Abstract
Introduction
Aspirin’s effectiveness in reducing cardiovascular disease events is inadequate in some individuals, a phenomenon termed aspirin “resistance”. The hypothesis that combining low dose aspirin with eicosapentaenoic acid and docosahexaenoic acid (EPA+DHA) reduces platelet function in the acute setting has not been investigated.
Patients and methods
We conducted a clinical trial of EPA+DHA and aspirin ingestion in healthy adults. Fasting blood samples were drawn at baseline and 4 h after supplementation with EPA/DHA (3.4 g/d), aspirin (81 mg), and both. Platelet function was measured using the Platelet Function Analyzer-100 (PFA-100). Plasma lysophosphatidylcholine (LPC), lysophosphatidic acid (LPA), autotaxin, angiogenesis activators, and cytokines were measured.
Results
Platelet function decreased with the combination of aspirin+EPA/DHA (p=0.03) but not with either alone (p>0.05). EPA-LPC increased (p=0.002).
Discussion and conclusions
Our results demonstrate that a potentially beneficial effect on platelet function occurred within 4 h after ingestion of low-dose aspirin and EPA+DHA in healthy adults.
Keywords: Omega-3 fatty acids, Eicosapentaenoic acid, Docosahexaenoic acid, Aspirin, Acetylsalicylic acid, Platelet function, Platelet function analyzer-100
1. Introduction
Cardiovascular disease (CVD) is the leading cause of death in the United States and the most prevalent cause of death world-wide [1]. Although significant progress has been made in reducing rates of CVD events [1], with the population aging the public health burden attributable to CVD is increasing. Aspirin has long been a stalwart and inexpensive therapy for the prevention of CVD. However, a systematic review reported that the frequency of biochemical “resistance” to long-term aspirin therapy (defined by a variety of platelet function assays) was 28% within 20 studies totaling 2930 patients with CVD [2], meaning that its effects on reducing platelet aggregation are negligible or absent. The effects of aspirin in these studies were determined after chronic administration over days or weeks of use, not after acute ingestion. The risk for a CVD event in these aspirin-resistant individuals was significantly higher than in aspirin-responsive subjects, with 41% experiencing any event (odds ratio 3.85, 95%; CI 3.08–4.80), with death occurring in 5.7% (OR 5.99; CI 2.28–15.72), and an acute coronary syndrome occurring in 39% (OR 4.06; CI 2.96–5.56). Importantly, these patients did not benefit from other antiplatelet drugs, including clopidogrel, and tirofiban, a glycoprotein IIb/IIIa inhibitor. Thus, aspirin-resistant patients are at greater risk of clinically important CVD morbidity and mortality than aspirin-sensitive patients, and this risk is present in those with stable CVD, those having undergone coronary artery bypass surgery or percutaneous coronary intervention, those undergoing other vascular procedures, and those who have had a stroke [2].
The fish-derived omega-3 (ω3) fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have demonstrated strong cardioprotective effects [3-8]. EPA+DHA supplementation has been shown to reduce platelet aggregation, improve arterial endothelial function, and lower triglycerides and blood pressure [7]. Much of the cardioprotective effects have been attributed to the fact that EPA and DHA compete for the same metabolic pathways with arachidonic acid, an omega-6 fatty acid metabolized into primarily proinflammatory prostaglandins and leukotrienes through the actions of cyclooxygenase (COX)-1 and lipoxygenase pathways. Aspirin acetylates COX-1 and blocks the metabolism of arachidonic acid into a variety of mediators including thromboxane (a very potent platelet aggregator) [9-12]. One important fact is that low doses of aspirin (≤81 mg/d) are associated with a lower risk for clinically significant bleeding than higher doses [13,14]. In addition, synthesis of the vasodilatory prostaglandin PGI (PGI2) is less inhibited by low doses (≤81 mg) of aspirin than higher doses [15]. Low dose aspirin and ω3 fatty acids might work in parallel to shift the fatty acid metabolic balance toward a less inflammatory milieu as well as by interactions leading to the production of a variety of potent lipid mediator metabolites of EPA and DHA [16-18]. Although potential benefits of EPA and DHA in suppressing platelet function beyond that of aspirin alone have been demonstrated with chronic dosing [19,20], very little is known about acute effects of aspirin and ω3 fatty acids and more research is needed to define the precise mechanisms involved.
Lysophospholipids are potent lipid mediators with a diverse range of effects in a variety of tissues, and affect the growth, survival, migration and activation of many cell types [21]. Lysophosphatidic acid (LPA) and lysophosphatidylcholine (LPC) are increasingly linked with atherosclerosis by virtue of their effects on endothelial cells, monocytes and smooth muscle cells [22-25]. A diverse group of LPA and LPC species exist with each including a particular fatty acid such as EPA or DHA, and unsaturated LPA species have demonstrated proinflammatory effects whereas this is not the case with saturated LPA species [26]. Studies in vitro and in mouse models suggest that extracellular LPA is primarily generated by hydrolysis of LPC by autotaxin, an endothelial expressed enzyme, but this has not been well studied in humans [27,28]. CVD has strong inflammatory components, and several cytokines/chemokines and angiogenesis factors have been associated with its progression [29]. These include, most prominently, tumor necrosis factor-α, IL-6, IL-8, IL-1β, macrophage chemoattractant protein (MCP), platelet-derived growth factor (PDGF), basic fibroblast growth factor (b-FGF), and vascular endothelial growth factor (VEGF), whereas IL-10 tends to be atheroprotective [30].
We conducted a clinical trial to learn more about potential interactions between aspirin and ω3 fatty acids. Our primary hypothesis was that treatment with low-dose aspirin (81 mg) plus EPA and DHA would acutely reduce platelet function more than the treatment with aspirin alone. Secondary hypotheses were that treatment with aspirin plus EPA+DHA would alter concentrations of lysophospholipids, reduce proinflammatory cytokines and angiogenesis stimulators more than aspirin alone.
2. Patients and methods
2.1. Patients
Twenty-five healthy volunteers were recruited for this study to allow for an assessment of fatty acid and aspirin effects without undue influence by factors that would alter lysophospholipid metabolism. Non-smoking male and female subjects between the ages of 18 and 50 not taking any medications, vitamin pills, nutritional supplements, or herbal preparations were recruited. Subjects with a history of chronic diseases (e.g. cardiovascular, renal, hepatic, neurodegenerative, neoplastic, metabolic {diabetes}, hypertension; based on screening medical history, a complete blood count, and comprehensive metabolic profile), or allergic reactions to aspirin, fish, fish oils, or non-steroidal anti-inflammatory drugs were excluded. Other exclusions included drinking more than three alcoholic beverages a day, or having any of the following conditions: an ulcer or bleeding in the stomach, liver or kidney disease, bleeding or blood clotting disorder (e.g. hemophilia), congestive heart failure, fluid retention, high blood pressure, gout, asthma, arthritis, or nasal polyps. The study was approved by the University of Rochester’s Research Subjects’ Review Board (IRB) and written informed consent was obtained from each subject. Subjects were told to notify the study coordinator by telephone if they noticed any unusual symptoms during the study.
2.2. Protocol
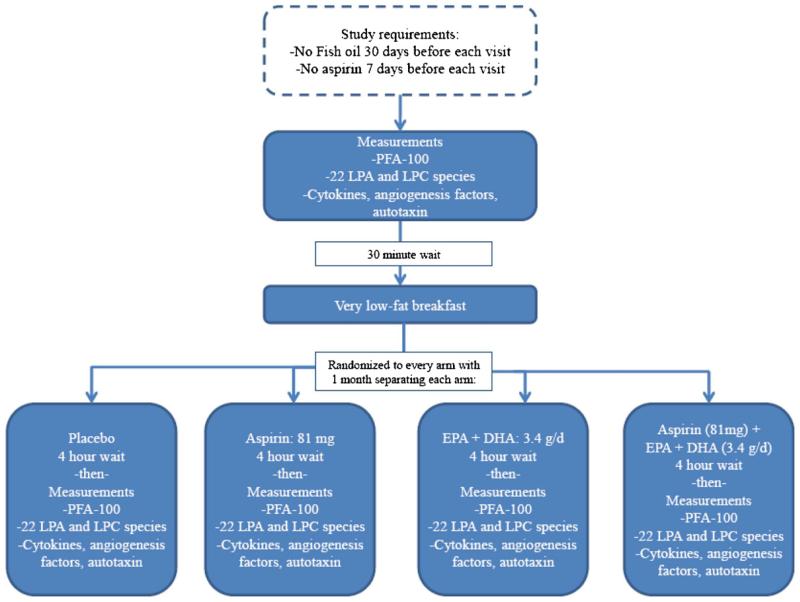
This was a randomized, placebo-controlled, double-blinded trial with a cross-over design (ClinicalTrials.gov identifier: NCT01181882). Each subject served as his/her own control. The study (see Fig. 1) involved four visits 4 weeks apart, all hosted in the University of Rochester Clinical Research Center. At each separate study visit, each subject received (using a randomized protocol) placebo, 81 mg aspirin, 4 g Lovaza®(3.4 g of EPA+DHA), or both aspirin and Lovaza®. Thus, each subject received each of these treatments individually during randomly chosen visits. Subjects, Center staff, and investigators were blinded as to which treatment was given at each visit and this ensured by the study pharmacist making the tablets and capsules for each treatment appear identical. Prior to each visit, subjects ate a standard low-fat dinner the prior evening, then fasted for at least 8 h prior to arrival at the Center. Subjects were required to abstain from taking aspirin or non-steroidal anti-inflammatory drugs for 10 day prior to each visit and ω3 fatty acids for 30 day prior to the baseline study visit, and all subsequent clinic visits. Visits lasted approximately 6 h, with subjects at bedrest. A venous catheter was placed in a peripheral vein (saline lock, 18 gauge or larger, {no heparin used} in the forearm) with blood drawn, at baseline and 4 h post-treatment, into citrated tubes at each visit for Platelet Function Analyzer-100 (PFA-100-Siemens, Deerfield, IL) closure time testing, and EDTA tubes for plasma, in that order. Plasma from EDTA containing tubes was separated via centrifugation and frozen at −80 °C for lysophospholipid, autotaxin, and cytokine measurements. Subjects were provided with a standard low-fat breakfast after the baseline phlebotomy.
Fig. 1.
Study design.
Lovaza® (Reliant Pharmaceuticals) is an FDA-regulated ω3 fatty acid supplement and the only prescription EPA+DHA product available in the US. Each 1-g capsule of Lovaza® contains at least 900 mg of the ethyl esters of ω3 fatty acids sourced from fish oils. These are predominantly a combination of ethyl esters of eicosapentaenoic acid (EPA-approximately 465 mg) and docosahexaenoic acid (DHA-approximately 375 mg). One gram of a virtually identical supplement has been shown to reduce CVD events within 3 months of use in individuals post-myocardial infarction [31]. We chose to use a higher dose of 4000 mg to investigate fairly rapid metabolic changes and because 4000 mg/d is an approved and safe daily treatment for hypertriglyceridemia. Note that the absorption of fatty acids, including ω3’s, occurs within 1 h and peaks between 4 and 6 h in healthy adults [32].
2.3. Laboratory methods
2.3.1. PFA-100 testing
The PFA-100 system (PFA-100-Siemens, Deerfield, IL) uses a disposable analysis cartridge in order to simulate an injured blood vessel [33]. The PFA-100 system has been extensively described by Kundu and co-workers [34]. It simulates primary hemostasis by flowing whole blood at a high shear rate through an aperture (147 μm diameter) cut into a collagen-coated membrane coated with either epinephrine (10 μg) or ADP (50 μg), where it interacts with the membrane surface and aggregates. A platelet plug forms, with occlusion of the aperture and cessation of blood flow. The closure time reflects platelet function in the sample evaluated, with shorter closure times indicating increased platelet aggregation. Since it measures the ability of blood to pass through an aperture (not clot) after platelet activation in the presence of erythrocytes, it can be considered more clinically relevant than platelet aggregrometry, which is conducted in the absence of erythrocytes and blood flow. In addition, the PFA-100 method has been shown to correlate with an increased risk for CVD events [2]. ADP with collagen (non-specific) and epinephrine with collagen (aspirin-specific) were the primary platelet aggregation agonists used for PFA-100 testing [35], and the epinephrine–collagen disposable cartridge was used for the detection of aspirin “resistance” [36,37]. There is no consensus about how to define aspirin “resistance”, which can suggest failure of aspirin to prevent clinical events associated with vascular occlusion, failure of aspirin to inhibit platelet function in vivo or in vitro, and failure of aspirin to inhibit thromboxane A2 production [38]. For the purposes of this study, we define aspirin resistance as “acute failure of aspirin to inhibit platelet function”. More specifically the metric for this outcome was defined as a closure time of <181 s after aspirin, by PFA-100 testing with epinephrine, as per the clinical laboratory at the University of Rochester. This was based on the Siemens expected reference range of upper limit <193 s (90% central interval) [36] and the <181 s threshold internally was established from an in-house study involving 52 subjects. The lower cut off of 181 s was related to the different collection tubes used in the Siemens study (3.8% Na Citrate) and 3.2% Na Citrate internally.
2.3.2. Lysophospholipid testing
LPC and LPA were analyzed according to procedures identical to those that we employed and described in detail previously [39]. Briefly, a modified Bligh and Dyer extract of plasma was directly infused into an ABI QTrap 2000 via an Advion Nanomate robotic device. Signals were calibrated against internal standards LPA-17:0 and LPC-17:0 obtained from Avanti Polar Lipids (Alabaster, AL). LPA and LPC were analyzed in negative and positive ion modes, respectively. Calibration on a molecular species by species basis was accomplished with an external standard mixture to obtain relative response factors. After relative response was calibrated, concentrations were calibrated against the internal standards.
2.3.3. Autotaxin testing
We used a quantitative assay for plasma autotaxin mean fluorescence units/lL (activity) using a fluorescently labeled LPC analog as substrate. Recombinant autotaxin was expressed in insect cells and used as a positive control to generate a standard curve (courtesy of Dr. Andrew Morris, University of Kentucky). The LPC analog (termed FS-3, 10 μM, Echelon Biosciences) [40] uses a fluorescence “dequenching” motif, in which a fluorophore (fluorescein) that is silent because of intramolecular fluorescence resonance energy transfer to a nonfluorescing quencher (dabcyl) becomes fluorescent once enzymatic hydrolysis cleaves the substrate. The autotaxin assay was performed in triplicate in black 96-well microtiter plates using assay buffer, FS-3, and sample. The assay buffer was prepared as a 5 × working solution of 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 50 mM TRIS with a final pH of 8. Fluorescence was analyzed after 1 and 2 h at an incubation temperature of 37 °C by a Biotex FLX-800 plate reading fluorimeter/shaker/incubator equipped with a 485/20 nm excitation filter and a 528/20 nm emission filter.
2.3.4. Cytokine and angiogenesis factor testing
The effects of aspirin and EPA+DHA, alone or in combination, on plasma levels of cytokines and angiogenesis factors were examined using the BioPlex suspension array system (BioRad Life Sciences, Hercules, CA), according to the manufacturer’s instructions. Briefly, beads coated with specific antibodies [anti-tumor necrosis factor alpha TNF-α, platelet-derived growth factor (PDGF), interleukins (IL-6, IL-8, IL-1β, IL-10), macrophage chemoattractant protein (MCP), basic fibroblast growth factor (b-FGF), and vascular endothelial growth factor (VEGF)] were immobilized on a 96-well plate and allowed to react with sample (50 L) containing unknown amounts of cytokine. After a series of washes to remove unbound protein, a biotinylated detection antibody specific for a different epitope on the cytokine was added to the plate. Streptavidin-PE was added to the wells, and the reaction was quantified based on bead color and fluorescence intensity. Cytokine concentrations were calculated using BioPlex Manager software (Bio-Rad).
2.4. Statistical methods
PFA-100 results and concentrations of LPC, LPA acid species, and autotoxin activity were tested for normality using the ShapiroWilk statistic. As many variables were not normally distributed, the Wilcoxon signed-rank test was used to determine changes in these variables due to study treatments, relative to placebo, and also differences between baseline closure time values. Pearson correlation coefficients were used to determine the relationship between PFA-100 effects with aspirin and aspirin+EPA+DHA. A p-value <0.05 was used to define statistical significance and was not adjusted for multiple comparisons for any analyses since we had a priori hypotheses for each analyte examined. Analyses were performed using SAS software (version 9.2; SAS Institute Inc., Cary, NC).
3. Results
3.1. Baseline characteristics and study design
The baseline characteristics of all subjects who were enrolled and completed the study are described (Table 1). An overview of the study design is shown in Fig. 1. Subjects were provided with placebo, aspirin, EPA+DHA, or aspirin plus EPA/DHA in a randomized and blinded fashion. Four hours after the ingestion of each agent, a sample of peripheral blood was collected and analyzed for platelet function, lysophospholipid species, and cytokine and angiogenesis factor testing as outlined in the Methods.
Table 1.
Baseline demographic and clinical characteristics.
| Variable | Mean (N=25) |
|---|---|
| Age in years (SD) a | 28.8 (8.3) |
| Male (%) | 40 |
| Race (%) | |
| Caucasian | 64 |
| Black | 4 |
| Asian | 32 |
| Non-fried fish and tuna intake (%) | |
| Less than once a month | 40 |
| 1–3 day a month | 36 |
| Once a week | 20 |
| Two times a week | 4 |
| Alcohol consumption (%) | |
| Less than once a month | 40 |
| 1–3 days a month | 32 |
| 1–4 days a week | 28 |
| 5 or more days a week | 0 |
| Average # of drinks when drinking alcohol, (SD) | 1.5 (1.5) |
| Average exercise frequency/week, (SD) | 4.2 (2.5) |
| Education (% high school or more) | 100 |
| Smoking status (%) | |
| Current | 0 |
| Former | 16 |
| Never | 84 |
SD=Standard deviation.
3.2. Treatment effects
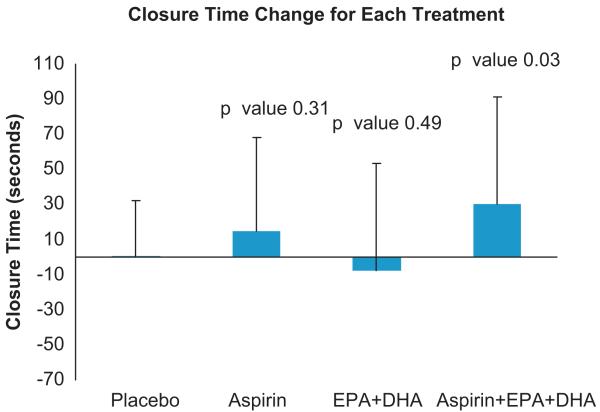
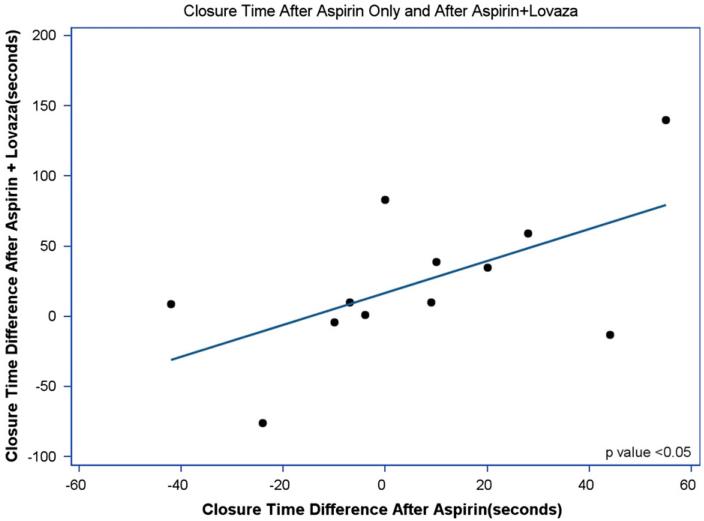
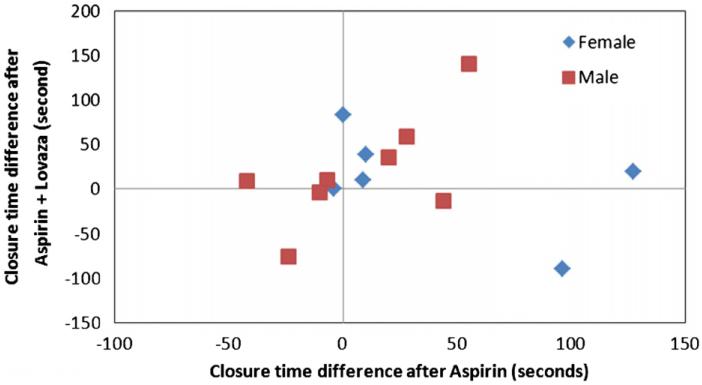
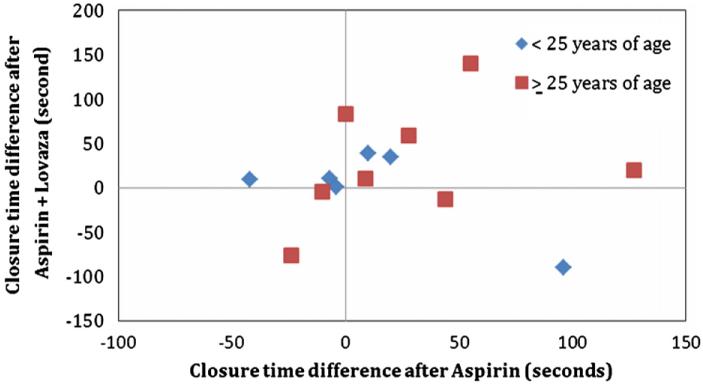
The mean closure time for all time points (baseline and after each treatment) was 131±1SD (36). Mean closure times at baseline were 129±32.5, 126±34, 136±48, and 132±29 for placebo, aspirin, EPA+DHA, and aspirin+EPA+DHA, respectively (p>0.05). There was a wide range of closure times after aspirin therapy (136 s±1SD{43.8}, range: 56–259), with most subjects (14/16; 88%) demonstrating evidence of acute failure of aspirin to inhibit platelet function (closure time <181 s). The treatment effects on platelet function measured by the PFA-100 closure time using epinephrine–collagen as substrates are displayed in Fig. 2. Treatment with either aspirin or EPA+DHA alone did not significantly increase closure time (p>0.05) compared with placebo. In contrast, treatment with both EPA+DHA and aspirin increased the PFA-100 closure time relative to placebo (p<0.05). Fig. 3 shows the positive relationship between PFA-100 closure times with aspirin treatment alone compared with placebo and closure times with both aspirin and EPA+DHA treatments compared with placebo (R2=0.34, p<0.05), for those 14 individuals who were classified as aspirin resistant (closure time <181 s). This is a significantly positive relationship (p<0.05, R2=0.34). This was not significant when the entire study sample of participants was included (data not shown: p>0.05). In Fig. 4 are the same data for all participants in which, although no significant difference (p>0.05) between males and females was present, a more linear and positive trend for males than females exists. In Fig. 5 are the same data for all participants in which, although no significant difference (p>0.05) between those age <25 years and those age ≥25 years was present, a more linear and positive trend for those ≥age 25 exists.
Fig. 2.
PFA-100 closure platelet function time Results. p-Values represent effects relative to placebo. Bars represent means and error bars represent standard deviations. Twenty-four participants had PFA-100 data from the placebo-only treatment visit. Since a result was not available for treatments as well as placebo in all individuals, the values are 16 for aspirin alone and 21 for EPA+DHA alone and both, compared to placebo.
Fig. 3.
PFA-100 closure platelet function time relationships between aspirin alone and aspirin+lovaza for those with closure time ≤181 s 4 h after aspirin dose. Pearson correlation coefficients were used to determine the relationship between PFA-100 effects with aspirin and aspirin+EPA+DHA. The p-value indicates the significance of the R2.
Fig. 4.
PFA-100 closure platelet function time scatterplot relationships between aspirin alone and aspirin+lovaza for all participants by sex.
Fig. 5.
PFA-100 closure platelet function time scatterplot relationships between aspirin alone and aspirin+lovaza for all participants by age.
We measured in parallel the effects of each treatment on concentrations of different LPA and LPC species in plasma using quantitative mass spectrometry (Table 2). Interestingly, acute ingestion of EPA+DHA significantly increased the concentration of 20:5n3 (EPA) LPC (p<0.05), whereas this effect was lost following ingestion of EPA+DHA and aspirin. Aspirin alone did not acutely change any lysophospholipid species examined, and none of the LPA species changed following acute ingestion of either aspirin, EPA+DHA, or aspirin combined with EPA+DHA (p>0.05). Table 3 shows that none of the treatment arms significantly changed plasma concentrations of cytokines, angiogenesis activators, or autotaxin activity (all p>0.05).
Table 2.
The changes in fatty acids within corresponding LPC and LPA species with treatments.
| Baseline values | Effect of ASA compared to placeboa |
p-Value | Effect of EPA+DHA compared to placeboa |
p-Value | Effect of EPA+DHA+ASA compared to placeboa |
p-Value | |
|---|---|---|---|---|---|---|---|
| LPA (nM) | |||||||
| 16:00 | 36.80 (60.3) | 12.45 (65.4) | 0.66 | 16.79 (68.11) | 0.18 | 18.55 (98.18) | 0.95 |
| 16:1n7 | 6.63 (9.2) | 9.49 (10.34) | 0.34 | 1.57 (9.76) | 0.44 | 2.06 (13.91) | 0.57 |
| 18:00 | 46.45 (77.4) | 19.5 (94.26) | 0.37 | 9.02 (93.16) | 0.93 | 32.56 (141.74) | 0.93 |
| 18:1n9 | 7.14 (9.1) | 6.14 (28.59) | 0.47 | 3.60 (15.90) | 0.57 | 10.30 (44.94) | 0.98 |
| 18:2n6 | 7.68 (6.6) | 0.16 (13.71) | 0.87 | 3.23 (10.76) | 0.18 | 3.46 (15.50) | 0.86 |
| 18:3n3 | 16.21 (18.9) | −15.78 (97.73) | 0.76 | 9.11 (46.13) | 0.84 | 4.48 (28.74) | 0.81 |
| 20:4n6 | 19.66 (17.4) | 5.32 (23.65) | 1.00 | 4.74 (29.07) | 0.45 | 10.36 (39.0) | 0.98 |
| 22:4n6 | 17.21 (18.8) | −6.55 (54.22) | 0.87 | 2.48 (24.38) | 0.88 | 3.40 (26.88) | 0.77 |
| 20:5n3 | 17.62 (16.1) | 1.18 (18.33) | 0.62 | 1.81 (20.52) | 0.57 | 7.72 (30.42) | 0.75 |
| 22:5n3 | 29.00 (18.6) | −6.45 (49.45) | 0.85 | 0.35 (25.73) | 0.66 | 5.74 (31.99) | 0.66 |
| 22:6n3 | 30.46 (23.9) | 4.59 (31.17) | 0.74 | 1.52 (34.26) | 0.77 | 13.44 (43.95) | 0.31 |
| Total LPA | 234.88 (250.5) | 21.10 (356.42) | 0.68 | 54.21 (313.92) | 0.57 | 64.00 (297.58) | 0.84 |
| LPC (μM) | |||||||
| 16:00 | 76.13 (110.9) | −3.6 (95.6) | 0.72 | −2.1 (63.3) | 0.39 | −2.7 (72.3) | 0.84 |
| 16:1n7 | 3.51 (5.4) | −0.5 (3.2) | 0.69 | −0.42 (2.17) | 0.89 | −0.06 (2.8) | 0.55 |
| 18:00 | 39.00 (50.6) | 0.69 (29.9) | 0.14 | 3.59 (25.3) | 0.39 | 0.32 (21.4) | 0.81 |
| 18:1n9 | 31.73 (58.0) | −6.5 (29) | 0.72 | −7.5 (25.0) | 0.75 | −11.4 (32.3) | 0.27 |
| 18:2n6 | 72.94 (147.6) | −24.3 (96.2) | 0.85 | −16.0 (69.9) | 0.54 | −34.6 (117.0) | 0.70 |
| 18:3n3 | 2.99 (2.59) | −0.007 (6.4) | 0.27 | −0.55 (5.0) | 0.50 | −1.09 (6.1) | 0.28 |
| 20:4n6 | 91.42 (84.4) | −0.03 (121.1) | 0.72 | 2.4 (67.4) | 0.31 | −13.2 (95.3) | 0.91 |
| 22:4n6 | 6.63 (6.9) | 4.1 (26.7) | 0.53 | −0.72 (11.4) | 0.34 | −1.23 (9.6) | 0.85 |
| 20:5n3 | 5.60 (4.4) | 0.1 (6.6) | 0.3 | 2.2 (5.2) | 0.002 | 0.86 (7.7) | 0.13 |
| 22:5n3 | 13.17 (10.2) | −1.2 (14.4) | 0.89 | −0.73 (11.2) | 0.27 | −1.7 (12.0) | 0.70 |
| 22:6n3 | 20.40 (10.2) | −3.8 (48.0) | 0.76 | −3.7 (39.2) | 0.42 | −6.6 (49.8) | 1.00 |
| Total LPC | 363.53 (418.0) | −28.8 (369) | 0.85 | −23.5 (254.7) | 0.62 | −71.4 (328.6) | 0.62 |
p-Values were calculated using the Wilcoxon signed-rank test.
Baseline values were collected prior to study agent treatment at each participant’s first study visit when comparing the effects of aspirin to EPA+DHA, aspirin to aspirin+EPA+DHA, and EPA+DHA to EPA+DHA+aspirin, none were statistically significant (p≥0.05).
ASA=aspirin.
Change (SD).
Table 3.
Mean changes in plasma cytokines, angiogenesis factors, and autotaxin with treatments.
| Cytokines | Baseline values | ASA effect compared to placebo* |
p-value | EPA+DHA effect compared to placebo* |
p-value | EPA+DHA+ASA effect compared to placebo* |
p-value |
|---|---|---|---|---|---|---|---|
| IL-1B | 0.61 (0.3) | 0.21 (0.61) | 0.06 | −0.11 (0.62) | 0.84 | −0.05 | 0.70 |
| IL-6 | 2.47 (1.1) | −0.57 (4.7) | 0.86 | −0.18 (1.26) | 0.63 | −0.98 (5.1) | 0.77 |
| IL-8 | 2.84 (0.9) | −0.1 (3.5) | 0.88 | −0.13 (3.3) | 0.46 | −0.3 (3.73) | 0.43 |
| IL-10 | 2.53 (3.3) | −0.39 (1.4) | 0.31 | −0.52 (1.5) | 0.15 | −1.92 (6.1) | 0.12 |
| MCP-1 | 8.58 (5.8) | −3.8 (13.9) | 0.23 | −4.8 (15.8) | 0.20 | −3.4 (16.7) | 0.57 |
| INF-A | 6.96 (5.7) | 2.6 (5.6) | 0.23 | 0.96 (5.1) | 0.45 | −1.5 (8.94) | 0.96 |
| IFN-G | 36.20 (22.0) | 0.05 (25.2) | 0.84 | −2.3 (28.9) | 0.68 | −3.0 (31.4) | 0.72 |
| G-CSF | 10.44 (8.3) | −0.8 (4.9) | 0.73 | −0.9 (6.2) | 0.68 | −1.1 (5.85) | 0.50 |
| EOTAXIN | 23.26 (13.0) | −4.5 (20.1) | 0.28 | −3.0 (20.8) | 0.70 | −3.5 (18.0) | 0.87 |
| PDGF-BB | 371.56 (206.0) | 14.6 (156.2) | 0.93 | 9.4 (232.3) | 0.40 | 16.7 (298.7) | 0.56 |
| VGEF | 9.33 (7.9) | −2.06 (35.4) | 0.80 | −5.6 (32.1) | 0.41 | −6.1 (33.0) | 0.28 |
| FGF-BASIC | 13.66 (9.5) | 1.4 (7.2) | 0.56 | −0.49 (12.1) | 0.98 | −0.62 (10.7) | 0.75 |
| Autotaxin | 117.76 (18.2) | 1.3 (13.24) | 0.45 | −0.09 (12.8) | 0.82 | −1.21 (9.9) | 0.76 |
All means are differences between the effects of placebo, aspirin, EPA+DHA, or both, on plasma concentrations. Units for the cytokines are picogram/mL and mean flourescenceunits/lL for autotoxin.
p-values were calculated using the Wilcoxon signed-rank test.
Change (SD).
When comparing the effects of aspirin to EPA+DHA, aspirin to aspirin+EPA+DHA, and EPA+DHA to EPA+DHA+aspirin, none were statistically significant (p≥0.05) ASA=aspirin.
4. Discussion
The primary objective of our study was to determine if the combination of aspirin plus a pharmaceutical ω3 supplement is better than aspirin alone for inhibiting thrombosis acutely. We investigated the acute effects of aspirin with or without EPA+DHA on platelet function due to the wide use of aspirin in the prophylaxis and treatment of CVD events. Our results demonstrate that a beneficial effect on platelet function occurred within 4 h after ingestion of 81 mg of aspirin and 3.4 g of EPA+DHA ω3 fatty acids in healthy adult subjects. The closure time prolongation effects of aspirin and EPA+DHA in our group with acute failure of aspirin to inhibit platelet function were significantly positively correlated with prolongation effects with aspirin alone. This fact implies that the adjunctive antithrombotic effect of EPA+DHA on the antiplatelet potential of aspirin depends on the degree of aspirin’s effects. This was not significant when the entire study sample of participants was included (data not shown: p-value >0.05). By using healthy subjects and low-dose aspirin, we sought to take the novel approach of determining these effects in those who would experience the acute failure of aspirin to inhibit platelet function only 4 h after ingestion of these agents. We are not aware of other investigators determining such acute, within hours, effects of aspirin as their time-frames for determining aspirin effects on platelet function and aspirin “resistance” (measured by the PFA-100) were in the range of days, weeks, or months [37,41-45].
The limitations of aspirin monotherapy as primary prevention of CVD are being increasingly appreciated. For example, in the Japanese Primary Prevention of Atherosclerosis with Aspirin for Diabetes (JPAD) trial [46], patients who took 81 mg or 100 mg of aspirin each day for close to 5 years experienced CVD events at a rate that was not statistically different than those who did not take aspirin. The exceptions were CVD death, as rates were lower in those taking aspirin, and total CVD events in individuals 65 years of age and older. Statistical power to detect a difference in the primary endpoint of CVD events (fatal and nonfatal ischemic heart disease, fatal and nonfatal stroke, and peripheral vascular disease) was very limited due to rates of the endpoint being about 1/3 predicted. Despite the limitations of the JPAD study (e.g. open-label study design), the results of it and others [47,48] such as the Primary Prevention Project (PPP) trial provide evidence that in patients with a wide spectrum of CVD risk [2,49], aspirin may exert no substantial cardioprotective effects. Given the increasing prevalence of diabetes mellitus and CVD globally [50,51], the public health implications of aspirin “resistance” are substantial. Current data exist for the potential benefits of the combination of aspirin and ω3 fatty acids on platelet function [19,20,52], but studies have not specifically assessed the acute effects of this treatment.
Implications of the acute effects of a low-dose of aspirin include that the risk of bleeding is lower with low (≤81 mg/d) than with higher doses [13,14]. When aspirin is used in the setting of an acute CVD event its effects are critical and physicians are encouraged to use the lowest effective dose of aspirin in a clinical setting [13]. In fact, the daily administration of 30 mg of aspirin results in virtually complete suppression of platelet TXA2 production after 1 week [53] through a cumulative process of fractional acetylation of roughly 50% of unacetylated platelet COX-1 by successive daily doses of aspirin [54]. Despite the fact that TXA2 is a prostanoid derived predominantly from COX-1 (mostly from platelets) and its biosynthesis is highly regulated by aspirin inhibition of COX-1 [54,55], vascular prostaglandin PGI (PGI2) is synthesized primarily by COX-2 [56-58] and is less inhibited by low doses (≤80 mg) of aspirin than higher doses [55]. Such low doses of aspirin have no measurable effects on prostaglandin I2(PGI2)-dependent endothelial function beneficial effects but do have other important vascular functions, including no increase in blood pressure [15], decline of renal function [59], or interference with the antihypertensive effects of diuretics and angiotensin-converting-enzyme (ACE) inhibitors [60]. This means that typical regimens of higher doses of aspirin per day clearly exceed the minimal effective dose required for a vascular pharmacodynamic effect. Aspirin-resistant patients do not benefit from other antiplatelet drugs and the combination of aspirin with other antiplatelet drugs, such as clopidogrel, is associated with a significantly higher risk of major bleeding than with aspirin alone [61]. In contrast, although the safety of EPA+DHA plus aspirin is supported by the sum of the data available from clinical trials and observational studies [62], the benefits of this combination of antiplatelet agents has not received much attention. Means of overcoming aspirin “resistance” with therapies that lack significant drug–drug interactions with prescription medications used in patients with or at risk for CVD events have so far been lacking [2], and thus novel approaches are needed. The effects of aspirin and EPA+DHA on the cardiovascular system extend to more than platelet function alone and thus the potential adjunctive effects of EPA+DHA on the benefits of aspirin may be broader than anticipated [18]. At the same time, peroxidation tends to occur with doses of EPA+DHA>1 g/d which could adversely affect platelet function and increase risk for CVD [63,64]. We hypothesize that the anti-inflammatory properties of aspirin may negate these oxidative effects and reduce platelet function. Emerging evidence from laboratory experiments has shown that EPA and DHA, in the presence of aspirin, are the precursors to potent bioactive mediators that possess both protective and anti-inflammatory properties [65]. Our approach is novel because we hypothesized that a dose of only 81 mg of aspirin (commonly prescribed in the US) can exert platelet function inhibitory effects when combined with EPA and DHA acutely. This combinatory effect is important because it may provide more potential benefits than higher doses of aspirin commonly used to overcome aspirin “resistance” and have implications for treatments of individuals with acute CVD events including myocardial infarction and stroke.
In a prior study we had shown that EPA+DHA ω3 fatty acid supplementation for 28 days leads to a significant increase in plasma EPA and DHA LPC species but no effect on EPA- and DHA-LPA species or autotaxin [39]. Current thinking is that hydrolysis of LPC by autotaxin is the major source of extracellular LPA [21]. Aspirin at a dose of 650 mg (alone and when combined with EPA+DHA) in this prior study had no effect on any of the LPCs, LPAs, or autotaxin 24 h after ingestion. In the present study, we observed a small but statistically significant acute effect of EPA+DHA ingestion on 20:5n3 LPC, indicating that free fatty acids can be rapidly incorporated into plasma LPC. These are the first data that we are aware of demonstrating that diet acutely influences plasma LPC remodeling. This same effect was not noted when EPA+DHA was combined with aspirin. We are not able to delineate why this occurred from the data we obtained. Whether alterations in specific LPC species acutely underlie any of the cardioprotective effects of ω-3 fatty acid supplementation will require further study. As both aspirin and LPA are known to affect platelet function [17,52,66,67], and platelet activation leads to LPA production, it is likely that the lack of effect of these agents on LPA was independent of their effects on platelet function in the current study. The ingestion of 100 mg of aspirin for 1 month has been associated with a reduction in plasma LPA in individuals with cerebrovascular disease [68], but we did not observe acute effects of aspirin in our current and prior studies. Our findings also suggest that the production of EPA and DHA LPA species is a highly regulated process, and that steady-state LPA levels are not influenced by the ingestion and concentration of these dietary fatty acids. Note that recent studies suggest that plasma LPA has a very short half-life in vivo [69]. As we did not conduct an extensive kinetic analysis of LPC or LPA species after ingestion of the dietary supplement, we cannot absolutely exclude the possibility that a very transient spike in plasma EPA or DHA LPA species occurs soon after ingestion (e.g. within minutes). With regard to our study findings of no effects of EPA+DHA or aspirin on autotaxin, we did not analyze its expression (e.g. by Western blot analysis of plasma samples), thus although it remains possible that EPA+DHA or aspirin may affect ATX expression per se, we conclude that this did not translate into effects on activity, arguably a more relevant biological readout.
Our study has several strengths, including the use of a clinical research center in which the study design was highly controlled and subject adherence to the treatments was ensured. We measured platelet function using the Platelet Function Analyzer (PFA-100), which is designed to be a more valid estimator of the effects of disease states and treatments on thrombus generation than other assays [33] and has been shown to correlate better with COX-1 inhibition than platelet aggregometry [70,71]. Platelet function analyzed using the PFA-100 has also correlated with risk for events in humans [2]. However, it is sensitive to many variables, including platelet function, platelet count, red blood cells, and plasma von Willebrand factor (VWF) [38]. We also investigated the combinatory effects of aspirin with EPA+DHA acutely on lysophospholipids, a variety of cytokines, and angiogenesis factors, which are associated with CVD risk. However, our study also has some limitations. The sample size was relatively small, and PFA-100 values were not obtainable for all subjects, limiting statistical power. Platelet function was measured using only the PFA-100 and these data were not compared to other valid platelet function and thrombus-stimulating analytes. The mechanisms by which the combination of low-dose aspirin plus EPA/DHA inhibited platelet function cannot be determined by our current data. Future studies investigating the potential roles of resolvins or other oxylipins should be worthwhile.
We conclude that the combination of aspirin together with EPA+DHA ω3 fatty acid supplementation demonstrates superior efficacy in attenuating platelet function that aspirin alone in healthy adult subjects. Future studies exploring the mechanisms of these effects and longer-term consequences for CVD endpoints would be worthwhile. In particular, similar studies in those who have a higher risk for CVD events, such as those with diabetes, are important. In addition, investigation of the dose- and time-dependent effects of aspirin and EPA+DHA on platelet function will be important.
Acknowledgments
The authors thank Kelly Keating, Ph.D., of the Albany College of Pharmacy and Health Sciences, for manuscript editing. We are indebted to Kelly Thevenet-Morrison, MS, for her assistance with improving the quality of the figures and tables.
Sources of support
The project described in this publication was supported by the University of Rochester CTSA award number KL2 RR024136 from the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The project described in this publication was supported by the University of Rochester CTSA award number UL1 RR024160 from the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures
None.
References
- [1].Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970–2002. J. Am. Med. Assoc. 2005;294(10):1255–1259. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- [2].Krasopoulos G, Brister SJ, Beattie WS, Buchanan MR. Aspirin “resistance” and risk of cardiovascular morbidity: systematic review and meta-analysis. Br. Med. J. 2008;336(7637):195–198. doi: 10.1136/bmj.39430.529549.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bucher HC, Hengstler P, Schindler C, Meier G. n-3 Polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am. J. Med. 2002;112(4):298–304. doi: 10.1016/s0002-9343(01)01114-7. [DOI] [PubMed] [Google Scholar]
- [4].Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS. n-3 Polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the cardiovascular health study. Am. J. Clin. Nutr. 2003;77(2):319–325. doi: 10.1093/ajcn/77.2.319. [DOI] [PubMed] [Google Scholar]
- [5].Iso H, Rexrode KM, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, et al. Intake of fish and omega-3 fatty acids and risk of stroke in women. J. Am. Med. Assoc. 2001;285(3):304–312. doi: 10.1001/jama.285.3.304. [DOI] [PubMed] [Google Scholar]
- [6].GISSI, -Prevenzione Investigators Dietary supplementation with n-3 poly-unsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- [7].Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- [8].Block RC, Harris WS, Reid KJ, Sands SA, Spertus JA. EPA and DHA in blood cell membranes from acute coronary syndrome patients and controls. Atherosclerosis. 2008;197(2):821–828. doi: 10.1016/j.atherosclerosis.2007.07.042. [DOI] [PubMed] [Google Scholar]
- [9].Gurbel PA, Bliden KP, DiChiara J, Newcomer J, Weng W, Neerchal NK, et al. Evaluation of dose-related effects of aspirin on platelet function: results from the Aspirin-Induced Platelet Effect (ASPECT) study. Circulation. 2007;115(25):3156–3164. doi: 10.1161/CIRCULATIONAHA.106.675587. [DOI] [PubMed] [Google Scholar]
- [10].Ohmori T, Yatomi Y, Nonaka T, Kobayashi Y, Madoiwa S, Mimuro J, et al. Aspirin resistance detected with aggregometry cannot be explained by cyclooxygenase activity: involvement of other signaling pathway(s) in cardiovascular events of aspirin-treated patients. J. Thromb. Haemost. 2006;4(6):1271–1278. doi: 10.1111/j.1538-7836.2006.01958.x. [DOI] [PubMed] [Google Scholar]
- [11].Dyerberg J, Bang HO, Stoffersen E, Moncada S, Vane JR. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 1978;2(8081):117–119. doi: 10.1016/s0140-6736(78)91505-2. [DOI] [PubMed] [Google Scholar]
- [12].Terano T, Hirai A, Hamazaki T, Kobayashi S, Fujita T, Tamura Y, et al. Effect of oral administration of highly purified eicosapentaenoic acid on platelet function, blood viscosity and red cell deformability in healthy human subjects. Atherosclerosis. 1983;46(3):321–331. doi: 10.1016/0021-9150(83)90181-8. [DOI] [PubMed] [Google Scholar]
- [13].Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N. Engl. J. Med. 2005;353(22):2373–2383. doi: 10.1056/NEJMra052717. [DOI] [PubMed] [Google Scholar]
- [14].Campbell CL, Smyth S, Montalescot G, Steinhubl SR. Aspirin dose for the prevention of cardiovascular disease: a systematic review. J. Am. Med. Assoc. 2007;297(18):2018–2024. doi: 10.1001/jama.297.18.2018. [DOI] [PubMed] [Google Scholar]
- [15].Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the hypertension optimal treatment (HOT) randomised trial, HOT Study Group. Lancet. 1998;351(9118):1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- [16].Fredman G, Van Dyke TE, Serhan CN. Resolvin E1 regulates adenosine diphosphate activation of human platelets. Arterioscler. Thromb. Vasc. Biol. 2010;30(10):2005–2013. doi: 10.1161/ATVBAHA.110.209908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, et al. Resolvin E1, an EPA-derived mediator in whole blood, selectively counter-regulates leukocytes and platelets. Blood. 2008;112(3):848–855. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008;8(5):349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gajos G, Rostoff P, Undas A, Piwowarska W. Effects of polyunsaturated omega-3 fatty acids on responsiveness to dual antiplatelet therapy in patients undergoing percutaneous coronary intervention: the OMEGA-PCI (OMEGA-3 Fatty Acids after PCI to Modify Responsiveness to Dual Antiplatelet Therapy) study. J. Am. Coll. Cardiol. 2010;55(16):1671–1678. doi: 10.1016/j.jacc.2009.11.080. [DOI] [PubMed] [Google Scholar]
- [20].Lev EI, Solodky A, Harel N, Mager A, Brosh D, Assali A, et al. Treatment of aspirin-resistant patients with omega-3 fatty acids versus aspirin dose escalation. J. Am. Coll. Cardiol. 2010;55(2):114–121. doi: 10.1016/j.jacc.2009.08.039. [DOI] [PubMed] [Google Scholar]
- [21].Aoki J. Mechanisms of lysophosphatidic acid production. Semin. Cell Dev. Biol. 2004;15(5):477–489. doi: 10.1016/j.semcdb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- [22].Wu WT, Chen CN, Lin CI, Chen JH, Lee H. Lysophospholipids enhance matrix metalloproteinase-2 expression in human endothelial cells. Endocrinology. 2005;146(8):3387–3400. doi: 10.1210/en.2004-1654. [DOI] [PubMed] [Google Scholar]
- [23].Lucas A, Grynberg A, Lacour B, Goirand F. Dietary n-3 polyunsaturated fatty acids and endothelium dysfunction induced by lysophosphatidylcholine in Syrian hamster aorta. Metabolism. 2008;57(2):233–240. doi: 10.1016/j.metabol.2007.09.006. [DOI] [PubMed] [Google Scholar]
- [24].Panchatcharam M, Miriyala S, Yang F, Rojas M, End C, Vallant C, et al. Lysophosphatidic acid receptors 1 and 2 play roles in regulation of vascular injury responses but not blood pressure. Circ. Res. 2008;103(6):662–670. doi: 10.1161/CIRCRESAHA.108.180778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schmitz G, Ruebsaamen K. Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis. 2010;208(1):10–18. doi: 10.1016/j.atherosclerosis.2009.05.029. [DOI] [PubMed] [Google Scholar]
- [26].Yoshida K, Nishida W, Hayashi K, Ohkawa Y, Ogawa A, Aoki J, et al. Vascular remodeling induced by naturally occurring unsaturated lysophosphatidic acid in vivo. Circulation. 2003;108(14):1746–1752. doi: 10.1161/01.CIR.0000089374.35455.F3. [DOI] [PubMed] [Google Scholar]
- [27].Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae D, Takio K, et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J. Cell Biol. 2002;158(2):227–233. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP, et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol. Cell. Biol. 2006;13:5015–5022. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Celletti FL, Waugh JM, Amabile PG, Brendolan A, Hilfiker PR, Dake MD. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat. Med. 2001;4:425–429. doi: 10.1038/86490. [DOI] [PubMed] [Google Scholar]
- [30].Lamon BD, Hajjar DP. Inflammation at the molecular interface of atherogenesis: an anthropological journey. Am. J. Pathol. 2008;173(5):1253–1264. doi: 10.2353/ajpath.2008.080442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Marchioli R, Barzi F, Bomba E. On behalf of the GISSI-Prevenzione Investigators. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- [32].Garaiova I, Guschina IA, Plummer SF, Tang J, Wang D, Plummer NT. A randomised cross-over trial in healthy adults indicating improved absorption of omega-3 fatty acids by pre-emulsification. Nutr. J. 2007;6:4. doi: 10.1186/1475-2891-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sanderson S, Emery J, Baglin T, Kinmonth AL. Narrative review: aspirin resistance and its clinical implications. Ann. Intern. Med. 2005;142(5):370–380. doi: 10.7326/0003-4819-142-5-200503010-00012. [DOI] [PubMed] [Google Scholar]
- [34].Kundu SK, Heilmann EJ, Sio R, Garcia C, Davidson RM, Ostgaard RA. Description of an in vitro Platelet Function Analyzer-PFA-100. Semin. Thromb. Hemost. 1995;21(Suppl. 2):106–112. doi: 10.1055/s-0032-1313612. [DOI] [PubMed] [Google Scholar]
- [35].Mammen EF, Comp PC, Gosselin R, Greenberg C, Hoots WK, Kessler CM, et al. PFA-100 system: a new method for assessment of platelet dysfunction. Semin. Thromb. Hemost. 1998;24(2):195–202. doi: 10.1055/s-2007-995840. [DOI] [PubMed] [Google Scholar]
- [36].Gum PA, Kottke-Marchant K, Poggio ED, Gurm H, Welsh PA, Brooks L, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am. J. Cardiol. 2001;88(3):230–235. doi: 10.1016/s0002-9149(01)01631-9. [DOI] [PubMed] [Google Scholar]
- [37].Borna C, Lazarowski E, van Heusden C, Ohlin H, Erlinge D. Resistance to aspirin is increased by ST-elevation myocardial infarction and correlates with adenosine diphosphate levels. Thromb. J. 2005;3:10. doi: 10.1186/1477-9560-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cattaneo M. Aspirin and clopidogrel: efficacy, safety, and the issue of drug resistance. Arterioscler. Thromb. Vasc. Biol. 2004;24(11):1980–1987. doi: 10.1161/01.ATV.0000145980.39477.a9. [DOI] [PubMed] [Google Scholar]
- [39].Block RC, Duff R, Lawrence P, Kakinami L, Brenna JT, Shearer GC, et al. The effects of EPA, DHA, and aspirin ingestion on plasma lysophospholipids and autotaxin. Prostaglandins Leukot. Essent. Fatty Acids. 2010;82(2-3):87–95. doi: 10.1016/j.plefa.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ferguson CG, Bigman CS, Richardson RD, van Meeteren LA, Moolenaar WH, Prestwich GD. Fluorogenic phospholipid substrate to detect lysophospholipase D/autotaxin activity. Org. Lett. 2006;8(10):2023–2026. doi: 10.1021/ol060414i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Andersen K, Hurlen M, Arnesen H, Seljeflot I. Aspirin non-responsiveness as measured by PFA-100 in patients with coronary artery disease. Thromb. Res. 2002;108(1):37–42. doi: 10.1016/s0049-3848(02)00405-x. [DOI] [PubMed] [Google Scholar]
- [42].Hobikoglu GF, Norgaz T, Aksu H, Ozer O, Erturk M, Nurkalem Z, et al. High frequency of aspirin resistance in patients with acute coronary syndrome. Tohoku J. Exp. Med. 2005;207(1):59–64. doi: 10.1620/tjem.207.59. [DOI] [PubMed] [Google Scholar]
- [43].McCabe DJ, Harrison P, Mackie IJ, Sidhu PS, Lawrie AS, Purdy G, et al. Assessment of the antiplatelet effects of low to medium dose aspirin in the early and late phases after ischaemic stroke and TIA. Platelets. 2005;16(5):269–280. doi: 10.1080/09537100400020567. [DOI] [PubMed] [Google Scholar]
- [44].Abaci A, Caliskan M, Bayram F, Yilmaz Y, Cetin M, Unal A, et al. A new definition of aspirin non-responsiveness by Platelet Function Analyzer-100 and its predictors. Platelets. 2006;17(1):7–13. doi: 10.1080/09537100500163358. [DOI] [PubMed] [Google Scholar]
- [45].Pamukcu B, Oflaz H, Onur I, Oncul A, Umman B, Koylan N, et al. Aspirin-resistant platelet aggregation in a cohort of patients with coronary heart disease. Blood Coagul. Fibrinolysis. 2007;18(5):461–465. doi: 10.1097/MBC.0b013e32814db7e7. [DOI] [PubMed] [Google Scholar]
- [46].Ogawa H, Nakayama M, Morimoto T, Uemura S, Kanauchi M, Doi N, et al. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. J. Am. Med. Assoc. 2008;300(18):2134–2141. doi: 10.1001/jama.2008.623. [DOI] [PubMed] [Google Scholar]
- [47].Anfossi G, Russo I, Trovati M. Resistance to aspirin and thienopyridines in diabetes mellitus and metabolic syndrome. Curr. Vasc. Pharmacol. 2008;6(4):313–328. doi: 10.2174/157016108785909760. [DOI] [PubMed] [Google Scholar]
- [48].Sacco M, Pellegrini F, Roncaglioni MC, Avanzini F, Tognoni G, Nicolucci A, et al. Primary prevention of cardiovascular events with low-dose aspirin and vitamin E in type 2 diabetic patients: results of the Primary Prevention Project (PPP) trial. Diabetes Care. 2003;26(12):3264–3272. doi: 10.2337/diacare.26.12.3264. [DOI] [PubMed] [Google Scholar]
- [49].Hovens MM, Snoep JD, Eikenboom JC, van der Bom JG, Mertens BJ, Huisman MV. Prevalence of persistent platelet reactivity despite use of aspirin: a systematic review. Am. Heart J. 2007;153(2):175–181. doi: 10.1016/j.ahj.2006.10.040. [DOI] [PubMed] [Google Scholar]
- [50].Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: Part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104(22):2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- [51].Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case–control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- [52].Larson MK, Ashmore JH, Harris KA, Vogelaar JL, Pottala JV, Sprehe M, et al. Effects of omega-3 acid ethyl esters and aspirin, alone and in combination, on platelet function in healthy subjects. Thromb. Haemost. 2008;100(4):634–641. [PubMed] [Google Scholar]
- [53].Patrignani P, Filabozzi P, Patrono C. Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. J. Clin. Invest. 1982;69(6):1366–1372. doi: 10.1172/JCI110576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Patrono C, Ciabattoni G, Patrignani P, Pugliese F, Filabozzi P, Catella F, et al. Clinical pharmacology of platelet cyclooxygenase inhibition. Circulation. 1985;72(6):1177–1184. doi: 10.1161/01.cir.72.6.1177. [DOI] [PubMed] [Google Scholar]
- [55].FitzGerald GA, Oates JA, Hawiger J, Maas RL, Roberts LJ, 2nd, Lawson JA, et al. Endogenous biosynthesis of prostacyclin and thromboxane and platelet function during chronic administration of aspirin in man. J. Clin. Invest. 1983;71(3):676–688. doi: 10.1172/JCI110814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].McAdam BF, Catella-Lawson F, Mardini IA, Kapoor S, Lawson JA, FitzGerald GA. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc. Natl. Acad. Sci. USA. 1999;96(1):272–277. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Cullen L, Kelly L, Connor SO, Fitzgerald DJ. Selective cyclooxygenase-2 inhibition by nimesulide in man. J. Pharmacol. Exp. Ther. 1998;287(2):578–582. [PubMed] [Google Scholar]
- [58].Belton O, Byrne D, Kearney D, Leahy A, Fitzgerald DJ. Cyclooxygenase-1 and -2-dependent prostacyclin formation in patients with atherosclerosis. Circulation. 2000;102(8):840–845. doi: 10.1161/01.cir.102.8.840. [DOI] [PubMed] [Google Scholar]
- [59].Pierucci A, Simonetti BM, Pecci G, Mavrikakis G, Feriozzi S, Cinotti GA, et al. Improvement of renal function with selective thromboxane antagonism in lupus nephritis. N. Engl. J. Med. 1989;320(7):421–425. doi: 10.1056/NEJM198902163200703. [DOI] [PubMed] [Google Scholar]
- [60].Mene P, Pugliese F, Patrono C. The effects of nonsteroidal anti-inflammatory drugs on human hypertensive vascular disease. Semin. Nephrol. 1995;15(3):244–252. [PubMed] [Google Scholar]
- [61].Usman MH, Notaro LA, Nagarakanti R, Brahin E, Dessain S, Gracely E, et al. Combination antiplatelet therapy for secondary stroke prevention: enhanced efficacy or double trouble? Am. J. Cardiol. 2009;103(8):1107–1112. doi: 10.1016/j.amjcard.2009.01.003. [DOI] [PubMed] [Google Scholar]
- [62].Bays HE. Safety considerations with omega-3 fatty acid therapy. Am. J. Cardiol. 2007;99(6A):35C–43C. doi: 10.1016/j.amjcard.2006.11.020. [DOI] [PubMed] [Google Scholar]
- [63].Lagarde M, Calzada C, Guichardant M, Vericel E. Dose-effect and metabolism of docosahexaenoic acid: pathophysiological relevance in blood platelets, Prostaglandins Leukot. Essent. Fatty Acids. 2012 Apr 18; doi: 10.1016/j.plefa.2012.04.001. [DOI] [PubMed] [Google Scholar]
- [64].Guillot N, Caillet E, Laville M, Calzada C, Lagarde M, Vericel E. Increasing intakes of the long-chain omega-3 docosahexaenoic acid: effects on platelet functions and redox status in healthy men. FASEB J. 2009;23(9):2909–2916. doi: 10.1096/fj.09-133421. [DOI] [PubMed] [Google Scholar]
- [65].Serhan CN. Novel eicosanoid and docosanoid mediators: Resolvins, docosatrienes, and neuroprotectins. Curr. Opin. Clin. Nutr. Metab. Care. 2005;8(2):115–121. doi: 10.1097/00075197-200503000-00003. [DOI] [PubMed] [Google Scholar]
- [66].Antithrombotic Trialists’ Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. Br. Med. J. 2002;324(7329):71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Harris WS. Expert opinion: omega-3 fatty acids and bleeding-cause for concern? Am. J. Cardiol. 2007;99(6A):44C–46C. doi: 10.1016/j.amjcard.2006.11.021. [DOI] [PubMed] [Google Scholar]
- [68].Li ZG, Yu ZC, Wang DZ, Ju WP, Zhan X, Wu QZ, et al. Influence of acetylsalicylate on plasma lysophosphatidic acid level in patients with ischemic cerebral vascular diseases. Neurol. Res. 2008;30(4):366–369. doi: 10.1179/174313208X300369. [DOI] [PubMed] [Google Scholar]
- [69].Tomsig JL, Snyder AH, Berdyshev EV, Skobeleva A, Mataya C, Natarajan V, et al. Lipid phosphate phosphohydrolase type 1 (LPP1) degrades extracellular lysophosphatidic acid in vivo. Biochem. J. 2009;419(3):611–618. doi: 10.1042/BJ20081888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].de Meijer A, Vollaard H, de Metz M, Verbruggen B, Thomas C, Novakova I. Meloxicam, 15 mg/d, spares platelet function in healthy volunteers. Clin. Pharmacol. Ther. 1999;66(4):425–430. doi: 10.1053/cp.1999.v66.a101063. [DOI] [PubMed] [Google Scholar]
- [71].Jilma B, Fuchs I. Detecting aspirin resistance with the Platelet Function Analyzer (PFA-100) Am. J. Cardiol. 2001;88(11):1348–1349. doi: 10.1016/s0002-9149(01)02143-9. [DOI] [PubMed] [Google Scholar]