Abstract
Introduction
EBV has been a leading candidate as a trigger for several autoimmune diseases. We describe an antineutrophil cytoplasmic autoantibody (ANCA) -associated systemic vasculitis as the initial presenting illness of AIDS.
Case report and results
The patient was diagnosed ANCA -associated systemic vasculitis in the setting of HIV infection because of a high level of ANCA level, crescent glomerulonephritis in pathology, and clinical signs and symptoms compatible with systemic vasculitis. He also had HIV associated lymphadenopathy with scattered. EBV-RNA positive cells and reactive germinal centers.
Conclusion
EBV genome was found in reactive lymph nodes and, therefore, may be associated with the immunopathogenesis of vasculitis.
Keywords: EBV, ANCA-Associated Vasculitis, HIV
Introduction
Epstein-Barr virus (EBV) is a human herpesvirus that causes persistent human infection in most individuals by adulthood., Primary EBV infection is usually clinically silent or causes the self-limiting lymphoproliferative disorder infectious mononucleosis 1. EBV infection is associated with severe disease, particularly in HIV patients, and is implicated in the pathogenesis of human malignancies such as T cell lymphoma, Burkitt’s lymphoma, Hodgkin disease, pyothorax-associated pleural lymphoma, nasopharyngeal carcinoma, and gastric carcinoma 1.
The term antineutrophil cytoplasmic autoantibody (ANCA)-associated systemic vasculitis (AASV) describes a group of diseases characterized by the necrotizing inflammation of small blood vessels with paucity of immune deposits 2. AASV generally includes most types of small-vessel vasculitis, which should be suspected in any patient who presents with a multisystem disease such as renal dysfunction, skin rashes, pulmonary manifestations, or neurologic manifestation 3.
AASV is usually diagnosed by the detection of antineutrophilic cytoplasmic antibodies (ANCA). Antibodies directed against proteinase 3 (PR3-ANCA) and myeloperoxidase (MPO-ANCA) are highly specific for this condition 3.
The association between EBV and vasculitis has been documented in previous studies 4. However, to our knowledge, the association between EBV infection and AASV in patients with HIV infection has not previously been described.
Patient and methods
The patient is a 25-year-old African-American male who presented to the emergency room for a 2-day history of cough, hemoptysis, subjective fevers and chills. He complains of nausea, vomiting, generalized weakness, periumbilical abdominal pain, and reduced urine output over the last two weeks. Immediately prior to admission, he noticed passing a minimal amount of dark brown urine. He denied any shortness of breath, chest pain, dysuria, and changes in bowel movement.
The patient’s past medical history was uneventful with the exception of a recent diagnosis of hypertension. He denied the use of alcohol, tobacco or illicit drugs. Patient noted weight loss of and estimated 20 pounds over the past month. Patient admitted he was MSM.
On physical examination, patient was ill appearing, afebrile with a respiratory rate of 15, blood pressure of 144/101, pulse of 102, and pulse oxygen of 99% on room air. His BMI was 22. Eye exam revealed a markedly injected sclera. He had marked dryness of the oropharynx. The abdomen was mild to moderate epigastric tenderness without masses or organomegaly. Significant generalized lymphadenopathies in neck, armpits and groins were found. The testicles were nontender without any mass. Extremities were without clubbing, cyanosis, or edema. The remainder of the exam was unremarkable. The patient’s laboratory results showed in table 1. CT of thorax without IV contrast showed mediastinal lymphadenopathy without active pulmonary disease. Abdominal and pelvic CT-scan showed diffuse retroperitoneal lymphadenopathy and bilateral kidney enlargement. Lymph node biopsy of posterior chain of the neck and CT-guided kidney biopsies were performed. AIDS was diagnosed with positive ELISA and Western blot tests. Absolute CD4 cell count was 147/ml. Diagnosis was supported by high titer positive serum ANCA.”
Table 1.
Laboratory characteristics of the patient
| Results | |
|---|---|
| Hematological variables | |
| White cell count (per mm3) | 9.1× 103 |
| Hematocrit (%) | 31.6 |
| Hemoglobin (g/d) | 10.9 |
| Neutrophils (%) | 79 |
| Lymphocytes (%) | 10.4 |
| Platelet count (per mm3) | 380× 103 |
| Erythrocytesedimentation rate (mm/hour) | > 150 |
| C-Reactive protein (CRP) (mg/dL) | 5.8 |
| Absolute Retic Count | 0.34 |
| Immunological variables | |
| Immunoglobulin G (SRID) (mg/dL) | 6209 |
| Immunoglobulin A (SRID) (mg/dL) | 268 |
| Immunoglobulin M (SRID) (mg/dL) | 162 |
| C3 (SRID) (mg/dL) | 95 |
| C4 (SRID) (mg/dL) | 18 |
| Flowcytometry | |
| CD3 absolute count & percentage | 710 (71 %) |
| CD4 absolute count & percentage | 147(13%) |
| CD8 absolute count & percentage | 575(53%) |
| CD4/CD8 | 0.26 |
| CD19 absolute count & percentage | 200(22%) |
| Protein electrophoresis | |
| Total protein (g/dL) | 9.8 |
| Alpha 1 globulin (g/dL) | 0.27 |
| Alpha 2 globulin (g/dL) | 1.04 |
| Beta globulin (g/dL) | 0.77 |
| Gamma globulin (g/dL) | 5.04 |
| FANA | positive |
| Rheumatoid factor (RF) (U/mL) | 5 |
| Anti DNA ds Ab (U/ml) | <4 |
| Anti glomerular basement membrane Ab(U/mL) | <3 |
| Myeloperoxidase Ab* (U/mL) | 68 |
| Cytoplasmic neutrophil Ab** (U/mL) | 9 |
| Haptoglobin (mg/dL) | 307 |
| Ferritine (ng/mL) | 949 |
| Cryoglubulin | negative |
| Urinary Random Protein (mg/dL) | 1163 |
| Urinary Random Albumin (mg/dL) | 564.2 |
| Urinary Random Globulin (mg/dL) | 598.8 |
| Urinary Random A/G ratio | 0.9 |
| Mono Clonal spike | no M spike |
| Creatinine Clearance (mL/min) | 18 |
| Blood chemical variables | |
| Calcium (mEq/dL) | 7.2 |
| Sodium (mEq/dL) | 129 |
| Potassium (mEq/dL) | 4.6 |
| Chloride (mEq/dL) | 69 |
| Carbon Dioxide(mEq/dL) | 30 |
| BUNl(mg/dL) | 231 |
| Creatinine (mg/dL) | 37.6 |
| Fasting blood glucose (mg/dL) | 121 |
| Magnesium (mEq/dL) | 2.7 |
| Phospherous (mEq/dL) | 19.3 |
| Aspartae amino transferase (IU/L) | 18 |
| Alanin amino transferase (IU/L) | 24 |
| Alkaline phosphatase (IU/L) | 62 |
| Bilirubin Total (mg/dL) | 0.64 |
| Pt (second) | 16.6 |
| PTT (second) | 53.7 |
| INR | 1.25 |
| Total protein (mg/dL) | 8.8 |
| Albumin (mg/dL) | 2.4 |
| Virologic study | |
| HBS AG (ELISA) | Negative |
| Anti-HBC (ELISA) | Negative |
| Anti-HCV (ELISA) | Negative |
| Anti-HAV (ELISA) | Negative |
| Anti-HIV (ELISA) | Positive |
| Anti B19 Ab IgG | Negative |
| Anti B19 Ab IgG | Negative |
| Anti-HIV (ELISA) | Positive |
| Western blot Ab | Positive |
| HIV RNA quantitive PCR (copy/ml) | 91469 |
| Log copies/ml (utra) | 4.96 |
| Urine analysis | |
| Color: | Dark-Brown |
| PH | 5.5 |
| Protein | 2+ |
| Glucose | Negative |
| WBC | TNTC |
| RBC | TNTC |
| Occult Blood | 3+ |
| Epithelial cells | 0 |
| Granular cast | present |
| RBC cast | present |
Myeloperoxidase Ab (MPO-ANCA or P-ANCA) normal serum rang is 0–8 EU/ml.
Cytoplasmic neutrophil Ab (C-ANCA) normal serum rang is 0 EU/ml
He was treated with prednisone and cyclophosphamide. He improved dramatically. He was given Highly Active Anti Retroviral Therapy later on.
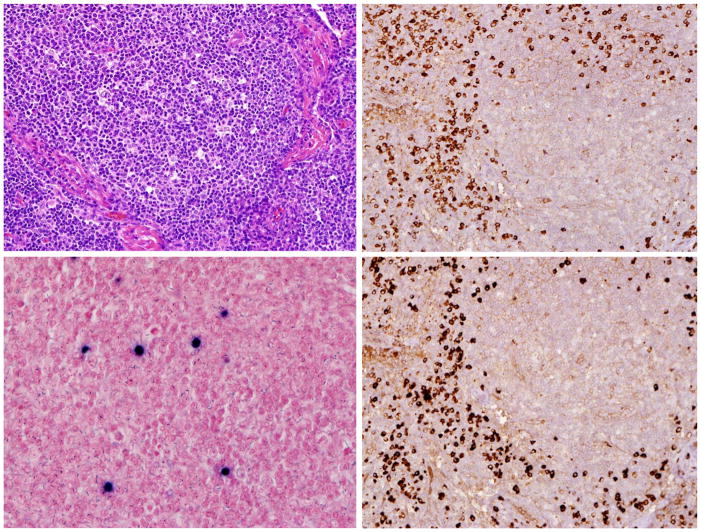
Histological examination of the lymph node revealed multiple enlarged naked reactive germinal centers. Marked medulary plasmocytosis was present. By Immunohistochemical stains, CD20 highlighted the germinal center B-cells. CD3 highlighted the normal paracortical T-cells. There was polyclonal population of plasma cells staining with kappa and lambda stains. EBV-RNA (EBER1) stain showed scattered positive cells, up to five per high power field (Figure 1). HHV-8 stain was negative.
Figure 1.
Left up showed lymph node, Left down showed EBER + cells in lymph node. Right up showed Kappa staining and Right down showed Lambda staining
Light microscopy study of kidney biopsies showed globally sclerosed glomeruli. The glomeruli showed mild enlargement, normal cellularity, and unremarkable mesangium. Notably, crescent formation (cellular or very early fibrocellular crescents), intermediate fibrocellular crescents associated with loop compression, and early segmental sclerosis were found. Glomerular basement membranes were mildly prominent without spike, holes, or duplication. No endocapillary proliferation or fibrin thrombus was seen. There was mild interstitial lymphocytic infiltration with very rare PMNs, very rare eosinophils and mild interstitial edema. Arterioles showed mild hyalinosis. Interlobular and large arteries show focal eccentric moderate to severe intimal fibrosis. In the immunofluorescence study, there was extensive crescent formation. There was granular mesengial staining with rare loop extension for IgG (2-3+), IgM (trace to 1+), C3 (2-3+), and C1q (very trace). Polyvalent staining is similar to IgG. Remaining stains were essentially negative.
Electron microscopy showed the glomerular basement membranes were of normal thickness of lamina densa with collapse and corrugation. There were rare subendothelial ill-defined electron dense deposits and no subepithelial deposits seen. The visceral epithelial cells showed virtually complete foot process effacement. Endothelial cells were swollen with occasional reticular aggregates and no fibrin tactoids. Mesangium showed occasional ill-defined electron dense deposits. There was no cellular interposition. There was no tubular basement membrane deposition.
Discussion
The patient was diagnosed AASV in the setting of HIV infection because of a high level of ANCA level, crescent glomerulonephritis in pathology, and clinical signs and symptoms compatible with systemic vasculitis. He also had HIV associated lymphadenopathy with scattered EBER+ cells and reactive germinal centers.
Although vasculitis may be a manifestation of opportunistic infections, it remains a possibility that HIV by itself is causally related to vasculitis 5. In this patient, EBV is the most likely cause for AASV.
EBV has been a leading candidate as a trigger for several autoimmune diseases since the initial description of raised EBV-specific antibody titers in patients with SLE 6. EBV is a biologically plausible candidate because it establishes lifelong dormant infection in most humans. Also EBV is capable of continuous virus production due to reactivation and it has the ability to modulatethe human immune system. In its immune-modifying function, EBV rescues infected B cells via latent antigen expression and assists their differentiation into memory B cells in which it persists. In addition, the virus continuously stimulates strong T-cell responses via chronic antigen presence, a key component in preventing EBV-associated malignancies. Recent studies indicate that EBV-specific cellular and humoral immune responses and the regulation of viral persistence in EBV-infected memory B cells are altered in non-HIV patients with autoimmune diseases 7. The molecular pathogenesis of EBV in systemic vasculitis was discussed recently in X-linked lymphoproliferative syndrome. Dutz JP et al 8 proposed that a functional inactivation of a protein that expresses primarily in T cells could impair the immunologic response to EBV, resulting in systemic vasculitis. The same mechanism might be effective in HIV/AIDS patients.
In the setting of HIV infection, EBV control is much more complicated. Once the immune system is weakened, EBV is associated with a number of diseases including as many as 50% of all AIDS-associated lymphoma 5, collagen vascular diseases and vasculitis.
EBV genomes were detected in lymph node tissue from this patient without evidence of lymphoma. This is consistent with paradoxical reports concerning the existence of EBV in lymph nodes of HIV/AIDS patients without the presence of lymphoma. Dolcetti et al 9 studied the presence of HIV and EBV antigens and genome in 50 lymph nodes with persistent generalized lymphadenopathy (PGL). Few cells with positive results for EBV antigens were found in only two of 50 lymph nodes. These rare EBV-positive centrocyte-like cells were mainly located in the germinal centers. On the other hand, F. Mampaso et al 10 demonstrated EBV nucleic acid in scattered germinal centre cells in eight of the 11 HIV patients with PGL. A recently published study of Ugandan patients could not find any statistically significant association between the presence of EBV in reactive lymphadenopathy in the setting of HIV infection. 11
The exact mechanism of AASV processes occurring in the HIV setting are still somewhat speculative. Cell mediated immune dysregulation is suspected by Hagen et al which supports the role of quantitative T cells dysfunction in the pathogenesis of AASV. This study also revealed that disease remission could be induced by antibodies directed at T cells. 12 In this case, the patient’s advanced HIV infection would cause an altered CD+T-cell allowing EBV to be proliferated in lymph nodes and vessels. Further, AIDS may also increase the patient’s susceptibility to AASV with immunologic alteration as Hagen et al described.
Conclusion
We can conjecture that EBV persistent replication in B-cells located in lymph nodes in addition to the continuous stimulation of T cells by HIV and EBV might be the ethiopathogenesis of AASV. However, there is little supportive evidence for this hypothesis. Indeed, to clarify the pathognomonic significance of scattered EBER+ cells in HIV lymphoproliferative lymphadenopathy associated with AASV, further study is needed.
Acknowledgments
The authors would like to thank Drs Michael Brancaccio, Jose Bordon, James Roberson, Abrahim Dabela for their assistance in management of the patient. We also appreciate the excellent comments given by Dr. Mohhamd Sajadi and Dr. Mary Beth Allen for preparing final version of manuscript.
Footnotes
Conflict of interest statement: All authors: no conflicts.
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- 1.Khanna R, Burrows SR, Moss DJ. Immune regulation in Epstein-Barr virus-associated diseases. Microbiol Rev. 1995;59:387–405. doi: 10.1128/mr.59.3.387-405.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337:1512–23. doi: 10.1056/NEJM199711203372106. [DOI] [PubMed] [Google Scholar]
- 3.Savage CO, Harper L, Adu D. Primary systemic vasculitis. Lancet. 1997;349:553–8. doi: 10.1016/s0140-6736(97)80118-3. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman GS, Franck WA. Infectious mononucleosis, autoimmunity, and vasculitis. A case report. JAMA. 1979;241:2735–6. [PubMed] [Google Scholar]
- 5.Gherardi R, Belec L, Mhiri C, Gray F, Lescs MC, Sobel A, Guillevin L, Wechsler J. The spectrum of vasculitis in human immunodeficiency virus-infected patients. A clinicopathologic evaluation. Arthritis Rheum. 1993;36:1164–74. doi: 10.1002/art.1780360818. [DOI] [PubMed] [Google Scholar]
- 6.Evans AS, Rothfield NF, Niederman JC. Raised antibody titres to E.B. virus in systemic lupus erythematosus. Lancet. 1971;1:167–8. doi: 10.1016/s0140-6736(71)91937-4. [DOI] [PubMed] [Google Scholar]
- 7.Gross AJ, Hochberg D, Rand WM, Thorley-Lawson DA. EBV and systemic lupus erythematosus: a new perspective. J Immunol. 2005;174:6599–607. doi: 10.4049/jimmunol.174.11.6599. [DOI] [PubMed] [Google Scholar]
- 8.Dutz JP, Benoit L, Wang X, Demetrick DJ, Junker A, de Sa D, Tan R. Lymphocytic vasculitis in X-linked lymphoproliferative disease. Blood. 2001;97:95–100. doi: 10.1182/blood.v97.1.95. [DOI] [PubMed] [Google Scholar]
- 9.Dolcetti R, Gloghini A, De Vita S, Vaccher E, De Re V, Tirelli U, Carbone A, Boiocchi M. Characteristics of EBV-infected cells in HIV-related lymphadenopathy: implications for the pathogenesis of EBV-associated and EBV-unrelated lymphomas of HIV-seropositive individuals. Int J Cancer. 1995;63:652–9. doi: 10.1002/ijc.2910630509. [DOI] [PubMed] [Google Scholar]
- 10.Mampaso F, Bellas C, Molina A, Quereda C, Bricio T, Buzon L. In situ demonstration of Epstein-Barr virus in intravenous drug abusers with generalized lymphadenopathy. Postgrad Med J. 1992;68:739–41. doi: 10.1136/pgmj.68.803.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalungi S, Wabinga H, Bostad L. Reactive lymphadenopathy in Ugandan patients and its relationship to EBV and HIV infection. APMIS. 2009;117:302–7. doi: 10.1111/j.1600-0463.2009.02444.x. [DOI] [PubMed] [Google Scholar]
- 12.Hagen EC, de Keizer RJ, Andrassy K, van Boven WP, Bruijn JA, van Es LA, van der Woude FJ. Compassionate treatment of Wegener’s granulomatosis with rabbit anti-thymocyte globulin. Clin Nephrol. 1995;43:351–9. [PubMed] [Google Scholar]