | 45 (26–76) | – (3–87) | 51 – |
| Gender (women:men) | 7:10 | 8:3 | 2:3 | 22:29 | 4:12 | – | 5:7 |
| Duration of achalasia, months (range) | 100.8 (6–360) | 21.5 | – | – | – | 132 (6–600) | – |
| Type of achalasia | |||||||
| Nonsigmoid (N) | 12 N | 10 N | 5 N | – | – | 9 N | |
| Sigmoid (S) | 5 S | 0 S | 0 S | 16 S | 3 S | ||
| Esophageal diameter | |||||||
| Grade I (<3.5 cm) | 3 | – | – | – | – | 1 | |
| Grade II (3.5–6 cm) | 11 | 9 | |||||
| Grade III (>6 cm) | 3 | 0 | 2 | ||||
| Prior treatment | |||||||
| Botox (BT) | 1 BT | 4 BT | – | 1 BT | 0 BT | ||
| Pneumatic dilation (PD) | 3 PD | 1 PD | 1 PD | 9 PD | 0 PD | ||
| Both BT + PD | 1 BT+PD | ||||||
| Heller (HM) | 5 HM | 0 HM | |||||
| Procedure time (min), mean (range) | 126 (100–180) | 100.7 (75–140) | (120–240) | 105( 57–237) | 114 (65–188) | 113.4 (56–240) | 150 (102–240) |
| Length of myotomy (cm), mean (range) | 8.1 N: 6.5 (3–14) S: 10.6 (7–15) | 10.2 | 7.5* (6–12) | 13 (7–23) | 12 (8–17) | 14 (3–23) | 5.5 (3–10) |
| Follow up (months) | 5 | 3 | 0.5 | 3 | 3 | 11$ | 9.5$ |
| POEM success|| (% of patients) | 100% | 91% 1 POEM aborted due to submucosal fibrosis – performed EBD | 100% | 94% 3 failed to have symptom improvement (Eckardt score ≤ 3) | 94% 1 retreatment with EBD | 99% 1 retreatment with EBD 1 retreatment with POEM | 92% 1 retreatment with EBD |
| Pre/post mean LES resting pressure (mmHg) | 52.4/19.8 (p = 0.0001) | 45.1/16.9 (p = 0) | 55.1*/36.5* | 27.4/10.2 (p < 0.001) | 27.2/11.8 (p < 0.001) | 26.8/12.6 (p < 0.001) | 48.2/22.08 (p = 0.0264) |
| Pre/post mean Eckardt score | 10/1.3‡ (p = 0.0003) | 7.1/1.1 (p = 0) | –/0 or 1‡ | 7.9/1.4 (p < 0.001) | 8.8/1.4 (p < 0.001) | 6.36/1.45 (p = 0.003) | 7.8/0.7 (p = 0.0001) |
| Length of stay (days), mean (range) | 4.8 (3–8) | 4 | 1.2 | – | – | 5.9 (3–10) | 2.2 (1–4) |
| GERD symptoms | 6% | 0% | - | 20% (7/35) | 0% | 10.5% | 0% |
| GERD by pH study | – | – | – | – | – | – | – |
| GERD endoscopic evidence (erosions) | 6% (1 reflux esophagitis LA classification B) | 0% | – | 17% (5/30 with follow up EGD) | 6% (1 erosive esophagitis LA classification A) | – | – |
Median; $mean; ‡non-Eckardt dysphagia score; §total number per communication with lead author (Eleftheriadis).
Failure defined as need to employ alternative achalasia treatments or failure to meet the criterion or criteria for successful treatment. It includes technical failure/inability to complete the procedure, failure to achieve appropriate symptoms improvement (e.g. Eckhardt score ≤ 3) or recurrence of symptoms during short follow up provided by these studies (e.g. initial improvement but substantial increase in Eckardt score to more than 3 on follow up).
GERD, gastroesophageal reflux disease; LES, lower esophageal sphincter ; MCT, multicenter trial; EBD, endoscopic balloon dilation; EGD, Esophagogastroduodenoscopy; LA, Los Angeles.
Adverse events
Data regarding adverse events (AEs) remain limited. We have had no significant AEs in the 43 POEMs performed to date, despite having included five patients older than 80 years, patients with significant comorbidities and patients with failed prior BTI or PD, and even a patient with failed prior LHM. Minor AEs included mainly tense capnoperitoneum and inadvertent injuries to the mucosal flap. Both of these minor AEs occurred in a small number of patients mainly early in our experience and were easily treated with needle decompression and placement of endoscopic clips respectively. These minor AEs had no significant clinical impact, except possibly for slight prolongation of hospitalization for an additional day on average for purely precautionary reasons.
The generally minor AEs reported by others are listed in Table 5 for published series and a summary of AE data from the international POEM survey are shown in Table 3. It should be noted that in the case series from Shanghai by Ren and colleagues the very high rate of pneumothorax of about 28% is at variance with all the other series and, along with the very high rates of pneumoperitoneum and subcutaneous and mediastinal emphysema reported by this group, are believed to be due to this group’s initial use of air instead of carbon dioxide for insufflation [Ren et al. 2012]. Use of carbon dioxide is essential to minimize such complications. Significant nonfatal AEs (international POEM survey, Table 3) have been reported in a small percentage of patients. Overall, largely self-limited AEs resulting from bleeding or gas in the thorax or abdomen appear to be the main significant complications.
Table 5.
Comparing selected published per oral endoscopic myotomy complications.
| Study | Complications | Cases (%) |
|---|---|---|
| Costamagna et al. [2012] (10 cases) | Intraoperative | |
| Junctional flap perforation | 2/10 (20%) | |
| Cervical emphysema | 2/10 (20%) | |
| Pneumomediastinum | 10/10 (100%) | |
| Postoperative | ||
| Mild chest pain | 10/10 (100%) | |
| von Renteln et al. [2012b] (16 cases) | Intraoperative | |
| Full thickness dissection into peritoneal cavity | 9/16 (56.3%) | |
| Full thickness dissection into mediastinum | 13/16 (81.3%) | |
| Postoperative | ||
| Cutaneous emphysema | 6/16 (37.5%) | |
| Pneumoperitoneum | 8/16 (50%) | |
| Mucosal perforation | 1/16 (6.3%) | |
| Superficial ulcer at cardia | 1/16 (6.3%) | |
| Ulcer in distal esophagus | 1/16 (6.3%) | |
| Follow up | ||
| Erosive esophagitis | 1/16 (6.3%) | |
| Yoshida et al. [2012] (161 cases) | Postoperative | |
| Aspiration pneumonia | 1/161 (0.6%) | |
| Lesser omentum inflammation | 1/161 (0.6%) | |
| Submucosal hematoma | 1/161(0.6%) | |
| Pneumothorax | 1/161 (0.6%) | |
| Follow up | ||
| GERD | 17/161(10.6%) | |
| Eleftheriadis et al. [2012] (236 cases) | Postoperative | |
| Local peritonitis | 1/197 (0.5%) | |
| Mucosal laceration in cardia | 3/197 (1.5%) | |
| Intramucosal hematoma | 1/197 (0.5%) | |
| Pneumothorax | 1/197 (0.5%) | |
| Ren et al. [2012] (119 cases) | Intraoperative | |
| Cutaneous emphysema | 27/119 (22.7%) | |
| Pneumothorax | 3/119 (2.5%) | |
| Postoperative | ||
| Pneumothorax | 30/119 (25.2%) | |
| Subcutaneous emphysema | 66/119 (55.5%) | |
| Mediastinal emphysema | 35/119 (29.4%) | |
| Delayed hemorrhage | 1/119 (0.8%) | |
| Pleural effusion | 58/119 (48.7%) | |
| Segmental atelectasis of the lungs | 59/119(49.6%) | |
| Aeroperitoneum | 47/119 (39.5%) | |
| Follow up | ||
| Esophageal stricture | 1/119 (0.8%) | |
| Dehiscence at tunnel entry | 1/119 (0.8%) | |
| Swanström [2012] (5 cases) | Intraoperative | |
| Gastric mucosotomies | 2/5 (40%) | |
| Pneumoperitoneum | 3/5 (60%) | |
GERD, gastroesophageal reflux disease
Interestingly, there are no reported instances of leaks and mediastinal sepsis, the initial paramount concern of most investigators embarking on POEM. It should be noted, however, that there was a rumored death of a 19-year-old woman from mediastinal sepsis 3 days after having had a POEM procedure. To the best of our knowledge, this rather alarming rumor has not been confirmed at this time several months since its first appearance [Swanström, 2012]. It should be noted that even if this event is confirmed, and assuming that there are no other unreported deaths at this time, this would put POEM mortality at approximately 1/900, which is similar to or even lower than the 0.2–0.8% mortality from LHM [Campos et al. 2009]. It should be emphasized here that POEM is an invasive procedure breeching the barrier between the gastrointestinal lumen and the mediastinum and peritoneum, and the experience with POEM at this time does not allow same-day discharge to be routinely considered, even in apparently healthy, young patients. More safety data are required before same-day discharge can be advocated for selected patients.
Extended indication patients
Very little data exist in the published literature regarding POEM in patients with nonachalasia hypercontractile conditions of the esophagus, age extremes, sigmoid and megaesophagus or in patients with prior treatment with BTI, PD or Heller myotomy. However, data from the international POEM survey show that POEM has been successfully applied to such extended indication patients.
Shiwaku and colleagues recently reported in a case report a long myotomy performed via POEM to successfully treat a patient with diffuse esophageal spasm (DES) [Shiwaku et al. 2012]. The international POEM survey data indicate that POEM was performed for DES, hypertensive LES or nutcracker esophagus in 189/841 patients (22.5%) (Table 3). Participants report that POEM efficacy for DES may be lower than that for achalasia whereas POEM efficacy for hypertensive LES and nutcracker esophagus may be higher than that of achalasia.
Regarding patients with prior failed treatments, among the five published series, four reported substantial numbers of patients undergoing POEM after prior BTI or PD: Costamagna 2/11 (18%) [Costamagna et al. 2012], Ren 36/119 (30%) [Ren et al. 2012], von Renteln 11/16 (69%) [von Renteln et al. 2012b] and Swanström 4/5 (80%) [Swanström et al. 2012]. None of the authors performed a formal subgroup analysis, but it appears that the statement from von Renteln that POEM in previously endoscopically treated patients is ‘more challenging but feasible’ captures the majority view [von Renteln et al. 2012b].
POEM after failed LHM presents special challenges given the long surgical intervention with scar formation, the dissection of structures around the gastroesophageal junction causing altered anatomy and possible neovascularization and frequent presence of an anterior or posterior fundoplication. Looking at the published series, there is a brief mention in one publication [Inoue et al. 2011] of seven patients undergoing POEM after failed LHM, out of a series of 105 POEMs, and in another series [Ren et al. 2012] six patients out of a series of 119 POEMs undergoing POEM after prior failed THM. Excellent general efficacy was reported but without specific subgroup analysis. Having successfully performed POEM in a patient with failed prior Heller myotomy as the 37th patient in our series we agree with the recommendation by the Shanghai and Yokohoma operators for significant POEM experience of 20–30 cases prior to tackling failed Heller myotomy cases.
Regarding patients with end-stage achalasia manifested as sigmoid or severely dilated esophagus, Inoue and colleagues reported POEM in 11/17 patients with grade II (3.5–6 cm) and 3/17 patients with grade III (>6 cm) disease without, however, offering specific subgroup analysis [Inoie et al. 2010]. They reported POEM in 5/17 patients with moderate (S1) sigmoid esophagus with good efficacy but a lower decrease in the LES pressure compared with the 12 patients without sigmoid esophagus [Inoue et al. 2010]. On extended data briefly presented in a 2011 review, Inoue and colleagues report successful POEM in 16/105 patients with sigmoid achalasia [Inoue et al. 2011]. Data from the LHM literature suggest that most patients with end-stage achalasia and megaesophagus or severe sigmoidization may ultimately require esophagectomy due to the low efficacy of Heller myotomy but an initial trial of myotomy is deemed reasonable as a less invasive approach. At our center and others around the world such patients are not excluded from POEM consideration and initial data on technical challenges and efficacy should be forthcoming.
There are very limited data in the published literature regarding POEM in patients at age extremes. POEM has been reported in pediatric patients as young as 3 [Eleftheriadis et al. 2012] and 6 [Zhou et al. 2012b]. Very few data are available on patients over 80 years old.
Training and accreditation
As we move towards POEM standardization, it should be emphasized that very large majorities of the 16 centers participating in the international POEM survey supported the need for preclinical lab training (87.5%), proctoring on the first human cases (100%) and IRB approval prior to initiating POEM at a center (62.5%). There was also high agreement among respondents in recommending a formal center accreditation for POEM (‘similar to that used for bariatric surgery centers in the US’) (68.8%), POEM operator certification (‘similar to that used for robotic surgery in the US’) (68.8%) and even mandatory reporting of severe AEs to an independent monitoring body (87.5%). The strong agreement with regard to these recommendations despite the disparate backgrounds of the respondents (including both surgeons and gastroenterologists with wide geographic distribution of centers in Asia, Europe and North America) underscores the fact that POEM is a novel invasive endoscopic surgical procedure that requires appropriate training and preparation, and rigorous accreditation and monitoring of outcomes to ensure safety and efficacy.
Potential advantages of POEM compared with LHM
It is anticipated that POEM will achieve results as durable as those of LHM but with less invasiveness, similar to that of endoscopic therapies. Two POEM centers where POEM is performed by surgeons with significant prior experience with LHM, one in Germany and one in the USA, have presented in lectures archived online unpublished data comparing recent consecutive LHMs with the initial experience with POEM by the same operator(s) that performed the LHMs (Table 6 and 7). Although these are relatively limited, retrospective data, they appear to suggest at least equivalence between the two procedures in terms of procedure duration, morbidity and efficacy. These are very encouraging data since equivalence at this point, with POEM being at a nascent stage and LHM at a very mature, fully developed stage, likely portends dominance of POEM in the not too distant future. Potential specific advantages of POEM may include the following:
Table 6.
Retrospective comparison of per oral endoscopic myotomy (POEM) with laparoscopic Heller myotomy (LHM).
| POEM | LHM | p | |
|---|---|---|---|
| Number of patients | 11 | 50 | |
| Age (years) | 36 ± 11 (18–85) | 50 ± 16 | p < 0.01 |
| Prior achalasia treatment | None | – | |
| Type of achalasia | 11 nonsigmoid | – | |
| Gender (men:women) | 7:4 | 26:24 | ns |
| Duration of symptoms (years) | 2 ± 5 | 2 ± 3 | ns |
| Operation time (min) | 121 ± 42 | 126 ± 29 | ns |
| Estimated blood loss (ml) | ≤10 | 91 ± 55 | p < 0.001 |
| Myotomy length (cm) | 8.0 ± 1.1 | 8.5 ± 0.7 | p = 0.04 |
| Pain score on day of surgery | 3.3 ± 3.1 | 2.1 ± 2.3 | ns |
| Pain score on postoperative day 1 | 2.5 ± 2.8 | 2.1 ± 2.3 | ns |
| Use of narcotics on day of surgery (mg morphine equivalents) | 4.8 ± 5.2 | 2.8 ± 4.3 | ns |
| Use of narcotics on postoperative day 1 (mg morphine equivalents) | 6.9 ± 7.7 | 4.6 ± 5 | ns |
| Length of hospitalization (days) | 2.3 ± 3.6 | 1.6 ± 2.9 | ns |
| Minor complication, number of cases (%) | 3 (27%) | 7 (14%) | ns |
| Major complication | A contained leak at the EGJ requiring laparoscopic drain placement | Delayed esophageal leak requiring thoracotomy for drainage and repair | |
| Preoperative versus 6-week postoperative LES basal and relaxation pressure (mmHg) | Basal: 25 ± 10 versus 12 ± 7 | – | p = 0.04 |
| Relaxation: 29 ± 17 versus 15 ± 3 | p < 0.05 | ||
| Timed barium: difference in contrast height (cm) at 1, 2 and 5 min | 4 versus 17
2 versus 16 2 versus 11 |
– |
p = 0.02
p = 0.04 |
Data from Hungness et al. [2012], Northwestern, USA.
Table 7.
Retrospective comparison of per oral endoscopic myotomy (POEM) to laparoscopic Heller myotomy (LHM).
| Characteristics | POEM | LHM |
|---|---|---|
| No. of patients | 12 | 151 |
| Prior failed achalasia treatment (%) | 80% | 94% |
| Morbidity | 2 cases | 5% |
| Need for reintervention | 0% | 3% |
| Mortality | 0 | 0 |
| Median pre/post Eckhardt score | 7/1 | 8/1 |
| Gastroesophageal reflux | ||
| No. of clinical cases (%) | 1/10 (10%) | (6%) |
| No. on pH study (%) | 3/8 (37.5%) | (5%) |
| Pre/post GIQLI | 105/117 | 97/117 |
Data from Fuchs [2012], Frankfurt, Germany.
GIQLI, Gastrointestinal Quality of Life Index.
Easy extension of the myotomy to any length, permitting long myotomies that may be optimal for patients with DES [Shiwaku et al. 2012] or other disorders with long hypercontractile esophageal segments.
Less risk of injury to the vagus nerve.
Less reflux since attachments of the esophagus such as the phrenoesophageal membrane are not disrupted as they are in LHM. LHM results in severe reflux in a majority of patients. Therefore, LHM is combined with a Dor or Toupet partial fundoplication. Even when LHM is combined with fundoplication, reflux disease is the most frequent cause of poor long-term LHM outcomes [Csendes et al. 2006]. Objective evidence of reflux has been recently reported in approximately 20% of POEM patients [von Renteln et al. 2012a], which, while not as favorable as initial reports [Inoue et al. 2010; von Renteln et al. 2012b] (see Table 4) is still similar to a LHM combined with fundoplication.
Less pain than in LHM has been noted in our POEM series, with only 14% requiring post-POEM narcotics and only for a day or two at most, and this has also been the experience of other POEM operators [Swanström et al. 2011; von Renteln et al. 2012b].
Given these emerging advantages of POEM and the minimal invasiveness of this NOTES approach to LES myotomy, it is not surprising that most POEM experts, including surgeons with extensive LHM experience, believe that POEM will largely replace LHM. POEM could plausibly become the first-line treatment approach for achalasia in all patients except those with very severe comorbidities or end-stage sigmoid esophagus or megaesophagus.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Stavros N. Stavropoulos, Winthrop University Hospital, 222 Station Plaza North Suite 429, Mineola, NY 11501, USA
David Friedel, Winthrop University Hospital, Mineola, NY, USA.
Rani Modayil, Winthrop University Hospital, Mineola, NY, USA.
Shahzad Iqbal, Winthrop University Hospital, Mineola, NY, USA.
James H. Grendell, Winthrop University Hospital, Mineola, NY, USA
References
- Ahmed A. (2008) Achalasia: what is the best treatment? Ann Afr Med 7: 141–148 [DOI] [PubMed] [Google Scholar]
- Alderliesten J., Conchillo J., Leeuwenburgh I., Steverberg E., Kulpers E. (2011) Predictors for outcome of failure dilatation in patients with achalasia. Gut 60: 10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M., Lundell L., Kostic S., Ruth M., Lönroth H., Kjellin A., et al. (2009) Evaluation of the response to treatment in patients with idiopathic achalasia by the timed barium esophagogram: results from a randomized clinical trial. Dis Esophagus 22: 264–273 [DOI] [PubMed] [Google Scholar]
- Annese V., Basciani M., Borrelli O., Leandro G., Simone P., Andriulli A. (1998) Intrasphincteric injection of botulinum toxin is effective in long-term treatment of esophageal achalasia. Muscle Nerve 21: 1540–1542 [DOI] [PubMed] [Google Scholar]
- Annese V., Basciani M., Lombardi G., Caruso N., Perri F., Simone P., et al. (1996) Per-endoscopic injection of botulinum toxin is effective in achalasia after failure of myotomy or pneumatic dilation. Gastrointest Endosc 44: 461–465 [DOI] [PubMed] [Google Scholar]
- Bell R. (1997) Laparoscopic closure of esophageal perforation following pneumatic dilation for achalasia. Report of two cases. Surg Endosc 11: 476–478 [DOI] [PubMed] [Google Scholar]
- Boeckxstaens G., Annese V., des Varannes S., Chaussade S., Costantini M., Cuttitta A., et al. ; European Achalasia Trial Investigators (2011) Pneumatic dilation versus laparoscopic Heller’s myotomy for idiopathic achalasia. New England Journal of Medicine 364(19):1807–16 [DOI] [PubMed] [Google Scholar]
- Bonavina L., Incarbone R., Antoniazzi L., Reitano M., Peracchia A. (1999) Previous endoscopic treatment does not affect complication rate and outcome of laparoscopic Heller myotomy and anterior fundoplication for oesophageal achalasia. Ital J Gastroenterol Hepatol 31: 827–830 [PubMed] [Google Scholar]
- Bonin E., Moran E., Bingener J., Knipschield M., Gostout C. (2012) A comparative study of endoscopic full-thickness and partial-thickness myotomy using submucosal endoscopy with mucosal safety flap (SEMF) technique. Surg Endosc 26: 1751–1758 [DOI] [PubMed] [Google Scholar]
- Borotto E., Gaudric M., Daniel B., Samama J., Quartier G., Chaussade S., et al. (1996) Risk factors for immediate complications after progressive pneumatic dilation for achalasia. Gut 39: 9–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos G., Vittinghoff E., Rabl C., Takata M., Gadenstätter M., Lin F., et al. (2009) Endoscopic and surgical treatments for achalasia: a systematic review and meta-analysis. Ann Surg 249: 45–57 [DOI] [PubMed] [Google Scholar]
- Chan K., Wong S., Lee D., Mui W., Chan A., Ng E., et al. (2004) Short-term and long-term results of balloon dilation for achalasia: 12 years’ experience. Endoscopy 36: 690–694 [DOI] [PubMed] [Google Scholar]
- Costamagna G., Marchese M., Familiari P., Tringali A., Inoue H., Perri V.(2012) Peroral endoscopic myotomy (P.O.E.M.) for oesophageal achalasia: preliminary results in humans. Dig Liver Dis 18 May 18 (Epub ahead of print). DOI: 10.1016/j.dld.2012.04003 [DOI] [Google Scholar]
- Csendes A., Braghetto I., Burdiles P., Korn O., Csendes P., Henriquez A. (2006) Very late results of esophagomyotomy for patients with achalasia. Ann Surg 243: 196–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb S., Deschamps C., Allen M., Nichols F., Cassivi S., Crownhart B., et al. (2005) Laparoscopic esophageal myotomy for achalasia: factors affecting functional results. Ann Thorac Surg 80: 1191–1194; discussion 1194–1195. [DOI] [PubMed] [Google Scholar]
- de Oliveira J., Birgisson S., Doinoff C., Einstein D., Herts B., Davros W., et al. (1997) Timed barium swallow: a simple technique for evaluating esophageal emptying in patients with achalasia. Am J Roentgenol 169: 473–479 [DOI] [PubMed] [Google Scholar]
- Dobrucali A., Erzin Y., Tuncer M., Dirican A. (2004) Long-term results of graded pneumatic dilation under endoscopic guidance in patients with primary esophageal achalasia. World J Gastroenterol 10: 3322–3327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dughera L., Cassolino P., Cisaro F., Chiaverina M. (2008) Achalasia. Minerva Gastroenterol Dietol 54: 277–285 [PubMed] [Google Scholar]
- Duranceau A., Liberman M., Martin J., Ferraro P. (2012) End-stage achalasia. Dis Esophagus 25: 319–330 [DOI] [PubMed] [Google Scholar]
- Eaker E., Gordon J., Vogel S. (1997) Untoward effects of esophageal botulinum toxin injection in the treatment of achalasia. Dig Dis Sci 42: 724–727 [DOI] [PubMed] [Google Scholar]
- Eckardt V., Goekel I., Bernhard G. (2004) Pneumatic dilation for achalasia: late results of a prospective follow up investigation. Gut 53: 629–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleftheriadis N., Inoue H., Ikeda H., Onimaru M., Yoshida A., Hosoya T., et al. (2012) Training in per oral endoscopic myotomy (P.O.E.M.) for esophageal achalasia. Ther Clin Risk Manag 8:329–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enestvedt B., Williams J., Sonnenberg A. (2011) Epidemiology and practice patterns of achalasia in a large multi-centre database. Aliment Pharmacol Ther 33: 1209–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhoomand K., Connor J., Richter J., Achkar E., Vaezi M. (2004) Predictors of outcome of pneumatic dilation in achalasia. Clin Gastroenterol Hepatol 2: 389–394 [DOI] [PubMed] [Google Scholar]
- Fuchs K. (2012) Postgraduate course: per oral endoscopic myotomy (P.O.E.M.): clinical experience in Europe, SAGES 2012 Meeting, San Diego, CA, 18 May [Google Scholar]
- Ghoshal U., Rangan M., Misra A. (2012) Pneumatic dilation for achalasia cardia: reduction in lower esophageal sphincter pressure in assessing response and factors associated with recurrence during long-term follow up. Dig Endosc 24: 7–15 [DOI] [PubMed] [Google Scholar]
- Gockel I., Junginger T., Eckhardt V. (2005) Effects of pneumatic dilation and myotomy on esophageal function and morphology in patients with achalasia. Am Surg 71: 128–131 [PubMed] [Google Scholar]
- Guardino J., Vela M., Connor J., Richter J. (2004) Pneumatic dilation for the treatment of achalasia in untreated patients and patients with failed Heller myotomy. J Clin Gastroenterol 38: 855–860 [DOI] [PubMed] [Google Scholar]
- Horgan S., Hudda K., Eubanks T., McAllister J., Pellegrini C. (1999) Does botulinum toxin injection make esophagomyotomy a more difficult operation? Surg Endosc 13: 576–579 [DOI] [PubMed] [Google Scholar]
- Howard J., Mongam A., Manning B., Byrne P., Lawler P., Ravi N., et al. (2010) Outcome in achalasia from a surgical unit where pneumatic dilation is first-line therapy. Dis Esophagus 23: 465–472 [DOI] [PubMed] [Google Scholar]
- Hulselmans M., Vanuytsel T., Degreef T., Sifrim D., Coosemans W., Lerut T.(2010) Long-term outcome of pneumatic dilation in the treatment of achalasia. Clin Gastroenterol Hepatol 8: 30–35 [DOI] [PubMed] [Google Scholar]
- Hungness E., Teitelbaum E., Santos B., Arafat F., Pandolfino J., Soper N. (2012) Comparison of perioperative outcomes after per-oral esophageal myotomy (P.O.E.M.) and laparoscopic Heller myotomy. Gastroenterology 142(Suppl. 1): S1035–S1036 [DOI] [PubMed] [Google Scholar]
- Inoue H., Minami H., Kobayashi Y., Sato Y., Kaga M., Suzuki M., et al. (2010). Per oral endoscopic myotomy (P.O.E.M.) for esophageal achalasia. Endoscopy 42: 265–271 [DOI] [PubMed] [Google Scholar]
- Inoue H., Minami H., Satodate H., Kudo S. (2009) First clinical experience of submucosal endoscopic myotomy for esophageal achalasia with no skin incision (with video). Gastrointest Endosc 69: AB122 [Google Scholar]
- Inoue H., Tianle K., Ikeda H., Hosoya T., Onimaru M., Yoshida A., et al. (2011) Per oral endoscopic myotomy for esophageal achalasia: technique, indication, and outcomes. Thorac Surg Clin 21: 519–525 [DOI] [PubMed] [Google Scholar]
- Jankovic J., Brin M. (1991) Therapeutic uses of botulinum toxin. N Engl J Med 324: 1186–1194 [DOI] [PubMed] [Google Scholar]
- Katz P., Gilbert J. (1998) Pneumatic dilation is effective long-term treatment for achalasia. Dig Dis Sci 43: 1973–1977 [DOI] [PubMed] [Google Scholar]
- Khan A., Shah S., Alam A., Butt A., Shafgat F., Castell D. (1998) Pneumatic balloon dilation in achalasia: a prospective comparison of balloon distension time. Am J Gastroenterol 93: 1064–1067 [DOI] [PubMed] [Google Scholar]
- Kostic S., Andersson M., Hellström M., Lönroth H., Lundell L. (2005) Timed barium esophagogram in the assessment of patients with achalasia: reproducibility and observer variation. Dis Esophagus 18: 96–103 [DOI] [PubMed] [Google Scholar]
- Kroupa R., Hep A., Dolina J., Valek V., Matyasova Z., Prokesova J., et al. (2010) Combined treatment of achalasia–botulinum toxin injection followed by pneumatic dilatation: long-term results. Dis Esophagus 23: 100–105 [DOI] [PubMed] [Google Scholar]
- Kwiatek M., Pandolfino J., Hirano I., Kahrilas P. (2010).Esophagogastric junction distensibility assessed with an endoscopic functional luminal imaging probe (EndoFLIP). Gastrointest Endosc 72: 272–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyden J., Moss A., MacMathuna P. (2006) Endoscopic pneumatic dilation versus botulinum injection in the management of primary achalasia. Cochrane Database Systems Review (4): CD005046. [DOI] [PubMed] [Google Scholar]
- Li Y., Tang G., Cheng Y., Chen N., Chen W., Zhao J. (2010) 13-year follow-up of a prospective comparison of the long-term clinical efficacy of temporary self-expanding metallic stents and pneumatic dilation for the treatment of achalasia in 120 patients. AJR Am J Roentgenol 195: 1429–1437 [DOI] [PubMed] [Google Scholar]
- Lopushinsky S., Urbach D. (2006) Pneumatic dilatation and surgical myotomy for achalasia. JAMA 296: 2227–2233 [DOI] [PubMed] [Google Scholar]
- Mayberry J. (2001) Epidemiology and demographics of achalasia. Gastrointest Endosc Clin N Am 11: 235–248 [PubMed] [Google Scholar]
- Mikaeli J., Bishehsari F., Montazeri E., Yaqhoobi M., Malekzadeh R. (2004) Pneumatic dilation in achalasia: a prospective comparison of safety and efficacy with different balloon diameters. Aliment Pharmacol Ther 20: 431–436 [DOI] [PubMed] [Google Scholar]
- Neubrand M., Scheurlen C., Schepke M., Sauerbruch T. (2002) Long-term results and prognostic factors in the treatment of achalasia with botulinum toxin. Endoscopy 34: 519–523 [DOI] [PubMed] [Google Scholar]
- Niknam R., Mikael J., Mehrabi N., Mahmoud L., Elahi E., Shirani S., et al. (2011) Ethanolamine oleate in resistant idiopathic achalasia: a novel therapy. Eur J Gastroenterol Hepatol 23: 1111–1115 [DOI] [PubMed] [Google Scholar]
- Novais P., Lemme E. (2010) 24-h pH monitoring patterns and clinical response after achalasia treatment with pneumatic dilation or laparoscopic Heller myotomy. Aliment Pharmacol Ther 32: 1257–1265 [DOI] [PubMed] [Google Scholar]
- Oelschlager B.K., Chang L., Pellegrini C.A. (2003) Improved outcome after extended gastric myotomy for achalasia. Archives of Surgery 138: 490–495 [DOI] [PubMed] [Google Scholar]
- Ortega J., Madureri V., Perez L. (1980) Endoscopic myotomy in the treatment of achalasia. Gastrointest Endosc 26: 8–10 [DOI] [PubMed] [Google Scholar]
- Parkman H., Reynolds J., Ouyang A., Rosato E., Eisenberg J., Cohen S. (1993) Pneumatic dilation or esophagomyotomy for idiopathic achalasia: clinical outcomes and cost analysis. Dig Dis Sci 38: 75–85 [DOI] [PubMed] [Google Scholar]
- Pasricha P., Hawari R., Ahmed I., Chen J., Cotton P., Hawes R., et al. (2007) Submucosal endoscopic esophageal myotomy: a novel experimental approach for the treatment of achalasia. Endoscopy 39: 761–764 [DOI] [PubMed] [Google Scholar]
- Pasricha P., Rai R., Ravich W., Hendrix T., Kalloo A. (1996) Botulinum toxin for achalasia: long term outcome and predictors of response. Gastroenterology 110: 1410–1415 [DOI] [PubMed] [Google Scholar]
- Pasricha P., Ravich W., Hendrix T., Sostre S., Jones B., Kalloo A. (1995) Intrasphincteric botulinum for the treatment of achalasia. N Engl J Med 332: 774–778 [DOI] [PubMed] [Google Scholar]
- Patti M., Feo C., Arcerito M., De Pinto M., Tamburini A., Diener U., et al. (1999) Effects of previous treatment on results of laparoscopic Heller myotomy for achalasia. Dig Dis Sci 44: 2270–2276 [DOI] [PubMed] [Google Scholar]
- Pretap N., Kalapala R., Darrissetty S., Joshi N., Ramchandani M., Banerjee R., et al. (2011) Achalasia cardia subtyping by high-resolution manometry predicts the therapeutic outcome of pneumatic balloon dilatation. J Neurogastroenterol Motil 17: 48–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai R., Shende A., Joshi A., Mathur A., Nijhawan S. (2005) Rigiflex pneumatic dilation of achalasia: a novel office procedure. Gastrointest Endosc 62: 427–431 [DOI] [PubMed] [Google Scholar]
- Ren Z., Zhong Y., Zhou P., Xu M., Cai M., Li L., et al. (2012) Perioperative management and treatment for complications during and after peroral endoscopic myotomy (P.O.E.M.) for esophageal achalasia (EA) (data from 119 cases). Surg Endosc 19 May (Epub ahead of print). DOI: [DOI] [PubMed] [Google Scholar]
- Richter J. (2008) Update on the management of achalasia: balloons, surgery and drugs. Exp Rev Gastroenterol Hepatol 2: 435–445 [DOI] [PubMed] [Google Scholar]
- Richter J. (2012) Recent research on pneumatic dilation versus laparoscopic Heller myotomy for achalasia treatment. Gastroenterol Hepatol 8: 330–332 [PMC free article] [PubMed] [Google Scholar]
- Richter J., Boeckxstaens G. (2011) Management of achalasia: surgery or pneumatic dilation. Gut 60: 869–876 [DOI] [PubMed] [Google Scholar]
- Rohof W., Hirsch D., Kessing B., Boeckxstaens G. 2012. Efficacy of treatment for patients with achalasia depends on the distensibility of the esophagogastric junction. Gastroenterology 143: 328–335 [DOI] [PubMed] [Google Scholar]
- Rosemurgy A., Villadolid D., Thometz D., Kalipersad C., Rakita S., et al. (2005) Laparoscopic Heller myotomy provides durable relief from achalasia and salvages failures after botox or dilation. Annals of Surgery 241(5): 725–733; discussion 733–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiwaku H., Inoue H., Beppu R., Nakashima R., Minami H., Shiroshita T., et al. (2012). Successful treatment of diffuse esophageal spasm by per oral endoscopic myotomy. Gastrointest Endosc 4 April (Epub ahead of print). DOI: 10.1016/j.gie.2012.02.008 [DOI] [PubMed] [Google Scholar]
- Smith C.D., Stival A., Howell D.L., Swafford V. (2006) Endoscopic therapy for achalasia before Heller myotomy results in worse outcomes than Heller myotomy alone. Annals of Surgery 243(5): 579–584; discussion 584–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder C., Burton R., Brown L., Kakade M., Finan K., Hawn M. (2009) Multiple preoperative endoscopic interventions are associated with worse outcomes after laparoscopic Heller myotomy for achalasia. J Gastrointest Surg 13: 2095–2103 [DOI] [PubMed] [Google Scholar]
- Spiess A., Kahrilas P. (1998) Treating achalasia: from whalebone to laparoscope. JAMA 280: 638–642 [DOI] [PubMed] [Google Scholar]
- Srinivasan R., Vela M., Tutuian R., Katz P., Castell D. (2000) Prior botulinum toxin injection may compromise outcome of pneumatic dilatation in achalasia. Am J Gastroenterol 95: 2436–2437 [Google Scholar]
- Stavropoulos S., Brathwaite C., Iqbal S., Ghevariya V., Dejesus D., Korrapati V., et al. (2012a) P.O.E.M. (per oral endoscopic myotomy), a U.S. gastroenterologist perspective: initial 2 year experience. Gastrointest Endosc 75: AB149 (oral and poster presentation). [Google Scholar]
- Stavropoulos S., Harris M., Hida S., Brathwaite C., Demetriou C., Grendell J. (2010) Endoscopic submucosal myotomy for the treatment of achalasia (with video). Gastrointest Endosc 72: 1309–1311 [DOI] [PubMed] [Google Scholar]
- Stavropoulos S., Iqbal S., Modayil R., Dejesus D. (2012b) Per oral endoscopic myotomy, P.O.E.M, equipment and technique. A step by step explanation. Video J Encycloped GI Endosc. VJGIEN-D-12-00541 (accepted). [Google Scholar]
- Stavropoulos S.N., Modayil R., Friedel D., Achalasia (2013) Gastrointestinal endoscopy clinics of North America. Endolumenal Therapy 23(1): 53–75 [DOI] [PubMed] [Google Scholar]
- Sumiyama K., Gostout C., Rajan E., Bakken T., Knipschield M. (2007) Transesophageal mediastinoscopy by submucosal endoscopy with mucosal flap safety valve technique. Gastrointest Endosc 65: 679–683 [DOI] [PubMed] [Google Scholar]
- Swanström L. (2012) POEM: North American Experience. In: SAGES 2012 Conference PG Course Per Oral Endoscopic Myotomy (POEM), San Diego, CA, USA, 8 March 2012 San Diego, CA: SAGES, pp 45–46 [Google Scholar]
- Swanström L., Rieder E., Dunst C. (2011) A stepwise approach and early clinical experience in Per oral endoscopic myotomy for the treatment of achalasia and esophageal motility disorders. J Am Coll Surg 213: 751–756 [DOI] [PubMed] [Google Scholar]
- Vela M., Richter J., Wachsberger D., Connor J., Rice T. (2004) Complexities of managing achalasia at a tertiary referral center: use of pneumatic dilation, Heller myotomy, and botulinum toxin injection. Am J Gastroenterol 99: 1029–1036 [DOI] [PubMed] [Google Scholar]
- von Renteln D., Fuchs K., Fockens P., Bauerfeind P., Vassiliou M., Breithaupt W., et al. (2012a) Per oral endoscopic myotomy for the treatment of achalasia: prospective international multi center study. Gastrointest Endosc 75: AB160 [Google Scholar]
- von Renteln D., Inoue H., Minami H., Werner Y., Pace A., Kersten J., et al. (2012b) Per oral endoscopic myotomy for the treatment of achalasia: a prospective single center study. Am J Gastroenterol 107: 411–417 [DOI] [PubMed] [Google Scholar]
- Walzer N., Hirano I. (2008) Achalasia. Gastroenterol Clin North Am 37: 807–825, viii [DOI] [PubMed] [Google Scholar]
- Wang L., Li Y., Li L. (2009) Meta-analysis of randomized and controlled treatment trials for achalasia. Dig Dis Sci 54: 2303–2311 [DOI] [PubMed] [Google Scholar]
- Wong R. (2006) Achalasia: should we or should we not follow the bag? Am J Gastroenterol 101: 698–700 [DOI] [PubMed] [Google Scholar]
- Yi A., Shin J., Song H., Jung H., Lee G., Yoon C., et al. (2008) Esophageal achalasia: comparison of fluoroscopically-guided double vs. endoscopically-guided single balloon dilation. Abdominal Imaging 33: 177–182 [DOI] [PubMed] [Google Scholar]
- Yoshida A., Inoue H., Ikeda H., Hosoya T., Onimaru M., Sudo K., et al. (2012) Clinical results of P.O.E.M. (per-oral endoscopic myotomy) for esophageal achalasia in 161 consecutive cases. Gastrointest Endosc 75: AB212 [Google Scholar]
- Zerbid F., Thetiot V., Richy F., Benejah D., Message L., Lamouliatte H. (2006) Repeated pneumatic dilations as long-term maintenance therapy for esophageal achalasia. Am J Gastroenterol 101: 692–697 [DOI] [PubMed] [Google Scholar]
- Zhou P., Yao L., Cai M., Zhong Y., Ren Z., Xu M., et al. (2012a) Water-jet assisted per oral endoscopic myotomy (P.O.E.M.) in comparison to conventional endoscopic myotomy technique for treatment of esophageal achalasia. Gastrointest Endosc 75: AB160–AB161 [Google Scholar]
- Zhou P., Yao L., Zhang Y., Cai M., Zhong Y., Ren Z., et al. (2012b) Per oral endoscopic myotomy (P.O.E.M.) for esophageal achalasia: 205 cases report. Gastrointest Endosc 75: AB132–AAB133 [Google Scholar]