Abstract
Hyaluronan (HA) has been shown to play an important role during early heart development and promote angiogenesis under various physiological and pathological conditions. In recent years, stem cell therapy, which may reduce cardiomyocyte apoptosis, increase neovascularization, and prevent cardiac fibrosis, has emerged as a promising approach to treat myocardial infarction (MI). However, effective delivery of stem cells for cardiac therapy remains a major challenge. In this study, we tested whether transplanting a combination of HA and allogeneic bone marrow mononuclear cells (MNCs) promotes cell therapy efficacy and thus improves cardiac performance after MI in rats. We showed that HA provided a favorable microenvironment for cell adhesion, proliferation, and vascular differentiation in MNC culture. Following MI in rats, compared with the injection of HA alone or MNC alone, injection of both HA and MNCs significantly reduced inflammatory cell infiltration, cardiomyocyte apoptosis, and infarct size and also improved cell retention, angiogenesis, and arteriogenesis, and thus the overall cardiac performance. Ultimately, HA/MNC treatment improved vasculature engraftment of transplanted cells in the infarcted region. Together, our results indicate that combining the biocompatible material HA with bone marrow stem cells exerts a therapeutic effect on heart repair and may further provide potential treatment for ischemic diseases.
Introduction
Coronary artery disease is the most common type of heart disease. Coronary artery disease occurs when the coronary arteries become narrow as a result of atherosclerosis.1 In this condition, the blood flow that supplies the heart muscle is decreased, which leads to myocardial infarction (MI).2 Unfortunately, the standard treatments, which include early revascularization using coronary intervention followed by pharmaceutical administration support,3 are not sufficient. Eventually, the accumulated cardiomyocyte loss contributes to heart failure. The American Heart Association has estimated that there are approximately 6 million patients with heart failure each year in the United States.4 Therefore, the ultimate goal of coronary artery disease treatment is to reduce cardiomyocyte death and to prevent the further occurrence of heart failure.5
Previous studies have shown that cell therapy is a promising approach for heart repair post-MI. Some clinical trials have also demonstrated that autologous stem cell therapy can improve cardiac function after MI. These stem cells can participate in angiogenesis and provide paracrine factors that protect cardiomyocytes from the damage of ischemia. However, the results of these clinical studies remain controversial.6 The major reason for this controversy may be that only a small portion of the cells survive and are retained in the ischemic region after cell transplantation. For example, studies have revealed that >90% of the cells are lost during the intramyocardial injection due to contractions of the heart, and the survival of the few remaining cells is poor due to the highly hypoxic conditions of the ischemic region.6,7 As a result, tissue engineering has become the focus for improving the efficacy of cell therapy and cardiac regeneration in recent years.
Hyaluronan (HA), also referred to as hyaluronic acid, is a nonsulfated glycosaminoglycan and a natural component of the extracellular matrix. HA is distributed throughout the epithelial, neuronal, and connective tissue8 and plays an important role in the regulation of tissue homeostasis, inflammatory responses,8 and embryonic and neonatal tissue development.9 High molecular weight HA provides a unique microenvironment during cardiac morphogenesis for progenitor cell migration, whereas low molecular weight HA has been shown to activate the proliferation of noninflammatory cells, such as endothelial cells and smooth muscle cells, and participate in tissue remodeling.10,11 Under physiological conditions, HA fragments or HA oligomers increase the proliferation of endothelial cells and promote angiogenesis.12 Under pathological conditions, as in cases of hindlimb ischemia, HA also promotes angiogenesis to support muscle regeneration.13 In a previous study, HA production was shown to be regulated by hyaluronan synthase 2, and the knockout of this enzyme from the embryo resulted in cardiac and vascular abnormalities,14 suggesting that HA also plays an important role during heart development. Therefore, HA is a suitable biomaterial to increase angiogenesis, deliver stem cells, and promote heart regeneration.
Bone marrow mononuclear cells (MNCs) consist of a mix of cell types, including hematopoietic stem cells,15 mesenchymal stem cells,16 and endothelial lineage cells,17 all of which have been shown to benefit ischemic cardiovascular diseases.2 Therefore, we conducted a series of experiments to study the interaction between two established materials, HA and MNCs, with the hope of developing a novel approach for treating ischemic diseases.
Results
HA promotes bone marrow MNC adhesion and proliferation
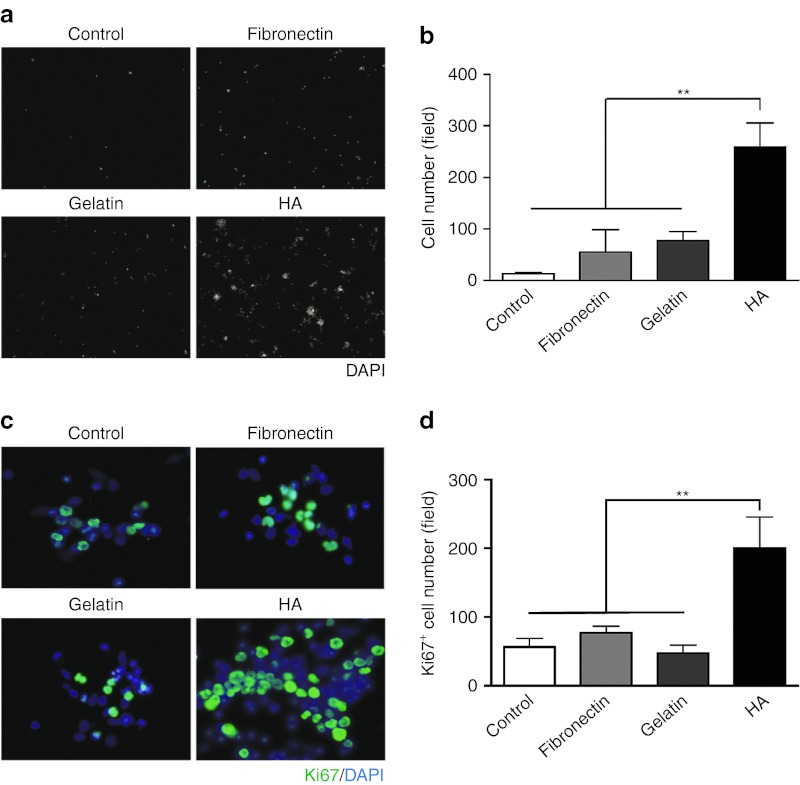
Most of the bone marrow MNCs were in suspension during in vitro culture. Therefore, in the transplanted region, the binding capacity of these cells is important for cell delivery and cell retention. To examine whether HA improves the adhesion of MNCs in vitro, the total bone marrow cells were isolated, purified by gradient centrifugation, and then seeded onto plates coated with different materials, including fibronectin and gelatin, which are commonly used in cell cultures to favor cell adhesion. We found that the number of adherent MNCs increased significantly after 60 minutes in the HA-coated group compared with the control, fibronectin- and gelatin-coated groups (Figure 1a,b), suggesting that HA facilitates MNC binding. We thus examined the expression of HA binding receptors including CD44, ICAM-1, and RHAMM in MNCs. Flow cytometric analysis showed that 56%, 3.9%, and 0.6% of MNCs expressed CD44, ICAM-1, and RHAMM, respectively (Supplementary Figure S1). Next, to examine whether the cell cycle of the adherent cells was affected, MNCs were stained with the proliferation maker Ki67 after 24 hours of culture. The quantified results showed that there were more proliferating MNCs in the HA-coated condition (Figure 1c,d). Together, these results demonstrate that HA coating promotes the adhesion and proliferation of MNCs.
Figure 1.
HA promotes the adhesion and proliferation of bone marrow mononuclear cells in culture. (a) Freshly isolated MNCs were cultured on dishes coated with fibronectin, gelatin or HA. The adhered cells were identified using nuclear staining with DAPI (white). (b) The quantification of the adhered cells in the different coating conditions. Data are presented as the mean ± SEM. n = 4 per group. **P < 0.01 (c) The identification of proliferating MNCs under different coating conditions using Ki67 immunostaining (green). Nuclei (blue). (d) The quantification of Ki67-positive cells under the different coating conditions. The data are presented as the mean ± SEM. n = 4 per group. **P < 0.01. DAPI, 4′, 6-diamidino-2-phenylindole fluorescent dye; HA, Hyaluronan; MNC, mononuclear cell.
HA modulates bone marrow MNC differentiation, upregulates paracrine factor gene expression and reduces MNC apoptosis
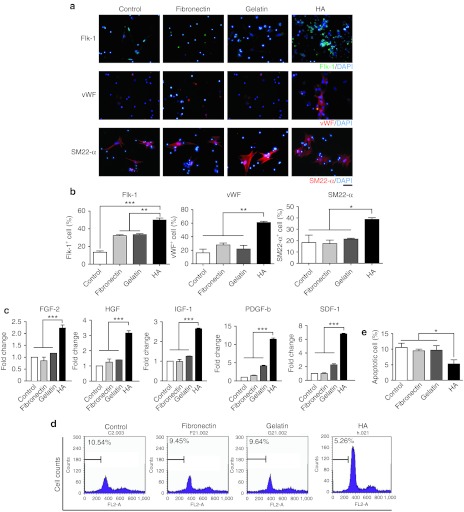
Bone marrow MNCs are a population of mixed cells. They include several types of progenitor cells that can differentiate into various cell types, including vascular lineage cells, which are important for therapeutic angiogenesis. To examine whether different coating conditions affect the differentiation of vascular lineage cells from MNCs, MNCs were cultured under different coating conditions and stained with the mature endothelial cell marker von Willebrand Factor (vWF), the smooth muscle cell marker smooth muscle 22-α (SM22-α), the endothelial progenitor cell marker fetal liver kinase 1 (Flk-1), the hematopoietic progenitor cell markers CD34 and CD133 and the monocyte/macrophage markers CD68 and CD163 using real-time reverse-transcription PCR. Our results revealed that the attached MNCs were capable of differentiating into vascular lineage cells, including smooth muscle cells and endothelial cells (Figure 2a). We also observed some small Flk-1+ colonies 4 days after plating (Figure 2a). Furthermore, quantification showed that HA coating significantly improved the differentiation of vascular lineage cells from MNCs (Figure 2b) and increased the expression of CD34 and CD133, but not the expression of CD68 or CD163 (Supplementary Figure S2), the fibrotic genes DDRII and Acta2 (Supplementary Figure S3), or the cardiomyocyte-specific genes α-myosin heavy chain (α-MHC), Nkx2.5 and Troponin I (cTnI) (Supplementary Figure S4). These observations indicated that some of the MNCs differentiate into vascular lineage cells, while other cells that originated from the MNCs may participate in different therapeutic effects. Therefore, we studied the effect of HA on the secretion of angiogenic and antiapoptotic factors. The MNCs were cultured on the different coating materials under hypoxic conditions for 2 days to mimic the ischemic condition in vivo and then subjected to gene expression analysis. The results showed that HA significantly elevated the gene expression level of paracrine factors, including fibroblast growth factor-2, hepatocyte growth factor, insulin-like growth factor-1, platelet-derived growth factor-b, and stromal cell-derived factor-1 (P < 0.001) (Figure 2c). Next, doxorubicin was used to induce MNC apoptosis. The treated MNCs were then collected, stained with propidium iodide, and subjected to flow cytometry to measure apoptosis. We found that the apoptotic cell number was reduced in the HA group compared with the other experimental groups (Figure 2d,e). These results suggest that HA regulates MNCs vascular differentiation and paracrine-associated gene expression and reduces MNC apoptosis.
Figure 2.
HA promotes MNC differentiation, paracrine genes expression, and survival. (a) Immunocytochemistry was performed to identify vascular cell differentiation from the MNCs after they were cultured under the different coating conditions for 4 days. Nuclei (blue); Flk-1 (green); vWF (red); SM22-α (red). (b) The quantification of MNC vascular differentiation under the different coating conditions. The data are presented as the mean ± SEM. n = 4 per group. *P < 0.05, **P < 0.01, ***P < 0.001. Scar bar: 50 µm (c) The quantitative reverse-transcription PCR results showing the paracrine gene expression profiles of MNCs cultured under the different coating conditions during hypoxia. (d) Representative propidium iodine staining, which was analyzed using flow cytometry to identify doxorubicin-induced MNC apoptosis. (e) The quantification of doxorubicin-induced MNC apoptosis. The data are presented as the mean ± SEM. n = 4 per group. DAPI, 4′, 6-diamidino-2-phenylindole fluorescent dye; FGF-2, fibroblast growth factor-2; Flk-1, fetal liver kinase 1; HA, hyaluronan; HGF, hepatocyte growth factor; IGF-1, insulin-like growth factor-1; MNC, mononuclear cell; PDGF-b, platelet-derived growth factor-b; SDF-1, stromal cell-derived factor-1; SM22-α, smooth muscle 22-α vWF, von Willebrand Factor.
HA/MNC injection reduces cardiomyocyte apoptosis in vivo
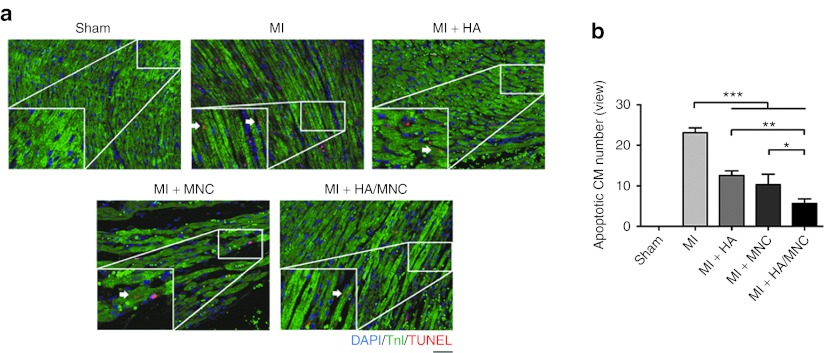
To further understand the effect of HA/MNC injection in vivo, we performed coronary artery ligation surgery in rats and injected HA, MNCs, or a combination of HA and MNCs into the ischemic heart. One day post-MI, the hearts were harvested, and a TUNEL/cTnI staining was performed to quantify the number of cardiomyocytes undergoing apoptosis (Figure 3a). The staining showed that there were fewer TUNEL/cTnI double-positive apoptotic cardiomyocytes in the HA/MNC group compared with the groups treated with HA or MNCs alone (Figure 3b).
Figure 3.
HA/MNC injection reduces cardiomyocyte apoptosis following infarction. (a) Representative TUNEL staining (red) co-stained with troponin I (green) showing cardiomyocyte apoptosis in heart sections taken from different treatment groups 1 day post-MI. Scale bar: 100 µm for all panels. (b) Quantification of cardiomyocyte apoptosis. Data are means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. n = 6 per group. CM, cardiomyocyte; DAPI, 4′, 6-diamidino-2-phenylindole fluorescent dye; HA, hyaluronan; MI, myocardial infarction; MNC, mononuclear cell; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
HA/MNC treatment reduces the inflammatory response after MI
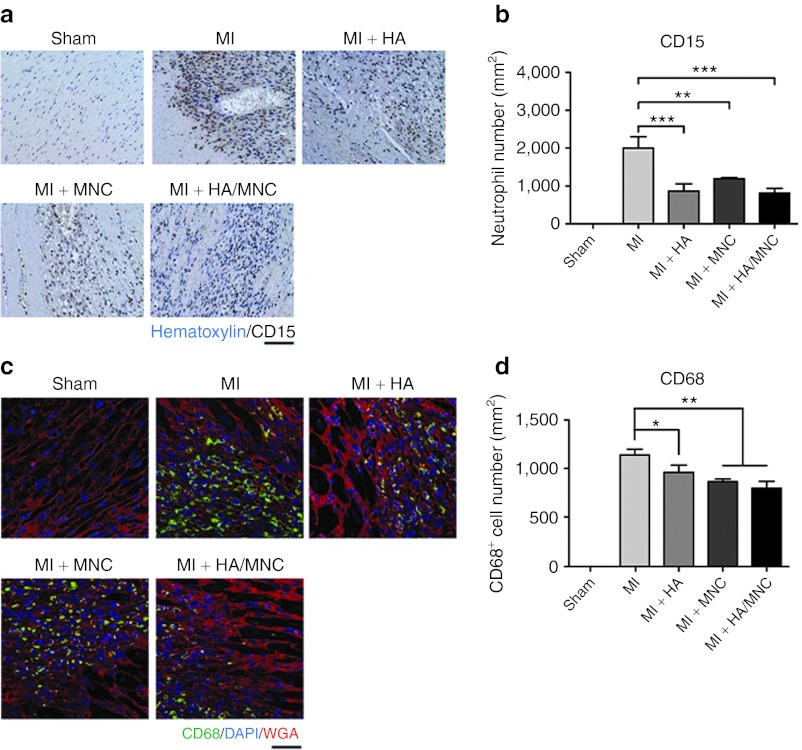
Inflammation is a part of the healing process post-MI. However, strong and regional inflammation may damage myocytes and enlarge the size of scar tissue, resulting in changes in the long-term prognosis. To understand whether the combined treatment modulates the inflammatory response, we quantified the number of infiltrating neutrophils and macrophages in the peri-infarct region using immunostaining of CD15 and CD68 (neutrophil and macrophage-specific antigen), respectively. Interestingly, we observed that the injection of HA and HA/MNC reduced neutrophil infiltration in the peri-infarct region (Figure 4a,b). The number of macrophages was also significantly reduced in the combined treatment group (Figure 4c,d).
Figure 4.
HA/MNC injection decreases neutrophil and macrophage infiltration after infarction. (a) Representative CD15 staining of neutrophils at the border zone from each group 3 days post-MI. Scale bar: 20 µm for all panels. (b) The number of infiltrated neutrophils at the border zone from each group. Data are presented as mean ± SEM. ** P < 0.01, ***P < 0.001. n = 6 per group. (c) Representative CD68 (green) staining of macrophages at the border zone from each group at 3 days post-MI. Membrane (red). Scale bar: 50 µm for all panels. (d) Quantification of infiltrated macrophages in different experimental groups. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. n = 8 per group. DAPI, 4′, 6-diamidino-2-phenylindole fluorescent dye; HA, Hyaluronan; MI, myocardial infarction; MNC, mononuclear cell; WGA, wheat germ agglutinin.
The combined HA/MNC injection improves heart function after MI
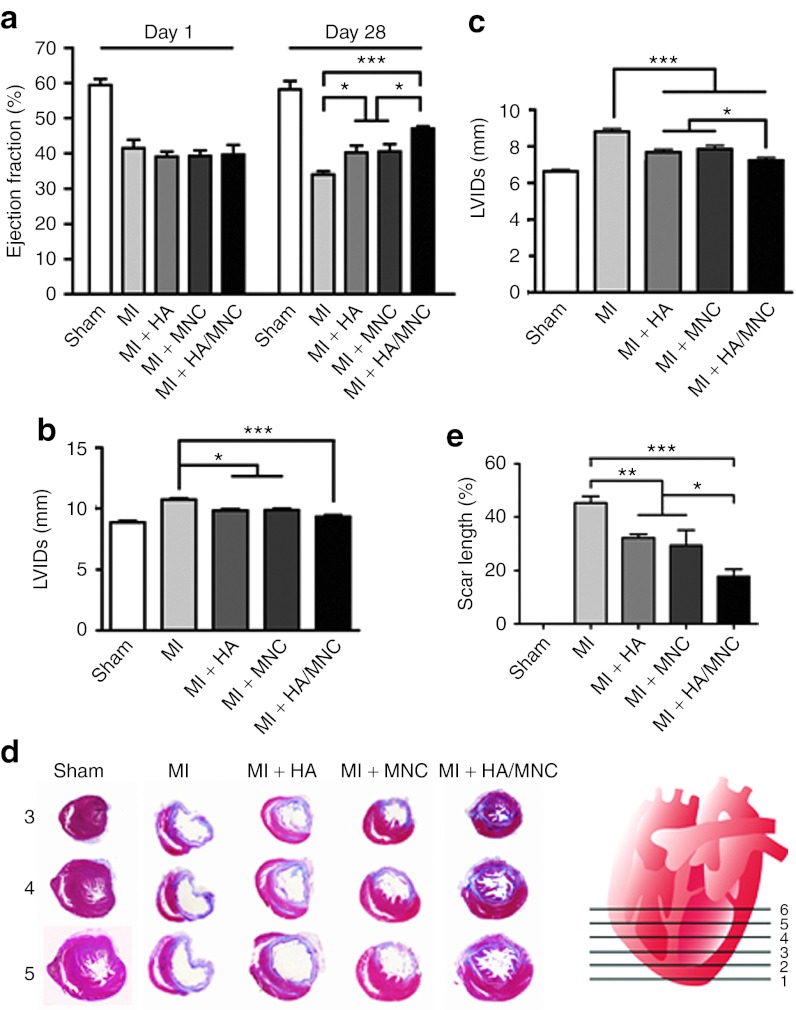
To determine whether the HA/MNC combined treatment improves heart function post-MI, echocardiography was performed. After 28 days, we found that the ejection fraction was dramatically decreased in the MI-only group. Although the heart function was slightly improved in the HA- and MNC-alone treatment groups, only the combined treatment significantly increased the ejection fraction 28 days post-MI (Figure 5a). Consistent with this finding, the ventricular dilatation, as indicated by left ventricle internal dimensions at diastole and left ventricle internal dimensions at systole, was significantly improved by the HA/MNC injection (Figure 5b,c), suggesting that this combined treatment decreases pathological remodeling after infarction.
Figure 5.
HA/MNC injection improves heart performance and reduces scar formation post-MI. (a) Histograms depicting the left ventricle ejection fraction 1 day and 28 days post-MI in the sham and other experimental groups. *P < 0.05, ***P < 0.001. (b, c) The statistical analysis of the echocardiographic results of the left ventricle internal dimensions at diastole (LVIDd, b) and systole (LVIDs, c) in the sham and other treatment groups. The data are presented as the mean ± SEM. *P < 0.05, ***P < 0.001. n = 8 per group. (d) The infarct size of Sections 3, 4, and 5 from the left ventricle regions is indicated in the artist's rendition and was determined using Masson's trichrome staining. (e) The statistical analysis of scar length in various groups. The data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. n = 8 per group. HA, Hyaluronan; MI, myocardial infarction; MNC, mononuclear cell.
HA/MNC injection reduces scar formation and does not induce chondrogenesis
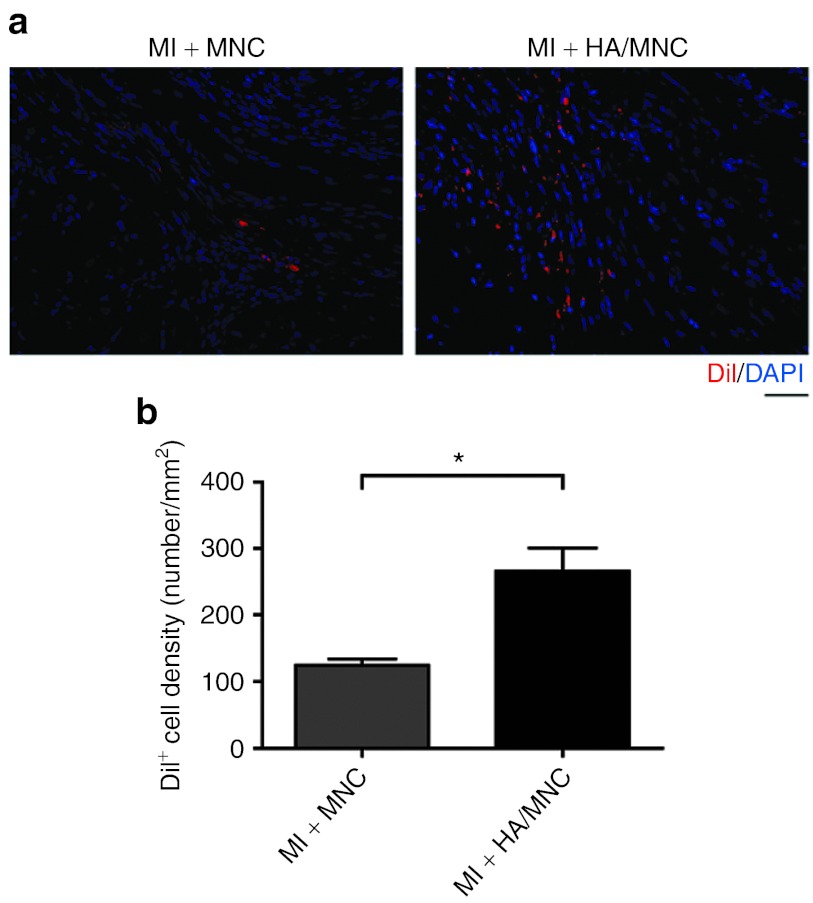
To determine whether improved cardiac function is accompanied by a reduction in scar size, the scar length was measured following Masson's trichrome staining (Figure 5d). We found that the size of the scar tissue decreased in both HA- and MNC-alone groups. Remarkably, the scar tissue and collagen accumulation were further minimized in the HA/MNC combined treatment group (P < 0.001 versus MI; P < 0.05 versus MI + HA or MI + MNC; Figure 5e). Based on this finding, we speculated that the HA/MNC combined treatment might ameliorate scar tissue size by reducing cardiomyocyte death. We therefore quantified the number of MNCs that was retained after injection. Not surprisingly, we observed that significantly more MNCs were retained in the ischemic heart 28 days after MI in the HA/MNC group compared with the MNC-alone group (Figure 6). Although HA may promote chondrogenesis,18 we did not detect any induction of chondrogenic genes from MNCs cultured with HA or chondrocyte formation in all experimental groups 28 days post-MI (Supplementary Figures S5 and S6).
Figure 6.
HA/MNC injection improves transplanted cell retention after MI. (a) Representative pictures of DiI+ MNC (red) in the area injected with cells alone (left panel) or with cells and HA (right panel). Nuclei are blue. (b) The cell retention rates as reflected by DiI+ cell counts. The data are presented as the mean ± SEM. *P < 0.05. n = 8 per group. Scale bar: 50 µm for all panels. DAPI, 4′, 6-diamidino-2-phenylindole fluorescent dye; HA, Hyaluronan; MI, myocardial infarction; MNC, mononuclear cell.
HA/MNC injection promotes angiogenesis and arteriogenesis after infarction
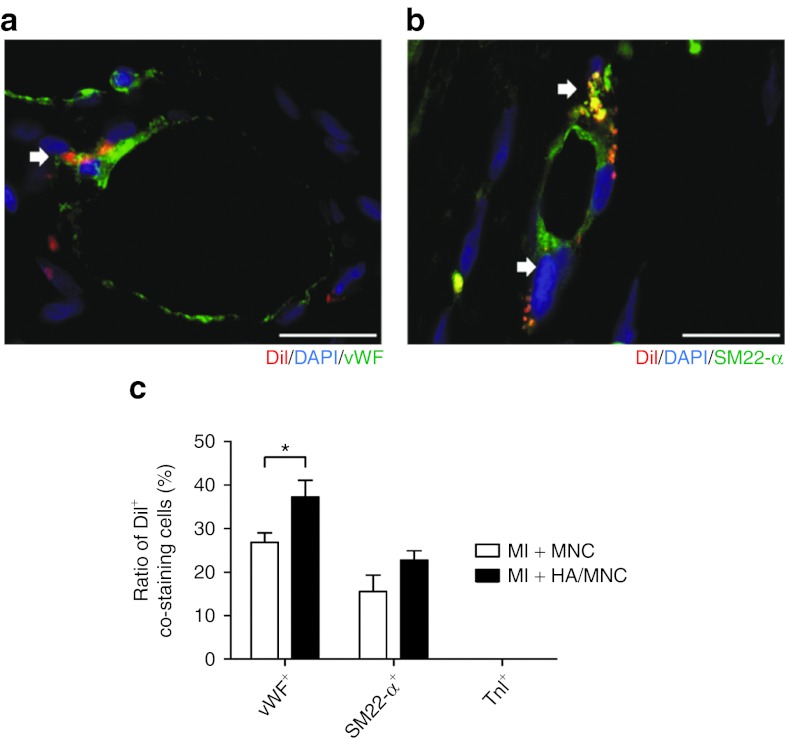
Given that the HA/MNC injection reduced the scar length after MI, we further investigated whether the HA/MNC combined treatment promoted angiogenesis and/or arteriogenesis of the ischemic heart. Immunostaining of the specific cardiomyocyte marker cTnI and the endothelial markers isolectin and RECA-1 was performed to reveal the peri-infarct area and the capillary density. The capillary density in the peri-infarct and infarct regions was quantified (Figure 7a). The results showed that the capillary density was only slightly increased in the HA or MNC group, whereas HA/MNC injection significantly enhanced the capillary density of both the peri-infarct and the infarct regions (Figure 7b,c and Supplementary Figure S7). Furthermore, a previous study showed that smooth muscle cells are important for stabilizing the de novo formation of blood vessels.19 Accordingly, the cells were stained with SM22-α to examine the degree of arteriogenesis (Figure 7d). The results indicated that the arteriole density in the HA/MNC group was significantly increased compared with the other experimental groups (Figure 7e,f), implying that the combined treatment had an impact on arteriogenesis. Finally, to investigate whether the MNCs differentiate into smooth muscle cell lineage or endothelial cell lineage or both, the cells were stained with SM22-α, RECA-1, and vWF overlapping with DiI. The percentages of DiI/SM22-α and DiI/vWF double-positive cells in the vessel were quantified, which would indicate injected MNC differentiation into vascular cells. Surprisingly, although we did detect DiI/SM22-α, DiI/RECA-1, and DiI/vWF double-positive cells integrating into the vessel at peri-infarct (Figure 8 and Supplementary Figure S8), we found that >70% of endothelial cells and >80% of smooth muscle cells were DiI-negative, indicating neovascularization originates from endogenous cells (Figure 8c). Interestingly, injection with HA further promoted MNC differentiation into endothelial cells, but not smooth muscle cells (Figure 8c).
Figure 7.
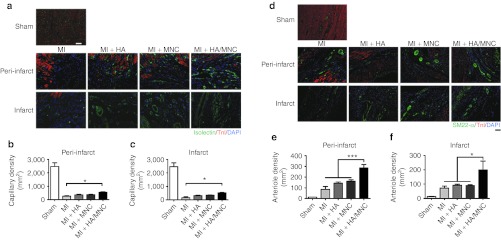
HA/MNC injection increases capillary and arteriole densities post-MI. (a) Representative immunostaining of isolectin (green) for endothelial cells, overlapped with cardiomyocytes stained with cardiac troponin I (cTnI, red) at the peri-infarct and the infarct from search group. Nuclei are blue. Scale bar: 50 µm for all panels. (b–c) The quantification of capillary density at the peri-infarct (b) and infarct (c) areas. The data are presented as the mean ± SEM.*P <0.05. n = 8 per group. (d) Representative immunofluorescence staining of smooth muscle 22 (SM22)-α and cTnI at the peri-infarct and infarct areas from each group. Scale bar: 100 µm for all panels. (e–f) The quantification of arteriole density at the (e) peri-infarct areas (f) and infarct areas. The data are presented as the mean ± SEM. *P < 0.05, ***P < 0.001. n = 8 per group. DAPI, 4′, 6-diamidino-2-phenylindole fluorescent dye; HA, Hyaluronan; MI, myocardial infarction; MNC, mononuclear cell; SM22-α, smooth muscle 22-α.
Figure 8.
Injection of HA/MNC enhances transplanted cell differentiation into vascular cells. (a, b) Representative images of red DiI fluorescence, overlapped with immunostaining of (a) vWF or (b) SM22-α in green. (c) Quantification of ratio of cells double positive with DiI and vWF, SM22-α or TnI. *P < 0.05. Scar bar: 50 µm. DAPI, 4′, 6-diamidino-2-phenylindole fluorescent dye; HA, Hyaluronan; MNC, mononuclear cell; SM22-α, smooth muscle 22-α vWF, von Willebrand Factor.
Discussion
The results described here document HA's role in promoting MNC adhesion, proliferation, differentiation, paracrine factor-associated gene expression and survival in vitro. Furthermore, intramyocardial injection of HA combined with MNCs improved heart function and reduced cardiomyocyte loss and scar formation, both at the early stage and at 28 days post-MI. More importantly, the HA hydrogel provided a suitable microenvironment for retaining the injected MNCs and recruiting host endothelial cells and smooth muscle cells to the infarct region to facilitate new vessel formation, as most of the newly formed vessels were DiI-negative. Some of the injected MNCs were also detected in the vessels.
It is noteworthy that there were more attached MNCs in the HA-coated plate and more proliferating MNCs in the presence of HA. Our data suggest that HA may provide more binding sites for MNC adhesion. Based on the flow cytometric analysis of common HA binding receptors, CD44, ICAM-1, and RHAMM, the result showed that 56% MNCs expressed CD44, 3.9% MNCs expressed ICAM-1 and 0.6% MNCs expressed RHAMM, all of which are important for cell adhesion, survival, and differentiation.20,21,22 Therefore, the binding of CD44, ICAM-1, or RHAMM to HA may have an impact on MNC adhesion and proliferation. We also found that MNCs cultured in HA-coated dishes were able to differentiate into endothelial cells and smooth muscle cells, as evidenced by vWF and SM22-α staining, respectively, and the progenitor cell markers CD34 and CD133 were upregulated. These results suggest that HA plays a role in MNC vascular cell differentiation.
Previous studies have indicated that bone marrow MNC therapy can protect the injured heart from hypoxic stress by releasing paracrine factors.23,24 We found that the MNCs cultured in the HA-coated group secreted more paracrine factors, including fibroblast growth factor-2, hepatocyte growth factor, insulin-like growth factor-1, platelet-derived growth factor-b, and stromal cell-derived factor-1, compared with the MNCs cultured under other coating conditions in vitro. These factors have been reported to participate in angiogenesis and confer cardiac protection. For example, fibroblast growth factor-2 has been shown to induce endothelial cell migration and increase angiogenesis,25 hepatocyte growth factor plays a role in smooth muscle cell recruitment,26 insulin-like growth factor-1 binds to tyrosine kinase to induce the PI3K-Akt and the MEK1/2-Erk1/2 pathways to achieve cardioprotection,27 platelet-derived growth factor-b regulates smooth muscle cell and pericyte recruitment,28 and stromal cell-derived factor-1 recruits circulating progenitor cells to participate in tissue regeneration.29 By contrast, the level of cardiogenic genes such as α-MHC, Nkx2.5, and cTnI did not increase in HA-coated group after hypoxia treatment, suggesting that HA did not promote MNC differentiation into cardiac lineage cells.
Upon hypoxia-induced injury, cardiomyocytes undergo apoptosis.30 When we induced MNC apoptosis with doxorubicin under the different coating conditions, we found that HA effectively reduced the percentage of apoptotic cells. Thus, HA may trigger survival signaling in MNCs and establish a microenvironment for cell survival. This finding is similar to that of Misra et al., who conclude that HA is able to upregulate COX2 signaling and prevent cells from undergoing apoptosis.31 In the in vivo study, TUNEL staining also revealed that the combined HA/MNC treatment reduced cardiomyocyte apoptosis 1 day after MI, which was consistent with the in vitro data showing that HA promoted MNC survival.
It has been shown that HA participates in the regulation of inflammation.32 Low molecular weight HA induces the production of pro-inflammatory cytokines and chemokines,33,34 and high molecular weight HA (>1000 kDa) is associated with anti-inflammatory effects.35 In our study, we found a decreased number of neutrophils and macrophages in all three treatment groups. These results were consistent with previous findings in failed back syndrome.36 Importantly, the heart function was ameliorated after HA/MNC injection, as evidenced by the increase in ejection fraction, which represents the heart's contraction ability, in this group compared with the other treatment groups. The end-systolic dimension and the end-diastolic dimension of the HA/MNC group also improved compared with the other treatment groups, suggesting that ventricular dilatation was prevented after MI. Furthermore, we found that the fibrotic gene expression was not increased in vitro and the HA/MNC injection group had a smaller scar length compared with the other groups. Consistently, a previous study by Austin et al. also reported that a HA hydrogel reduced scar formation in the severely injured spinal cord.37 Although some studies have shown that HA can promote chondrogenesis,18 we did not observe any chondrocyte formation in the HA/MNC group.
In addition, the capillary and arteriole densities at the peri-infarct and infarct regions of the injured heart following HA/MNC treatment were significantly increased. As was noted in our previous report, HA hydrogel promotes neovascularization in hindlimb ischemia when it is combined with another cell type–human umbilical vein endothelial cells.38 We also identified many DiI-negative vascular cells (isolectin and SM22-α positive cells) in the peri-infarct region in the HA/MNC group compared with the MI-only group. This result indicates that endogenous cells populated the injury region and mediated endogenous repair. Moreover, we noticed integration of injected DiI-labeled MNCs with the vessels, suggesting that the combination of HA and MNCs may enhance angiogenic activity post-MI. The injected MNCs may also benefit the heart through paracrine effects by recruiting endogenous stem/progenitor cells for heart regeneration post-MI.39 Because we used outbred Sprague-Dawley rats as both donors and recipients, one may questions whether immune rejection of the graft cells exists. Interestingly, even at 28 days post-MI, we still detected DiI-positive cells in the vessel, peri-infarct, and infarct regions (Figures 6 and 8 and Supplementary Figure S8). We also observed that the number of neutrophils, indication of immune rejection and inflammation, was actually reduced 3 days post-MI by HA, MNC, or HA/MNC treatment (Figure 4). To further confirm this, we examined the HA binding protein tumor necrosis factor-stimulated gene 6 which inhibits inflammation and reduces neutrophil infiltration.40 Twenty rats were randomly divided into five groups, sham, MI, MI + HA, MI + MNC, and MI + HA/MNC. The rats were killed and the hearts were harvested 3 days post-MI for RNA isolation. Real-time reverse-transcription PCR confirmed that tumor necrosis factor-stimulated gene 6 was significantly upregulated in both HA and HA/MNC groups (Supplementary Figure S9), suggesting that HA and MNC treatment may be immune privileged. Taken together, our results suggest that HA/MNC combined injection may improve heart regeneration post-MI. Nevertheless, the long-term effects of the combined therapy should be investigated in the future.
Conclusion
In this study, we demonstrate that HA promotes MNC survival and proliferation and endothelial and smooth muscle cell differentiation. The combination of HA therapy and MNCs improved cardiac function in rats through multiple mechanisms, including enhanced angiogenesis and arteriogenesis, increased cardiomyocyte survival, and reduced scar formation post-MI. These results may contribute to future studies that could translate into a clinical therapy.
Materials and Methods
Preparation of the coated plates. Noncoated tissue culture plates served as the control. Fibronectin (Millipore, Billerica, MA) was diluted with phosphate-buffered saline to make a 1% fibronectin solution. Gelatin powder (J. T. Baker, Phillipsburg, NJ) was dissolved in double-distilled water (ddH2O) to make a 1% (w/v) solution and was sterilized. HA powder (1,630 kDa; Sigma-Aldrich, St Louis, MO) was dissolved in phosphate-buffered saline at 4 °C for at least 24 hours to form a 1% (w/v) HA solution. Tissue culture plates were coated with all three solutions for at least 1 hour at 37 °C.
Experimental animals. All animal protocols were approved by the Institutional Animal Care and Use Committee at National Cheng Kung University, Tainan, Taiwan. Six-week-old male Sprague-Dawley rats (200–250 g) were acquired from the National Cheng Kung University Animal Center. All animals were anesthesized with Zoletil and Ronpum before surgery and killing.
Bone marrow MNC isolation, purification, and receptor analysis. Bone marrow MNCs were isolated from the femoral bones of 6-week-old normal adult male Sprague-Dawley rats. The cells were first flushed with Hank's buffered salt solution containing 10% fetal bovine serum from the femoral bone and purified using histopaque-1083 (Sigma) to remove the red blood cells. HA receptors such as CD44 (R & D Systems, Minneapolis, MN), ICAM-1(AbD Serotec, Oxford, UK), and RHAMM (Bioss, Freiburg, Germany) were analyzed by flow cytometry.
Bone marrow MNC apoptosis analysis. Freshly isolated MNCs were cultured on 10-cm culture plates with or without coating with 1% fibronectin, 1% gelatin, and 1% HA and then maintained at 37 °C in a 5% CO2 incubator overnight. The next day, 5 µmol/l doxorubicin was added to the medium to induce cell apoptosis. After 5 hours, the cells were harvested and fixed. Then, the MNCs were stained with propidium iodide (Sigma) and flow cytometry was used to quantify the cells undergoing apoptosis.
Bone marrow MNC adhesion, proliferation, differentiation and gene expression. The MNCs were cultured on 6-well plates without additional coating as a control. The other wells were coated with 1% fibronectin, 1% gelatin or 1% HA and then maintained at 37 °C in a 5% CO2 incubator for 24 hours. After 24 hours, all of the cells were fixed and stained with 4′, 6-diamidino-2-phenylindole fluorescent dye. The total cell number was counted using high-throughput screening microscopy (ImageXpress; Molecular Devices, Sunnyvale, CA) and analyzed using MetaXpress software (Molecular Devices). Cell proliferation was detected using Ki67 (GeneTex, San Antonio, TX) staining. MNCs were cultured in different coating condition plate, such as noncoating, 1% fibronectin, 1% gelatin or 1% HA in endothelial basal medium-2 (Lonza, Basel, Switzerland) and then maintained at 37 °C in a 5% CO2 incubator for 4 days to observe vascular lineage cell differentiation. The hypoxic condition was induced in a hypoxia chamber with CO2 and 5% nitrogen to decrease the oxygen concentration. The MNCs were cultured in the hypoxia chamber for 2 days. Total RNA was extracted from the MNCs using TRIzol (Invitrogen, Carlsbad, CA) and converted to cDNA using RevertAid Reverse Transcriptase (Fermentas, Vilnius, Lithuania). The expression patterns of paracrine factor-associated genes (fibroblast growth factor-2, hepatocyte growth factor, insulin-like growth factor-1, platelet-derived growth factor-b, and stromal cell-derived factor-1), progenitor cell and monocyte/macrophage genes (CD34, CD133, CD68, and CD163), fibrotic genes (DDRII and Acta2), cardiogenic genes (α-MHC, Nkx2.5, and cTnI) and chondrogenic genes (Sox9 and RUNX2) were determined using real-time PCR (Supplementary Table S1).
MI model and treatment. The MI model was created by performing a coronary artery ligation followed by an intramyocardial injection of therapeutics. A total of 40 rats were divided into 5 groups: sham, MI only, and MI with the treatment of 1% HA alone, MNCs alone (1 × 106 MNCs in 100 µl phosphate-buffered saline) or HA with MNCs (1 × 106 MNCs in 100 µl HA), n = 8 per group. In the cardiomyocyte apoptosis experiment, another 30 rats were divided into 5 groups: sham, MI only, and MI with HA alone, MNCs alone (1 × 106 MNCs in 100 µl phosphate-buffered saline), or HA with MNCs (1 × 106 MNCs in 100 µl HA), n = 6 per group.
Echocardiography. Heart function was evaluated using echocardiography (VisualSonics Vevo 770, Toronto, CA) 1 day and 28 days after coronary artery ligation. The rats were placed in a left lateral decubitus position. The anesthetic used during echocardiography was Isoflurane (Baxter, Deerfield, IL). Parasternal long-axis views were obtained with both the M-mode and the 2D echo images. The left ventricular end-diastolic diameter (LVEDD) and end-systolic diameter (LVESD) were measured perpendicular to the long axis of the ventricle at the papillary muscle insertion site. The left ventricle ejection fraction was calculated automatically by the echocardiography system as (LVEDV - LVESV)/LVEDV × 100%, where LVEDV is the left ventricular end diastolic volume, calculated as 7.0 × LVEDD3/(2.4 + LVEDD), and LVESV is the left ventricle end systolic volume, calculated as 7.0 × LVESD3/(2.4 + LVESD).
DiI labeling of MNCs. The MNCs used for in vivo studies were labeled with Celltracker CM-DiI fluorescence dye (Invitrogen) for 5 minutes at 37 °C and then for 15 minutes at 4 °C. After staining, the cells were washed three times with 10% fetal bovine serum/Hank's buffered salt solution.
Immunofluorescence and Masson's trichrome staining. The hearts were harvested and fixed with 4% parafolmadyhyde at 4 °C overnight, then dehydrated and paraffin embedded. The tissue was then sectioned, deparaffined, and rehydrated. All of the samples were boiled in sodium citrate buffer at pH 6 for 10 minutes to retrieve the antigen. The sections were incubated with antismooth muscle 22 α (1:200; Abcam, Cambridge, UK), anti-isolectin IB4 (1:100, Invitrogen), antitroponin I (1:200; DSHB, Iowa, IA) or anti-vWF (1:50; Millipore) antibodies overnight at 4 °C overnight. After three washes, the sections were incubated with the appropriate secondary antibody, which was either Alexa Fluor®488 or 568 (1:200; Invitrogen). The scar tissue was stained with Masson's trichrome stain (Sigma) according to the manufacturer's protocol, and the images were collected with TissueGnostics GmbH FACS-like tissue cytometry (TissueGnostics, Vienna, Austria) and analyzed using ImageJ software (National Institute of Mental Health, Bethesda, MD).
Statistical analysis. All of the measurements were presented as the mean ± SEM. The statistical significance was estimated using one-way or two-way analysis of variances. A value of P < 0.05 was considered statistically significant. Statistical analyses were performed with GraphPad Prism 5.0 software for Windows (GraphPad Software, San Diego, CA).
SUPPLEMENTARY MATERIAL Figure S1. Flow cytometric analysis of common HA receptors CD44, ICAM-1, and RHAMM. Figure S2. HA enhances gene expression of progenitor cell but not monocyte /macrophage. Figure S3. HA does not induce fibrotic gene expression of MNCs compared with the other coating conditions. Figure S4. HA does not induce cardiogenic gene expression of MNCs under hypoxic condition. Figure S5. HA reduces chondrogenesis-associated gene expression of MNCs. Figure S6. Chondrogenesis is not observed in all treatment groups and the MI alone group. Figure S7. HA/MNC injection increases capillary and arteriole densities post-MI. Figure S8. DiI-positive MNCs are integrated into vascular structure. Figure S9. Injection of HA or HA/MNC induces TSG-6 gene expression post-MI. Table S1. List of primers for real-time PCR.
Acknowledgments
We are grateful for support from the optic-image core lab at the Research Center of Clinical Medicine, National Cheng Kung University Hospital. This study was supported by the Department of Health Executive Yuan grants DOH100-TD-PB-111-TM018 and DOH100-TD-PB-111-TM019, the National Science Council grants 100-2314-B-006-046 and 101-2325-B-006-013 and the Academia Sinica Translational Medicine Program. The authors declared no conflict of interest.
Supplementary Material
Flow cytometric analysis of common HA receptors CD44, ICAM-1, and RHAMM.
HA enhances gene expression of progenitor cell but not monocyte /macrophage.
HA does not induce fibrotic gene expression of MNCs compared with the other coating conditions.
HA does not induce cardiogenic gene expression of MNCs under hypoxic condition.
HA reduces chondrogenesis-associated gene expression of MNCs.
Chondrogenesis is not observed in all treatment groups and the MI alone group.
HA/MNC injection increases capillary and arteriole densities post-MI.
DiI-positive MNCs are integrated into vascular structure.
Injection of HA or HA/MNC induces TSG-6 gene expression post-MI.
List of primers for real-time PCR.
References
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Strauer BE, Brehm M, Zeus T, Köstering M, Hernandez A, Sorg RV.et al. (2002Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans Circulation 1061913–1918. [DOI] [PubMed] [Google Scholar]
- Malik FI, Hartman JJ, Elias KA, Morgan BP, Rodriguez H, Brejc K.et al. (2011Cardiac myosin activation: a potential therapeutic approach for systolic heart failure Science 3311439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, American Heart Association Statistics Committee and Stroke Statistics Subcommittee et al. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii S., and, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- Segers VF., and, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- Laflamme MA., and, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- Knudson CB., and, Knudson W. Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB J. 1993;7:1233–1241. [PubMed] [Google Scholar]
- Jiang D, Liang J., and, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- Taylor KR., and, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB J. 2006;20:9–22. doi: 10.1096/fj.05-4682rev. [DOI] [PubMed] [Google Scholar]
- West DC, Hampson IN, Arnold F., and, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228:1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- Anttila MA, Tammi RH, Tammi MI, Syrjänen KJ, Saarikoski SV., and, Kosma VM. High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res. 2000;60:150–155. [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A., Jret al. (2000Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme J Clin Invest 106349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF., and, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ.et al. (2004Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction Am J Cardiol 9492–95. [DOI] [PubMed] [Google Scholar]
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L.et al. (2001Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth Nat Med 71194–1201. [DOI] [PubMed] [Google Scholar]
- Chung C., and, Burdick JA. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2009;15:243–254. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Miyake K, Medina KL, Hayashi S, Ono S, Hamaoka T., and, Kincade PW. Monoclonal antibodies to Pgp-1/CD44 block lympho-hemopoiesis in long-term bone marrow cultures. J Exp Med. 1990;171:477–488. doi: 10.1084/jem.171.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaillie CM, Benis A, Iida J, McGlave PB., and, McCarthy JB. Adhesion of committed human hematopoietic progenitors to synthetic peptides from the C-terminal heparin-binding domain of fibronectin: cooperation between the integrin alpha 4 beta 1 and the CD44 adhesion receptor. Blood. 1994;84:1802–1811. [PubMed] [Google Scholar]
- McCourt PA, Ek B, Forsberg N., and, Gustafson S. Intercellular adhesion molecule-1 is a cell surface receptor for hyaluronan. J Biol Chem. 1994;269:30081–30084. [PubMed] [Google Scholar]
- Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H.et al. (2005Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells Nat Med 11367–368. [DOI] [PubMed] [Google Scholar]
- Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S.et al. (2004Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms Circulation 1091543–1549. [DOI] [PubMed] [Google Scholar]
- Kanda S, Lerner EC, Tsuda S, Shono T, Kanetake H., and, Smithgall TE. The nonreceptor protein-tyrosine kinase c-Fes is involved in fibroblast growth factor-2-induced chemotaxis of murine brain capillary endothelial cells. J Biol Chem. 2000;275:10105–10111. doi: 10.1074/jbc.275.14.10105. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, DeBusk LM, Babichev YO, Dumont DJ., and, Lin PC. Hepatocyte growth factor mediates angiopoietin-induced smooth muscle cell recruitment. Blood. 2006;108:1260–1266. doi: 10.1182/blood-2005-09-012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy DJ., and, Yellon DM. Cardioprotective growth factors. Cardiovasc Res. 2009;83:179–194. doi: 10.1093/cvr/cvp062. [DOI] [PubMed] [Google Scholar]
- Hellström M, Kalén M, Lindahl P, Abramsson A., and, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- Stellos K, Bigalke B, Langer H, Geisler T, Schad A, Kögel A.et al. (2009Expression of stromal-cell-derived factor-1 on circulating platelets is increased in patients with acute coronary syndrome and correlates with the number of CD34+ progenitor cells Eur Heart J 30584–593. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Ito H, Adachi S, Akimoto H, Nishikawa T, Kasajima T.et al. (1994Hypoxia induces apoptosis with enhanced expression of Fas antigen messenger RNA in cultured neonatal rat cardiomyocytes Circ Res 75426–433. [DOI] [PubMed] [Google Scholar]
- Misra S, Obeid LM, Hannun YA, Minamisawa S, Berger FG, Markwald RR.et al. (2008Hyaluronan constitutively regulates activation of COX-2-mediated cell survival activity in intestinal epithelial and colon carcinoma cells J Biol Chem 28314335–14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble PW. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002;21:25–29. doi: 10.1016/s0945-053x(01)00184-6. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y.et al. (2005Regulation of lung injury and repair by Toll-like receptors and hyaluronan Nat Med 111173–1179. [DOI] [PubMed] [Google Scholar]
- McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C.et al. (1996Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44 J Clin Invest 982403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern R, Asari AA., and, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol. 2006;85:699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Austin JW, Kang CE, Baumann MD, DiDiodato L, Satkunendrarajah K, Wilson JR.et al. (2012The effects of intrathecal injection of a hyaluronan-based hydrogel on inflammation, scarring and neurobehavioural outcomes in a rat model of severe spinal cord injury associated with arachnoiditis Biomaterials 334555–4564. [DOI] [PubMed] [Google Scholar]
- Schimizzi AL, Massie JB, Murphy M, Perry A, Kim CW, Garfin SR.et al. (2006High-molecular-weight hyaluronan inhibits macrophage proliferation and cytokine release in the early wound of a preclinical postlaminectomy rat model Spine J 6550–556. [DOI] [PubMed] [Google Scholar]
- Tang ZC, Liao WY, Tang AC, Tsai SJ., and, Hsieh PC. The enhancement of endothelial cell therapy for angiogenesis in hindlimb ischemia using hyaluronan. Biomaterials. 2011;32:75–86. doi: 10.1016/j.biomaterials.2010.08.085. [DOI] [PubMed] [Google Scholar]
- Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD.et al. (2007Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury Nat Med 13970–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Liang J., and, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91:221–264. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RK, Mickle DA, Weisel RD, Mohabeer MK, Zhang J, Rao V.et al. (1997Natural history of fetal rat cardiomyocytes transplanted into adult rat myocardial scar tissue Circulation 96suppl. 9II–179. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow cytometric analysis of common HA receptors CD44, ICAM-1, and RHAMM.
HA enhances gene expression of progenitor cell but not monocyte /macrophage.
HA does not induce fibrotic gene expression of MNCs compared with the other coating conditions.
HA does not induce cardiogenic gene expression of MNCs under hypoxic condition.
HA reduces chondrogenesis-associated gene expression of MNCs.
Chondrogenesis is not observed in all treatment groups and the MI alone group.
HA/MNC injection increases capillary and arteriole densities post-MI.
DiI-positive MNCs are integrated into vascular structure.
Injection of HA or HA/MNC induces TSG-6 gene expression post-MI.
List of primers for real-time PCR.