Abstract
Protein S-nitrosylation (the binding of a nitric oxide group to a cysteine thiol) is a major mechanism through which the ubiquitous cellular influence of nitric oxide is exerted. Disruption of S-nitrosylation is associated with a wide range of pathophysiological conditions. Hemoglobin exemplifies both of these concepts. It is the prototypical S-nitrosylated protein in that it binds, activates, and deploys nitric oxide. Within red blood cells, hemoglobin is S-nitrosylated during the respiratory cycle and thereby conveys nitric oxide bioactivity that may be dispensed to regulate local blood flow in the physiological response known as hypoxic vasodilation. Hemoglobin thus both delivers oxygen directly and delivers vasoactivity to potentially optimize tissue perfusion in concert with local metabolic demand. Accordingly, decreased levels of S-nitrosylated hemoglobin and/or impaired delivery of red blood cell-derived nitric oxide bioactivity have been observed in a variety of disease states that are characterized by tissue hypoxemia. It has been shown recently that storage of blood depletes S-nitrosylated hemoglobin, accompanied by reduced ability of red blood cells to induce vasodilation. This defect appears to account in significant part for the impaired ability of banked red blood cells to deliver oxygen. Re-nitrosylation can correct this impairment and thus may offer a means to ameliorate the disruptions in tissue perfusion produced by transfusion.
In conjunction with the recognition of nitric oxide (NO) as the endothelium-derived relaxing factor (1–3) was the identification of its receptor: the heme center of soluble guanylyl cyclase (sGC). (4) Binding of NO leads to increased conversion of guanosine-5'-triphosphate (GTP) to cyclic guanosine monophophate (cGMP), which in turn activates protein kinase (PK) G. These steps served as an initial explanation for how NO produced vascular relaxation though it is now clear that the majority of the functions of NO in cellular regulation are carried out independently of sGC. Indeed, it seems protein hemes do not generally mediate NO-based signaling that involve post-translational protein modifications, but rather serve to promote the requisite redox chemistry of NO. Instead, the principal protein/peptide target(s) of NO are the thiol side-chains of cysteine residues, where NO covalently binds to generate S-nitrosothiols (SNOs) in a process termed S-nitrosylation. Over one thousand proteins have been identified as targets of S-nitrosylation where activity can increase or decease in response to the addition (or removal) of NO. (5) It is increasingly apparent that the breadth of cellular activities regulated by S-nitrosylation may rival that controlled by phosphorylation. It is also becoming apparent that disruption of S-nitrosylation is an important initiator or propagator of pathologic conditions. (6). As such, resolution of aberrant S-nitrosylation provides an attractive therapeutic target for disease amelioration.
The principal source of NO in mammalian cells comes from the oxidative conversion of L-arginine to L-citrulline, catalyzed by each of three isoforms of NO synthase: neuronal (nNOS/NOS1), inducible (iNOS/NOS2), and endothelial (eNOS/NOS3). (7) Direct interaction and/or sub-cellular compartmentalization of NOS with target proteins provides a basis for specificity of S-nitrosylation. In addition to direct modification by NO or nitrosylating equivalents, S-nitrosylation can also occur through thiol-to-thiol transfer of an NO group to the target protein from a small-molecular-weight S-nitrosothiol (most notably S-nitrosoglutathione, GSNO) or a SNO-protein. (8) Many SNO-proteins are in equilibrium with GSNO, as demonstrated by the increase in levels of both GSNO and SNO-proteins consequent upon genetic deletion of the principal GSNO-metabolizing enzyme, GSNO reductase. (9) Thus, denitrosylation of SNO-proteins can be mediated by transfer of the NO group to form GSNO. In addition, NO groups can be removed from SNO-proteins by de-nitrosylating enzymes including thioredoxin. (10) Thus, SNO-protein levels in situ reflect the operation of both nitrosylating and de-nitrosylating mechanisms.
Hemoglobin (Hb) is the prototypical S-nitrosylated protein in that it can bind, activate, and deploy NO as the red blood cells (RBCs) transit the circulatory system. (11) In the respiratory cycle, NO (itself or derived from nitrite or S-nitrosothiols) binds to heme iron (Fe2+ or Fe3+) of deoxy, T-state Hb mainly in the venous circulation to generate HbFeNO. (12). This FeNO, which resides primarily in the beta chains of Hb, shows behavior of Fe3+NO - a redox-activated form of NO in equilibrium with SNO. ((11, 13) Thus, in concert with oxygenation in the lungs and the transition of Hb from T-state to R-state, the NO group can be transferred to the highly-conserved Cysβ93 residue to generate SNO-Hb. So although FeNO cannot potentially release NO for export due to re-capture by hemes present in vast excess, the heme iron in Hb provides the redox requirements for S-nitrosylation that generates and preserves bioactivity. The transition from high to low oxygen tension in the arterial periphery (R to T transition in Hb) promotes the release from RBCs of SNO-based vasodilatory activity. Notably, only a small fraction of the NO bound to Hb is released (i.e. transferred to acceptor thiols); the remainder is auto-captured by hemes within the beta subunit. (14, 15) Thus, in systemic arterioles, SNO-Hb will elicit increases in blood flow, whereas SNO-Hb entering the lung (in T-state) may influence ventilation-perfusion matching. (16) RBCs thereby facilitate both the uptake and delivery of oxygen. (17) Alternative proposed mechanisms for RBC-mediated hypoxic vasodilation (including the postulate that Hb acts as a nitrite reductase to generate bioactive NO (18)) are unable to satisfy the physiologically relevant features of hypoxic vasodilation. (19) These features include the response of RBCs to lowered pO2 on a short time-scale compatible with arterial-venous transit times (seconds) and the precise gradation of vasodilatory activity linked to pO2. In addition, no mechanism has been demonstrated through which NO generated from nitrite by Hb could escape capture and inactivation by heme iron present in overwhelming excess within RBCs. (19) Conversely, nitrite reductase can furnish NO groups that may serve as a source of SNOs. (12, 20) In sum, RBCs are the only cells to recapitulate hypoxic vasodilation and SNO-based bioactivity is the mechanism most compatible with the data.
The re-conceptualization of the respiratory cycle as a three-gas system (NO along with oxygen and carbon dioxide), (16) driven by the accumulating evidence for the importance of NO bioactivity, provides the basis for understanding why therapeutic efforts to increase the oxygen content of blood can fail to improve delivery to tissues. (21) The disconnection between oxygen content and oxygen delivery reflects the fact that tissue blood flow and not blood oxygen content is the primary determinant of oxygen delivery. (11, 16) Tissue perfusion is regulated substantially by hypoxic vasodilation, which couples metabolic demand (oxygen requirement) to local blood flow, (19, 22, 23) and it has been established that the RBC itself is a principal transducer of this response. (24–26) Under normal physiological conditions, tissue pO2 is low, and falls further with local increases in metabolism, e.g., muscle activity. Thus, the release of vasodilatory NO bioactivity by RBCs sub-serves the graded increases in blood flow that are coupled to progressive decreases in the oxygen saturation of Hb. (17) RBC uptake of oxygen in the lung is optimized by analogous mechanisms that match flow to alveolar ventilation (V/Q matching), with localized hypoxic pulmonary vasoconstriction sub-serving this response to divert blood toward better-ventilated lung units. In this setting, NO trapping by hemoglobin is an important contributor to vasoconstriction. (16, 27) In sum, the binding and release of NO bioactivity in the form of SNOs is a central component of the physiologic response to local hypoxia. (16)
A thorough appreciation of the role played by SNOs in the respiratory cycle has allowed for a better understanding of an array of pathologic respiratory and circulatory conditions. Decreased levels and/or impaired bioavailability of SNO-Hb have been observed in a variety of disease states characterized by tissue hypoxemia, including pulmonary hypertension, (28) sickle cell disease, (29) diabetes, (30, 31) sepsis, (9, 32) and congestive heart failure. (33) Furthermore, several clinical (28, 34) and pre-clinical trials (35–38) have demonstrated the therapeutic benefits of restoration, maintenance, or enhancement of RBC-derived NO bioactivity. Based upon the role of NO/SNO in the delivery of oxygen by RBCs, we reasoned that the inability of banked blood to improve oxygen delivery might reflect a deficiency in SNO-Hb. (39)
Procured (i.e. donated) blood undergoes several time-dependent changes including decreased RBC flexibility, (40, 41) and increased RBC adhesiveness (42) as well as decreases in the concentration of molecular modulators of oxygen binding including 2,3-diphosphoglycerate. (43). It has been proposed that transfused blood, due to an increased affinity for oxygen combined with alterations in RBC rheology and adhesion, may exacerbate rather than correct ongoing ischemia (e.g. (44)) and thus account, at least partly, for the adverse effects of blood transfusion). However, it has been unclear how increases in oxygen affinity that are confined to a small percentage of the blood volume (transfused units) could impact overall oxygen delivery, and it remains to be shown that these biochemical or molecular measures of RBC function impacts oxygen delivery in vivo. Moreover, even freshly processed blood, which would not have undergone many of these storage-related biochemical changes, has been observed to decrease tissue oxygenation, (45) and very recent studies have found marked increases in mortality associated with transfusion of blood that is only a few days old. (46) Thus, it is likely that additional factors contribute to the storage-mediated alterations in RBC physiology that underlie the impairment in oxygen delivery, e.g. loss of SNO-Hb.
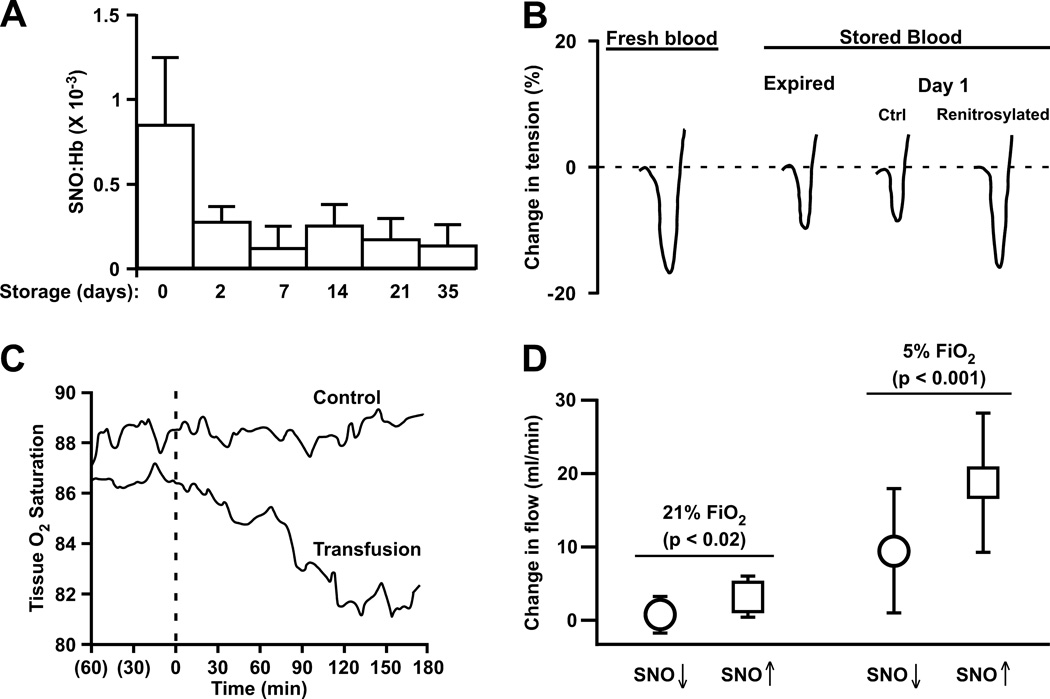
Donated blood is stored in an acidic-buffered isotonic solution that contains nutrients and an anti-coagulant; the pH is usually around 6.5, a condition previously shown to accelerate SNO-Hb decay. (47) Placing blood into this mixture may therefore accelerate the decline in RBC function (and venous blood is already depleted in SNO-Hb compared to arterial blood). (48) We used a combination of in vitro and in vivo techniques to determine that storage of blood leads to rapid losses in NO bioactivity, reflected by rapid losses in SNO-Hb, which are precisely paralleled by losses in the ability of RBCs to effect hypoxic vasodilation – these events are depicted in the figure. (39) (The findings were independently verified by another research team.) (49) We further showed that the defect in RBC vasodilation could be corrected by repleting SNO-Hb, raising the possibility that such an intervention might help prevent transfusion-associated ischemic morbidity. We did find that the ability to re-nitrosylate stored blood has a storage duration component in that older blood (i.e. at or approaching expiration) required additional manipulation (washing) and does not re-load SNO to the same magnitude as younger blood. Thus older blood would presumably be less capable of regenerating SNO-Hb in vivo.
Figure.
Blood Storage diminishes S-nitroso-hemoglobin (SNO-Hb) concentration, hypoxic vasodilation, and oxygen delivery (modified from references 39 and 44). A) Processing of donated blood under current AABB guidelines results in the loss of most SNO-Hb within 2 days and SNO-Hb levels remain low throughout the standard storage duration; B) The loss of SNO-Hb is pronounced after one day of storage and correlates directly with diminished hypoxic vasodilation by red blood cells (RBCs) in an in vitro bioassay (rabbit aortic ring segments).Vasorelaxation by RBCs is restored by repleting SNO-Hb; C) Consistent with diminished hypoxic vasodilation, peripheral tissue oxygen saturation in humans (measured with a trans-cutaneous probe placed on the thenar eminence) declines following transfusion with stored blood (21 days). D) Analysis of the effects on (canine) coronary artery blood flow produced by RBC infusion reveals that increases in flow elicited by re-nitrosylated RBCs were significantly greater than those produced by SNO-depleted (stored) RBCs, and that the degree of change was greater under hypoxic (5% fractional inspired oxygen; FiO2) than normoxic (21% FiO2) conditions. Thus, RBCs elicit vasodilation in vivo that is potentiated by hypoxia and dependent on SNO bioactivity, and RBCs depleted of SNO-Hb through storage can be re-nitrosylated to enhance blood flow (i.e., cardiac oxygen delivery).
Modifications made to date in blood storage conditions (e.g. inclusion of nutritional supplements in the blood bag and other re-workings of the storage media, leukocyte reduction, etc.) have been directed toward maintaining RBC metabolic status and/or enhancing shelf life - none have attempted to directly restore the RBC’s main function, which is tissue oxygen delivery. Similarly, although a number of storage-related deficits may at least partly resolve in viable RBCs within 24–48 h of transfusion (and 24 h in vivo survivability of 70% of transfused cells is the only FDA-mandated storage requirement with respect to RBC function), the observed losses in RBC SNO that occur with storage are very large relative to the amounts of NO produced in vivo (~1 µmol/day/70 kg). Thus, during the immediate post-transfusion period it is not anticipated that SNO levels would normalize in NO-deficient RBCs; and additional defects would further slow this process with older stored blood. Instead, stored, infused RBCs will act as overall sinks for NO, adversely affecting NO homeostasis and predisposing to vasoconstriction and ischemic insult at a time when the need is for vasodilation in the microcirculation to maintain or enhance end-organ oxygen delivery. At first pass, it may be difficult to rationalize transfusion-associated ischemic events based on depleted levels of SNO, increases in membrane rigidity or increases in oxygen affinity that characterize the small percentage of the circulating RBC pool that is contributed by transfused blood, in particular since oxygen availability is rarely limiting in vivo. However, increases in the affinity of Hb for oxygen are directly linked to increases in the affinity of SNO-Hb for NO, (3, 11, 16) and recent studies have reported that increases in Hb oxygen affinity are in fact associated with impaired vasodilation by RBCs. (31) Because RBCs traffic through the microcirculation in line, impaired vasodilation by a minor fraction would be expected to adversely influence oxygen delivery. In addition, there is reason to believe that infection (50, 51) and membrane rheological abnormalities (40, 41) associated with transfusion might also reflect the overall state of NO deficiency. Thus, NO deficiency may be linked to several storage defects. Conversely, enhanced oxygen delivery and restoration of NO homeostasis could result from reversing the SNO-Hb deficit at the time of transfusion.
Numerous studies across diverse patient populations indicate that transfusion is associated with a range of deleterious sequalae including pulmonary edema, renal failure, multi-organ failure, myocardial infarction, infection, increased hospital stay and death. (52–55) Notably, the Cochrane systematic review of multiple randomized trials found that liberal blood transfusion versus a more restrictive strategy is associated with a 20% increase in mortality and 56% increase in ischemic events. (56) Reports continue to accumulate describing clinical conditions where transfusion increases morbidity and mortality in adults following cardiac surgery, (46, 57) percutaneous coronary interventions, (58) and acute lung injury, (59) and in pediatric intensive care patients (60) – these are particularly striking findings in context with the increasing realization that even mild anemia is an adverse clinical predictor of outcome. (61, 62) Transfusion is also expensive. The cost of transfusion reflects not only the expense of procuring blood and ancillary expenditures (storage, cross-matching and staff wages), but also the costs incurred as a result of transfusion-related morbidity, including increased intensive care admissions and extended hospital stays. As a result, the projected cost of transfusing a single unit of packed RBCs is between $1,600 and $2,400, (63) which represents an annual expenditure of $22–34 billion in the United States. This dollar figure should be multiplied several fold to account for word-wide expenditures, and it brings into question society’s return on this investment.
Severe anemia poses a significant risk to patient survival and well being (64) and transfusion may, in some situations, be lifesaving. (65, 66) But even minor anemia may adversely impact outcomes (62) while at the same time, there is little doubt about the inability of stored blood to reverse this risk or replicate the functions of native RBCs, and accumulating evidence indicates that this difference is accentuated as the storage time increases. (52–55) Thus, currently, raising hematocrit into the normal range by RBC transfusion is not advocated. Instead, clinical interventions are focused on identifying anemic transfusion thresholds that do not produce adverse outcomes. (64, 65) (Indeed, part of the therapeutic benefit from the anti-thrombin agent bivalirudin may well come from reducing transfusions compared to the standard anti-coagulant therapy of low-dose heparin plus Gp IIb/IIIa blockade. (67) ). In principle, however, raising the hematocrit into the normal range should have beneficial effects if the oxygen-delivery function of the transfused blood could be restored. Restoration of RBC-derived NO vasoactivity through repletion of SNO-Hb appears to offer a means for blood transfusion to achieve its clinical purpose: vasodilation in the micro-circulation to maintain or enhance end-organ oxygen delivery in the anemic patient.
Acknowledgments
This was work was sponsored in part by NIH grants HL91876 and HL095463, a grant from the Case Western Reserve University/Cleveland Clinic CTSA (UL1RR024989), the Coulter-Case Translational Research Partnership, and by the Institute for Transformative Molecular Medicine at Case Western Reserve University.
Footnotes
JDR has a financial interest in N30. JSS has financial interests in LifeHealth, N30, and BioU. All are early stage biotech companies with interests in nitric oxide related technologies.
References
- 1.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 3.McMahon TJ, Exton Stone A, Bonaventura J, Singel DJ, Solomon Stamler J. Functional coupling of oxygen binding and vasoactivity in S-nitrosohemoglobin. J Biol Chem. 2000;275(22):16738–16745. doi: 10.1074/jbc.M000532200. [DOI] [PubMed] [Google Scholar]
- 4.Murad F. Cyclic Guanosine-Monophosphate as a Mediator of Vasodilation. Journal of Clinical Investigation. 1986;78(1):1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hess DT, Foster MW, Stamler JS. Assays for S-nitrosothiols and S-nitrosylated proteins and mechanistic insights into cardioprotection. Circulation. 2009;120(3):190–193. doi: 10.1161/CIRCULATIONAHA.109.876607. PMCID: 2879612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15(9):391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 8.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6(2):150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 9.Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, et al. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116(4):617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 10.Benhar M, Thompson JW, Moseley MA, Stamler JS. Identification of S-nitrosylated targets of thioredoxin using a quantitative proteomic approach. Biochemistry. 2010;49(32):6963–6969. doi: 10.1021/bi100619k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 12.Angelo M, Singel DJ, Stamler JS. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc Natl Acad Sci U S A. 2006;103(22):8366–8371. doi: 10.1073/pnas.0600942103. PMCID: 1482500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angelo M, Hausladen A, Singel DJ, Stamler JS. Interactions of NO with hemoglobin: from microbes to man. Methods Enzymol. 2008;436:131–168. doi: 10.1016/S0076-6879(08)36008-X. [DOI] [PubMed] [Google Scholar]
- 14.Pezacki JP, Ship NJ, Kluger R. Release of nitric oxide from S-nitrosohemoglobin. Electron transfer as a response to deoxygenation. J Am Chem Soc. 2001;123(19):4615–4616. doi: 10.1021/ja015716o. [DOI] [PubMed] [Google Scholar]
- 15.McMahon TJ, Stone AE, Bonaventura J, Singel DJ, Stamler JS. Functional coupling of oxygen binding and vasoactivity in S-nitrosohemoglobin. Journal of Biological Chemistry. 2000;275(22):16738–16745. doi: 10.1074/jbc.M000532200. [DOI] [PubMed] [Google Scholar]
- 16.Doctor A, Stamler JS. NO Transport in Blood: a third gas in the respiratory cycle. Comprehensive Physiology. doi: 10.1002/cphy.c090009. in press. [DOI] [PubMed] [Google Scholar]
- 17.Doctor A, Platt R, Sheram ML, Eischeid A, McMahon T, Maxey T, et al. Hemoglobin conformation couples erythrocyte S-nitrosothiol content to O2 gradients. Proc Natl Acad Sci U S A. 2005;102(16):5709–5714. doi: 10.1073/pnas.0407490102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9(12):1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 19.Allen BW, Stamler JS, Piantadosi CA. Hemoglobin, nitric oxide and molecular mechanisms of hypoxic vasodilation. Trends Mol Med. 2009;15(10):452–460. doi: 10.1016/j.molmed.2009.08.002. PMCID: 2785508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luchsinger BP, Rich EN, Gow AJ, Williams EM, Stamler JS, Singel DJ. Routes to S-nitroso-hemoglobin formation with heme redox and preferential reactivity in the beta subunits. Proc Natl Acad Sci U S A. 2003;100(2):461–466. doi: 10.1073/pnas.0233287100. PMCID: 141017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodges AN, Delaney S, Lecomte JM, Lacroix VJ, Montgomery DL. Effect of hyperbaric oxygen on oxygen uptake and measurements in the blood and tissues in a normobaric environment. Br J Sports Med. 2003;37(6):516–520. doi: 10.1136/bjsm.37.6.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duling BR, Berne RM. Longitudinal gradients in periarteriolar oxygen tension. A possible mechanism for the participation of oxygen in local regulation of blood flow. Circ Res. 1970;27:669–678. doi: 10.1161/01.res.27.5.669. [DOI] [PubMed] [Google Scholar]
- 23.Intaglietta M, Johnson PC, Winslow RM. Microvascular and tissue oxygen distribution. Cardiovasc Res. 1996;32(4):632–643. [PubMed] [Google Scholar]
- 24.Roach RC, Koskolou MD, Calbet JA, Saltin B. Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am J Physiol. 1999;276(2 Pt 2):H438–H445. doi: 10.1152/ajpheart.1999.276.2.H438. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res. 2002;91(11):1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Alonso J, Mortensen SP, Dawson EA, Secher NH, Damsgaard R. Erythrocytes and the regulation of human skeletal muscle blood flow and oxygen delivery: role of erythrocyte count and oxygenation state of haemoglobin. J Physiol. 2006;572(Pt 1):295–305. doi: 10.1113/jphysiol.2005.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deem S, Min JH, Moulding JD, Eveland R, Swenson ER. Red blood cells prevent inhibition of hypoxic pulmonary vasoconstriction by nitrite in isolated, perfused rat lungs. Am J Physiol Heart Circ Physiol. 2007;292(2):H963–H970. doi: 10.1152/ajpheart.00812.2006. [DOI] [PubMed] [Google Scholar]
- 28.McMahon TJ, Ahearn GS, Moya MP, Gow AJ, Huang YC, Luchsinger BP, et al. A nitric oxide processing defect of red blood cells created by hypoxia: deficiency of S-nitrosohemoglobin in pulmonary hypertension. Proc Natl Acad Sci U S A. 2005;102(41):14801–14806. doi: 10.1073/pnas.0506957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pawloski JR, Hess DT, Stamler JS. Impaired vasodilation by red blood cells in sickle cell disease. Proc Natl Acad Sci U S A. 2005;102(7):2531–2536. doi: 10.1073/pnas.0409876102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Padron J, Peiro C, Cercas E, Llergo JL, Sanchez-Ferrer CF. Enhancement of S-nitrosylation in glycosylated hemoglobin. Biochem Biophys Res Commun. 2000;271(1):217–221. doi: 10.1006/bbrc.2000.2617. [DOI] [PubMed] [Google Scholar]
- 31.James PE, Lang D, Tufnell-Barret T, Milsom AB, Frenneaux MP. Vasorelaxation by red blood cells and impairment in diabetes: reduced nitric oxide and oxygen delivery by glycated hemoglobin. Circ Res. 2004;94(7):976–983. doi: 10.1161/01.RES.0000122044.21787.01. [DOI] [PubMed] [Google Scholar]
- 32.Crawford JH, Chacko BK, Pruitt HM, Piknova B, Hogg N, Patel RP. Transduction of NO-bioactivity by the red blood cell in sepsis: novel mechanisms of vasodilation during acute inflammatory disease. Blood. 2004;104(5):1375–1382. doi: 10.1182/blood-2004-03-0880. [DOI] [PubMed] [Google Scholar]
- 33.Datta B, Tufnell-Barrett T, Bleasdale RA, Jones CJ, Beeton I, Paul V, et al. Red blood cell nitric oxide as an endocrine vasoregulator: a potential role in congestive heart failure. Circulation. 2004;109(11):1339–1342. doi: 10.1161/01.CIR.0000124450.07016.1D. [DOI] [PubMed] [Google Scholar]
- 34.Moya MP, Gow AJ, Califf RM, Goldberg RN, Stamler JS. Inhaled ethyl nitrite gas for persistent pulmonary hypertension of the newborn. Lancet. 2002;360(9327):141–143. doi: 10.1016/S0140-6736(02)09385-6. [DOI] [PubMed] [Google Scholar]
- 35.Auten RL, Mason SN, Whorton MH, Lampe WR, Foster WM, Goldberg RN, et al. Inhaled ethyl nitrite prevents hyperoxia-impaired postnatal alveolar development in newborn rats. Am J Respir Crit Care Med. 2007;176(3):291–299. doi: 10.1164/rccm.200605-662OC. PMCID: 1994219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali NA, Eubanks WS, Stamler JS, Gow AJ, Lagoo-Deenadayalan SA, Villegas L, et al. A method to attenuate pneumoperitoneum-induced reductions in splanchnic blood flow. Ann Surg. 2005;241(2):256–261. doi: 10.1097/01.sla.0000153034.54128.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimazutsu K, Uemura K, Auten KM, Baldwin MF, Belknap SW, La Banca F, et al. Inclusion of a nitric oxide congener in the insufflation gas repletes S-nitrosohemoglobin and stabilizes physiologic status during prolonged carbon dioxide pneumoperitoneum. Clin Transl Sci. 2009;2(6):405–412. doi: 10.1111/j.1752-8062.2009.00154.x. PMCID: 2895905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheng H, Reynolds JD, Auten RL, Demchenko IT, Piantadosi CA, Stamler JS, et al. Pharmacologically augmented S-nitrosylated hemoglobin improves recovery from murine subarachnoid hemorrhage. Stroke. 2010 doi: 10.1161/STROKEAHA.110.600569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, Stamler JS. S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci U S A. 2007;104(43):17058–17062. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lacelle P. Alterations of deformability of the erythrocyte membrane in stored blood. Transfusion. 1969;9:229–237. doi: 10.1111/j.1537-2995.1969.tb04930.x. [DOI] [PubMed] [Google Scholar]
- 41.Card RT, Mohandas N, Mollison PL. Relationship of post-transfusion viability to deformability of stored red cells. Br J Haematol. 1983;53(2):237–240. doi: 10.1111/j.1365-2141.1983.tb02016.x. [DOI] [PubMed] [Google Scholar]
- 42.Luk CS, Gray-Statchuk LA, Cepinkas G, Chin-Yee IH. WBC reduction reduces storage-associated RBC adhesion to human vascular endothelial cells under conditions of continuous flow in vitro. Transfusion. 2003;43(2):151–156. doi: 10.1046/j.1537-2995.2003.00310.x. [DOI] [PubMed] [Google Scholar]
- 43.Valeri CR, Hirsch NM. Restoration in vivo of erythrocyte adenosine triphosphate, 2,3-diphosphoglycerate, potassium ion, sodium ion concentrations following the transfusion of acid-citrate-dextrose-stored human red blood cells. J Lab Clin Med. 1969;73(5):722–733. [PubMed] [Google Scholar]
- 44.Kiraly LN, Underwood S, Differding JA, Schreiber MA. Transfusion of aged packed red blood cells results in decreased tissue oxygenation in critically injured trauma patients. J Trauma. 2009;67(1):29–32. doi: 10.1097/TA.0b013e3181af6a8c. [DOI] [PubMed] [Google Scholar]
- 45.Tsai AG, Cabrales P, Hangai-Hoger N, Intaglietta M. Oxygen distribution and respiration by the microcirculation. Antioxid Redox Signal. 2004;6(6):1011–1018. doi: 10.1089/ars.2004.6.1011. [DOI] [PubMed] [Google Scholar]
- 46.Hajjar LA, Vincent JL, Galas FR, Nakamura RE, Silva CM, Santos MH, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304(14):1559–1567. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- 47.Hausladen A, Rafikov R, Angelo M, Singel DJ, Nudler E, Stamler JS. Assessment of nitric oxide signals by triiodide chemiluminescence. Proc Natl Acad Sci U S A. 2007;104(7):2157–2162. doi: 10.1073/pnas.0611191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McMahon TJ, Moon RE, Luschinger BP, Carraway MS, Stone AE, Stolp BW, et al. Nitric oxide in the human respiratory cycle. Nat Med. 2002;8(7):711–717. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- 49.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A. 2007;104(43):17063–17068. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Camp ER, Yang A, Liu W, Fan F, Somcio R, Hicklin DJ, et al. Roles of nitric oxide synthase inhibition and vascular endothelial growth factor receptor-2 inhibition on vascular morphology and function in an in vivo model of pancreatic cancer. Clin Cancer Res. 2006;12(8):2628–2633. doi: 10.1158/1078-0432.CCR-05-2257. [DOI] [PubMed] [Google Scholar]
- 51.Whitaker BI, Sullivan M. The 2005 Nationwide Blood Collection and Utilization Survey Report. 2006 [Google Scholar]
- 52.Malone DL, Dunne J, Tracy JK, Putnam AT, Scalea TM, Napolitano LM. Blood transfusion, independent of shock severity, is associated with worse outcome in trauma. J Trauma. 2003;54(5):898–905. doi: 10.1097/01.TA.0000060261.10597.5C. discussion -7. [DOI] [PubMed] [Google Scholar]
- 53.Rao SV, Jollis JG, Harrington RA, Granger CB, Newby LK, Armstrong PW, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;292(13):1555–1562. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- 54.Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, et al. Anemia and blood transfusion in critically ill patients. Jama. 2002;288(12):1499–1507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 55.Tinmouth A, Fergusson D, Yee IC, Hebert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46(11):2014–2027. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 56.Hill SR, Carless PA, Henry DA, Carson JL, Hebert PC, McClelland DB, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2002;(2):CD002042. doi: 10.1002/14651858.CD002042. [DOI] [PubMed] [Google Scholar]
- 57.Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116(22):2544–2552. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 58.Kim P, Dixon S, Eisenbrey AB, O'Malley B, Boura J, O'Neill W. Impact of acute blood loss anemia and red blood cell transfusion on mortality after percutaneous coronary intervention. Clin Cardiol. 2007;30(10 Suppl 2):II35–II43. doi: 10.1002/clc.20231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Netzer G, Shah CV, Iwashyna TJ, Lanken PN, Finkel B, Fuchs B, et al. Association of RBC transfusion with mortality in patients with acute lung injury. Chest. 2007;132(4):1116–1123. doi: 10.1378/chest.07-0145. [DOI] [PubMed] [Google Scholar]
- 60.Kneyber MC, Hersi MI, Twisk JW, Markhorst DG, Plotz FB. Red blood cell transfusion in critically ill children is independently associated with increased mortality. Intensive Care Med. 2007;33(8):1414–1422. doi: 10.1007/s00134-007-0741-9. [DOI] [PubMed] [Google Scholar]
- 61.Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345(17):1230–1236. doi: 10.1056/NEJMoa010615. [DOI] [PubMed] [Google Scholar]
- 62.Wu WC, Schifftner TL, Henderson WG, Eaton CB, Poses RM, Uttley G, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007;297(22):2481–2488. doi: 10.1001/jama.297.22.2481. [DOI] [PubMed] [Google Scholar]
- 63.Basha J, Dewitt RC, Cable D, Jones GP. Transfusions and their costs: managing patients' needs and hospital economics. Inter J Emerg Intens Care Med. 2006;9:1–6. [Google Scholar]
- 64.Hebert PC, Fergusson DA. Do transfusions get to the heart of the matter? Jama. 2004;292(13):1610–1612. doi: 10.1001/jama.292.13.1610. [DOI] [PubMed] [Google Scholar]
- 65.Corwin HL, Carson JL. Blood transfusion--when is more really less? N Engl J Med. 2007;356(16):1667–1669. doi: 10.1056/NEJMe078019. [DOI] [PubMed] [Google Scholar]
- 66.Johansson PI, Hansen MB, Sorensen H. Transfusion practice in massively bleeding patients: time for a change? Vox Sang. 2005;89(2):92–96. doi: 10.1111/j.1423-0410.2005.00668.x. [DOI] [PubMed] [Google Scholar]
- 67.Lincoff AM, Bittl JA, Harrington RA, Feit F, Kleiman NS, Jackman JD, et al. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA. 2003;289(7):853–863. doi: 10.1001/jama.289.7.853. [DOI] [PubMed] [Google Scholar]