Abstract
Objective:
To quantitatively characterize domain-specific cognition in individuals with symptomatic lacunar stroke in a systematic review.
Methods:
Systematic searches of MEDLINE and EMBASE were conducted. Inclusion criteria were all articles published prior to December 2011 evaluating domain-specific cognitive status in individuals with a symptomatic lacunar infarct. Data extraction identified cognitive domains with reported impairment and effect size calculations and heterogeneity analyses were completed to assess the magnitude of this impairment for all studies with control group data.
Results:
Results of the search yielded 12 cross-sectional and 5 longitudinal studies that met inclusion criteria. Effect size calculations revealed small to medium effect sizes (ES) estimations for impairment after stroke in the domains of executive function (ES −0.44, 95% confidence interval [CI] −0.83, −0.50), memory (ES −0.55, 95% CI −0.96, −0.13), language (ES −0.63, 95% CI −0.92, −0.33), attention (ES −0.37, 95% CI −0.67, −0.07), and visuospatial abilities (ES −0.61, 95% CI −1.03, 0.19), and large effect sizes for global cognition (ES −0.90, 95% CI −1.48, −0.31) and information processing speed (ES −0.93, 95% CI −1.63, −0.23). Heterogeneity analyses revealed that a subset of these domains were heterogeneous and identified moderating factors accounting for this heterogeneity.
Conclusions:
Results of this systematic review are consistent with previous characterizations of cognitive impairment associated with lacunar strokes. However, impaired cognition in this stroke subtype appears less selective than previously thought, involving all major cognitive domains.
Lacunar stroke is the most common manifestation of cerebral small-vessel disease (SVD)1 and is an important predictor of poststroke cognitive decline and vascular dementia.2 Approximately one-third of individuals develop cognitive impairment after stroke.3 There is an estimated 11%–23% risk4,5 of developing dementia following a lacunar stroke, and this risk increases with recurrent lacunar events3 and the presence of concurrent white matter disease.6–8 Dementia is a leading cause of dependency after stroke and has a devastating impact on poststroke quality of life.9
The cognitive profile generally considered to be associated with cerebral SVD involves preserved memory with impairment in attentional and executive functioning.10,11 A number of cross-sectional studies have examined the relationship between lacunar stroke and cognitive impairment.12–15 In addition, relatively few studies have investigated longitudinal changes in cognitive function following lacunar stroke. To date, no quantitative systematic review has been conducted to investigate the cognitive impairment associated with lacunar stroke and its relation to the profile associated with SVD.
We present a quantitative systematic review of studies evaluating domain-specific cognition in individuals with symptomatic lacunar infarct. The objectives of this review were to identify and describe the cognitive domains subject to impairment in individuals with symptomatic lacunar infarct, quantify the degree of domain-specific impairment in individuals with lacunar stroke compared to healthy controls, identify factors that may moderate the magnitude of cognitive impairment estimated in individual studies, and characterize the longitudinal trajectory of cognitive deficits associated with lacunar stroke. Based on previous work identifying a distinct neuropsychological test (NPT) profile for cerebral SVD,10 we predict that the literature evaluating cognitive function in lacunar stroke populations will reveal impairments in attention/working memory and executive function, with relative sparing of episodic memory.
METHODS
The present systematic review was conducted in accordance with the guidelines outlined by the Center for Dissemination and Reviews.16
Inclusion/exclusion criteria
Published articles through December 2011 were evaluated for inclusion if the study 1) involved a study population with individuals with symptomatic lacunar infarct, 2) the stroke was confirmed on either CT or MRI, and 3) at least 2 NPTs were administered to evaluate cognitive status, including at least 1 domain-specific measure (table 1). Inclusion was also restricted to prospective observational studies involving cognitive assessments conducted either cross-sectionally or longitudinally. All longitudinal studies required a minimum of 2 prospective time points separated by a minimum of 6 months.
Table 1.
Inclusion criteria
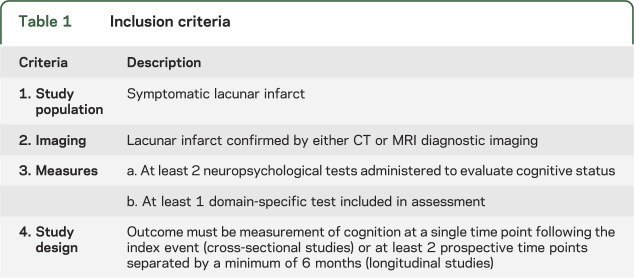
Studies investigating individuals with preexisting dementia, asymptomatic SVD, asymptomatic lacunes, or white matter changes only were excluded from this review. Studies where lacunar stroke was investigated as a subtype of a larger ischemic stroke sample were excluded unless results were reported separately for the lacunar subtype. In order to evaluate cognition specifically in relation to a documented clinical event, and ensure a homogenous population, we elected to exclude studies that did not confirm the presence of symptomatic lacunar infarction on CT or MRI. All retrospective studies, case studies, opinion and review articles, guidelines, published abstracts, and conference proceedings were excluded. Studies published in languages other than English were also excluded.
Search strategy
A systematic search of publications listed in MEDLINE and EMBASE databases was conducted using a combination of the following free text and MeSH search terms: (“small vessel disease” or “small vessel stroke” or “lacune” or “lacunar stroke” or “lacunar infarct” or “silent stroke” or “white matter lesion”) and (“cerebrovascular disease” or “cerebral infarction” or “vascular disease” or “cerebral small vessel disease” or “stroke”) and (“cognitive assessment” or “cognitive function” or “neuropsychological test”) and (“cognition” or “cognitive disorders”). All MeSH headings were exploded with subheadings included.
Citations were screened hierarchically by title, abstract, and full text. Initially, a title review was conducted to eliminate repeat and overlapping citations between databases and exclude studies that did not meet inclusion criteria. Next, for all remaining citations, primary raters (J.D.E., O.R.B.) conducted an abstract review and excluded those that did not meet inclusion criteria. All remaining citations then underwent a full text review and any conflicts in final article selection were resolved by consensus among the raters. Final studies were divided into cross-sectional and longitudinal studies and data extraction was completed separately for each category. After consensus, a third independent rater (B.P.) reviewed and identified studies for inclusion from a sample consisting of all final studies selected by the primary raters and a random subset of the excluded studies. Cohen κ and percent positive agreement were calculated to assess interrater reliability.17
Data analyses
For each study, demographic information and information on study design and setting were extracted (tables 2 and 3). In addition, prior to the quantitative analysis, data characterizing individual NPT utilized in each study were extracted and categorized into groups based on the domains they are known or assumed to measure (tables e-1 and e-2 on the Neurology® Web site at www.neurology.org).
Table 2.
Demographic information for cross-sectional studies of cognitive function after symptomatic lacunar infarct
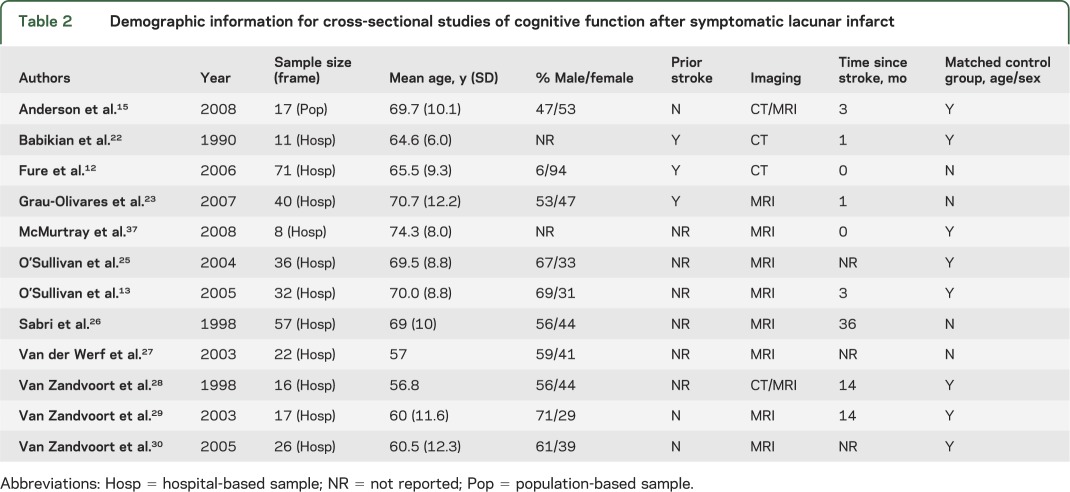
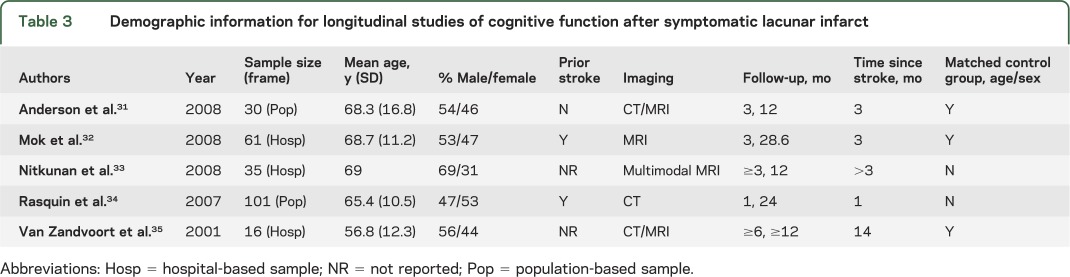
Table 3.
Demographic information for longitudinal studies of cognitive function after symptomatic lacunar infarct
To quantify the degree of domain-specific cognitive impairment for individuals with lacunar stroke compared to healthy controls, effect size (ES) estimates were calculated for all studies with data for both patient and control groups. Continuous data (mean, SD, n) from all relevant cross-sectional and longitudinal studies (baseline data only) were extracted for all reported neuropsychological outcome measures within each domain of cognitive impairment (tables e-1 and e-2). For each domain, reported test scores from the same tasks were pooled using DSTAT18 to generate a composite mean. Hedges g with a random-effects model ([MSubjects − MControls]/SDPooled)19 was then used to estimate an omnibus ES for each domain with a minimum of 3 included studies (table e-3). When multiple outcome measures were reported for a particular domain (e.g., memory), outcomes were pooled into a single effect for each task used in the study and a combined mean was used in the omnibus calculation. Additionally, for studies reporting a composite score only, that score was used to generate the omnibus ES for the domain. All effects were coded so that a negative effect size indicated impaired performance on that domain for the patient group compared to healthy controls. ES calculations were conducted using Comprehensive Meta-Analysis software.20
To evaluate the degree of heterogeneity across studies, effects for all cognitive domains were subjected to Cochran Q and I2 tests, where a significant Q test indicates that the variation among studies for a particular domain may be attributed to heterogeneity rather than chance and larger I2 values indicate increasing heterogeneity.21 For those domains that showed significant heterogeneity, the following potential moderating factors were identified: neuropsychological protocol (single vs composite measure), age, time since stroke, prior stroke, sampling frame (hospital-based vs population-based), and study design (cross-sectional vs longitudinal). All moderating factors were entered into the heterogeneity analyses in a stepwise fashion to determine which, if any, had an impact on the observed I2 values for domains with significant heterogeneity. Heterogeneity analyses were conducted using Comprehensive Meta-Analysis software.20
RESULTS
The initial search resulted in a total of 1,164 articles from Ovid (n = 483) and EMBASE (n = 681) databases (figure e-1). The title review led to the exclusion of 701 repeat or overlapping citations, or articles that did not meet inclusion criteria, resulting in a total of 463 remaining articles (Ovid [n = 252]; EMBASE [n = 211]). The abstract review resulted in the additional exclusion of 217 articles. A total of 246 articles remained, 69 longitudinal and 177 cross-sectional, for full review and consensus. After consensus, 5 longitudinal and 12 cross-sectional studies were identified that met review inclusion criteria.12,13,15,22–36 Results showed high interrater reliability for article selection (Cohen κ = 0.78). Percent positive agreement was also excellent, with 83.3% agreement on article selection between the primary and independent raters.
Data extraction
Cross-sectional studies
Twelve cross-sectional studies met inclusion criteria. Demographic information for these studies is presented in table 2. Results of the data extraction revealed that, in addition to global cognition, the following 8 cognitive domains were evaluated across studies: learning and memory; information/orientation; language; information processing speed; attention/working memory; executive functioning; praxis/motor function; and visuospatial/construction (table e-1). However, results also revealed that studies varied widely in their degree of domain-specific coverage. While some studies tested the majority of domains,13,22,23,25,26,28–30 others provided only acceptable12,27,37 or limited15 domain-specific coverage (table e-1). Importantly, no single study evaluated cognitive performance for all domains. Generally, the domains of learning and memory, language, attention/working memory, and executive functioning were more comprehensively evaluated than information/orientation, language, and praxis/motor function.
All cross-sectional studies included in this review reported impaired cognitive performance for individuals with lacunar stroke compared to normal controls,13,15,22,28–30,37 normative data,12,23,26 or clinical judgment.22,27 Consistent with our hypothesis, the data extraction revealed that, in addition to global cognition, the domains reported to be primarily affected in lacunar stroke patients were executive functioning and attention/working memory. Unlike the profile for impairment associated with SVD, deficits in the domains of learning and memory were also reported for individuals with lacunar stroke. However, this pattern of impairment was not consistent across studies and may be subject to bias, as some studies measured only these domains.
Longitudinal studies
Only 5 longitudinal studies met inclusion criteria. Demographic information for these studies is presented in table 3. Results of the data extraction revealed that the cognitive domains evaluated in the longitudinal studies included in this review were consistent with the domains evaluated cross-sectionally (table e-2). In addition, similar to the cross-sectional literature, the domains of learning and memory, language, attention/working memory, and executive functioning were more comprehensively evaluated than information/orientation, language, and praxis/motor function. Again, no single study evaluated cognitive performance across all domains.
All included longitudinal studies reported impairment at baseline in one or more cognitive domains for subjects with lacunar stroke in comparison to normal controls32,35 or normative data,33,34 with the exception of Anderson et al.,31 who reported no group-level differences at baseline for any domain (table e-2). Again, consistent with the study hypothesis, baseline impairment was most consistently reported for the domain of executive functioning32,33,35,36; however, deficits in global cognition,32 learning and memory,35 and language35 were also reported. At follow-up, regardless of the severity of baseline impairment or time between assessments, all studies reported that stroke patients showed similar performance to baseline across domains, whether assessed by comparison to controls,31,32,35 within-group,33 or as a diagnostic classification.34 Thus, data extraction revealed no consistent evidence that individuals with lacunar stroke show greater or different decline than controls over time.
Effect size calculations and heterogeneity analyses
Results from the ES calculations revealed that, for studies that included control groups (both cross-sectional and longitudinal [baseline only]), there were significant differences in cognitive performance between individuals with lacunar stroke and healthy controls for several cognitive domains (table 4). Specifically, small to medium estimates were observed for the domains of executive function (ES −0.44, 95% confidence interval [CI] −0.83, −0.50), memory (ES −0.55, 95% CI −0.96, −0.13), language (ES −0.63, 95% CI −0.92, −0.33), attention (ES −0.37, 95% CI −0.67, −0.07), and visuospatial abilities (ES −0.61, 95% CI −1.03, 0.19), while global cognition (ES −0.90, 95% CI −1.48, −0.31) and information processing speed (ES −0.93, 95% CI −1.63, −0.23) showed large effect size estimations (table 4). Across studies, lacunar stroke patients showed mild to moderate impairment compared to controls for these domains (figure 1).
Table 4.
Effect size calculations and heterogeneity analyses comparing domain-specific cognitive performance in lacunar stroke patients vs healthy controls for all cross-sectional and longitudinal studies (baseline data only) with control group data
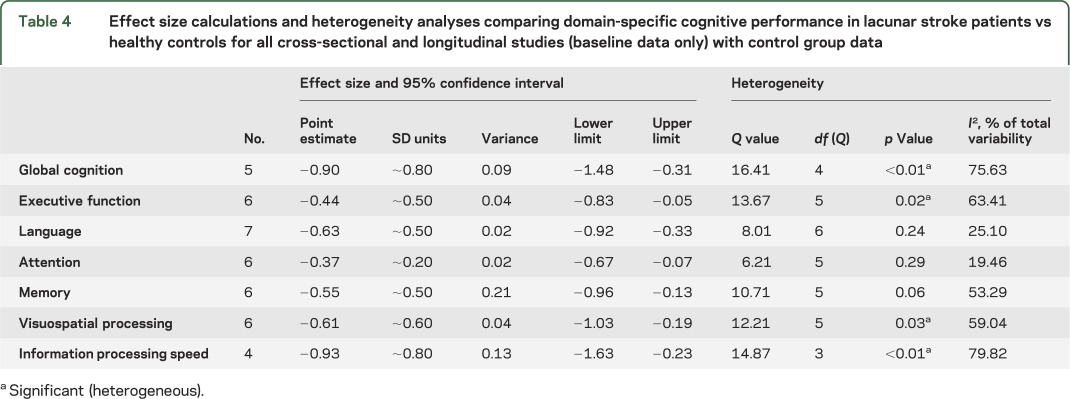
Figure 1. Forest plot representation of effect sizes (lacunar stroke vs control) as a function of cognitive domain and heterogeneity associated with each domain.
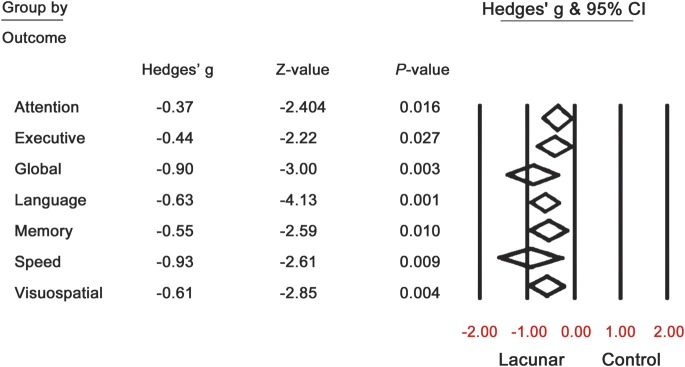
Results from the heterogeneity analyses indicated that, of the domains with significant effect sizes, global cognition, executive function, visuospatial processing, and information processing speed also showed significant heterogeneity (figure 1). To identify the source of this heterogeneity, analyses were adjusted for several potential moderating factors, including neuropsychological protocol (single vs composite measure), age, time since stroke, prior stroke, sampling frame (hospital-based vs population-based), and study design (cross-sectional vs longitudinal). The adjusted analyses revealed that the heterogeneity in effect sizes for information processing speed could not be attributed to any of these factors (table e-4). However, for global cognition and for the domains of executive function and visuospatial processing, a significant source of variability in effect sizes could be attributed to the type of neuropsychological protocol (single vs composite measure), time since stroke, and study design (cross-sectional vs longitudinal) (table e-4). Age and prior stroke did not significantly account for the heterogeneity present in any cognitive domain.
DISCUSSION
This is the first quantitative systematic review to evaluate and characterize domain-specific cognitive impairment associated with symptomatic lacunar infarct, quantify the degree of impairment after lacunar stroke across domains, and identify potential factors that may moderate observed effects. The main findings of this review were that the majority of studies (cross-sectional and longitudinal) reported impairment in multiple cognitive domains following lacunar stroke. As predicted, impairment after lacunar stroke was present in the domains of attention/working memory and executive functioning; however, impairment was also reported for additional domains, including memory, language, and visuospatial function. Further, the magnitude of impairment in stroke patients ranged from small to medium in the domains of executive function, memory, language, attention, and visuospatial abilities to large for global cognition and information processing speed. In addition, estimates for ES were homogeneous, with the exception of those for global cognition, executive function, visuospatial processing, and information processing speed, and neuropsychological protocol (single vs composite measure), time since stroke, and study design (cross-sectional vs longitudinal) appear to be sources of heterogeneity in the evaluation of global cognition, executive functioning, and visuospatial processing. Finally, descriptive evidence suggests no change in domain-specific cognitive function over time in individuals with lacunar stroke, with neither recovery nor further decline.
Results of this review implicate several cognitive domains potentially affected by lacunar stroke. Consistent with our hypothesis, ES calculations revealed mild to moderate impairment after stroke (ES −0.44 to −0.63) in the domains of executive function and attention/working memory and more severe impairment (ES −0.90 to −0.93) for global cognition and information processing speed. These findings are consistent with previous work showing that cognitive impairment associated with cerebral SVD occurs in the domains most susceptible to disruptions in subcortical gray and white matter, including processing speed and various aspects of executive function,38–40 and are also consistent with reports of frontal cognitive decline associated with the syndrome of capsular genu infarction.41–44 However, our findings depict a broader impairment profile, with involvement of memory, language, and visuospatial abilities, extending previous characterizations of subcortical vascular cognitive impairment.11,45 Importantly, domain impairments should not be understood as independent of each other and based on separable neuroanatomical substrates: while the exact contribution of information processing, attentional, and executive dysfunctions to other impairments remains to be delineated, there is a strong theoretical basis for positing an enabling and organizing role of these dysfunctions in cognition and memory.46,47
There was substantial variability across studies in the specific domains where impairment was reported. Although all the included studies employed some standardized measures in their neuropsychological assessments, critically, no single study (either cross-sectional or longitudinal) evaluated all cognitive domains. It is also possible that the studies included in this review were underpowered for analyses at the individual test level. The majority of studies included in this review had relatively small sample sizes (n < 50: range 16–101). Due to the small study samples, none of the analyses in included studies adjusted for potential covariates or conducted subgroup analyses, which may provide important information about potential within-group differences in domain-specific cognitive abilities. Thus effects for some domains may have been masked by variability. This variability may be attributed to the marked heterogeneity in neuropsychological instruments, study outcomes, and analytic techniques used across studies, or to potential heterogeneity across studies in the inclusion of patients of different ages and those with index vs recurrent, or prior, clinical events. This is particularly important given the results of the heterogeneity analysis in the present study.
Our findings indicated that the domain of global cognition showed significant heterogeneity attributable to the moderating factors of neuropsychological protocol and time since stroke (table e-4). Thus, given that many previous studies have evaluated cognition using only global measures (e.g., Mini-Mental State Examination)48–52 and have sample sizes unable to support adjusted analyses, it is possible that previous findings of cognitive status after lacunar stroke may be biased by the demonstrated heterogeneity for this domain, if results were not adjusted for these confounding variables. Although age and prior stroke did not significantly account for heterogeneity across studies in the present systematic review, these factors are well-recognized predictors of cognitive impairment. Specifically, both increasing age and increased lacunar burden are associated with greater cognitive decline53,54 and their potential impact on cognitive impairment merits further study. Thus, future work would benefit from the use of sample sizes sufficient to allow for both adjusted and subgroup analyses; study designs that account and report on factors shown to moderate potential factors of heterogeneity, including type of neuropsychological protocol (single vs composite measure), age, time since stroke, study design (cross-sectional vs longitudinal), and history of prior stroke; and the explicit exploration of the impact of these factors on measures of poststroke cognitive decline.
Effect sizes presented in this study involved cross-sectional data and baseline-only data from longitudinal studies. As a result, these analyses provide evidence for specific domains impacted at the outset after lacunar stroke; however, the longitudinal course of performance on these domains remains unclear. Thus, the inclusion of cognition as an outcome in future prospective observational studies and clinical trials is urgently required. The Secondary Prevention of Small Subcortical Strokes (SPS3) clinical trial55 (NCT:00059306), the first multicenter stroke prevention trial to prospectively examine domain-specific cognitive outcomes in 3,000 patients with symptomatic lacunar infarcts,55 will provide pivotal data regarding the long-term impact of lacunar stroke on domain-specific cognitive function.
Notwithstanding the noted paucity of longitudinal studies in this review, descriptive findings suggested that the cognitive impact associated with lacunar stroke remains stable over time. These results are consistent with previous reviews of poststroke dementia that demonstrated the longer-term rate of cognitive decline is largely accounted for by stroke recurrence and, in its absence, there appears to be a chronic nonprogressive course.3,56 Another potential explanation for the observed lack of change in cognitive performance was the length of study follow-up. Only 2 of the 5 longitudinal studies had follow-up times exceeding 1 year, with 2.5 years as the longest follow-up.32 Importantly, studies with short-term follow-up31,33,35 showed no significant changes in domain-specific cognitive function over time. Thus, it is possible that, in the literature to date, shorter follow-up times may have limited the ability to detect domain-specific changes in cognitive function.
Within a given cognitive domain, different NPTs may vary in their sensitivity to poststroke deficits.57 This important point was recently addressed in a proposal for harmonizing standards in the assessment of poststroke cognitive function, with the goal of defining a minimal uniform data set in clinical practice and research.58 The National Institute of Neurological Disorders and Stroke–Canadian Stroke Network Working Group in Neuropsychology recommended testing 4 domains (executive/activation, language, visuospatial, memory), and operationally defined the skills within each. For instance, within the executive/activation domain, measures of processing speed, working memory, set shifting, and executive control, derived from the administration of letter and category fluency, Wechsler Adult Intelligence Scale digit symbol, and trail making tasks, were recommended. Based on this systematic review, we would argue that the use of domain-specific neuropsychological assessments with extended follow-up times in future prospective observational work is essential to characterize the impact of lacunar stroke on cognition. Consistent with the recommendations of the National Institute of Neurological Disorders and Stroke–Canadian Stroke Network Working Group,58 our findings support the use of a comprehensive neuropsychological protocol in the assessment of cognitive impairment after lacunar stroke, with evaluation of global cognition in addition to domain-specific function. The use of a standardized neuropsychological protocol would be advantageous to improve comparability across studies and provide consensus for the definition and treatment of lacunar stroke and, more generally, SVD.
Results of this quantitative systematic review provide evidence for a cognitive impairment profile after symptomatic lacunar infarct that involves several domains, including information processing speed, attention/working memory, executive function, language, memory, and visuospatial processing. Our findings also indicated that there is currently no consistent evidence for change in domain-specific cognitive function over time after lacunar stroke. However, the variability in measures and effects in the available literature underscores the urgent need for guidelines regarding the use of a standardized neuropsychological protocol after stroke and the inclusion of cognition as an outcome in clinical trials to provide greater understanding of the cognitive domains vulnerable to impairment after lacunar stroke.
Supplementary Material
Glossary
- CI
confidence interval
- ES
effect sizes
- NPT
neuropsychological test
- SVD
small-vessel disease
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Ms. Edwards: study concept and design; article search, review, and selection; data analysis and interpretation; manuscript preparation and critical revision for important intellectual content. Dr. Jacova: study concept and design; data analysis and interpretation; critical revision of the manuscript for important intellectual content. Mr. Sepehry: data analysis and interpretation; manuscript revision. Ms. Pratt: article search, review, and selection; data analysis; manuscript revision. Dr. Benavente: study concept and design; article review and selection; data interpretation; critical revision of the manuscript for important intellectual content; study supervision.
STUDY FUNDING
Supported by funding from the NIH and National Institutes of Neurological Disorders and Stroke (NIH-NINDS) to O.R.B. (grant U01-NS38529-04A1), awards from the Canadian Institutes of Health Research to J.D.E. and A.A.S. and the Michael Smith Foundation for Health Research to J.D.E., and support from the Ralph Fisher and Alzheimer Society of BC Professorship in Alzheimer's Research Endowment Fund to C.J.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Micheli S, Agnelli G, Palmerini F, et al. Need for extensive diagnostic work-up for patients with lacunar stroke. J Neurol 2008;255:637–642 [DOI] [PubMed] [Google Scholar]
- 2.Aharon-Peretz J, Daskovski E, Mashiach T, et al. Natural history of dementia associated with lacunar infarctions. J Neurol Sci 2002;203–204:53–55 [DOI] [PubMed] [Google Scholar]
- 3.Morris HR, Waite AJ, Williams NM, Neal JW, Blake DJ. Recent advances in the genetics of the ALS-FTLD complex. Curr Neurol Neurosci Rep 2012;12:243–250 [DOI] [PubMed] [Google Scholar]
- 4.Ross GW, Petrovitch H, White LR, et al. Characterization of risk factors for vascular dementia: the Honolulu-Asia Aging Study. Neurology 1999;53:337–343 [DOI] [PubMed] [Google Scholar]
- 5.Barba R, Martinez-Espinosa S, Rodríguez-Garcia E, Pondal M, Vivancos J, Del Ser T. Poststroke dementia: clinical features and risk factors. Stroke 2000;31:1494–1501 [DOI] [PubMed] [Google Scholar]
- 6.Longstreth WT, Jr, Arnold AM, Beauchamp NJ, Jr, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke 2005;36:56–61 [DOI] [PubMed] [Google Scholar]
- 7.Schmidt R, Ropele S, Enzinger C, et al. White matter lesion progression, brain atrophy, and cognitive decline: the Austrian Stroke Prevention Study. Ann Neurol 2005;58:610–616 [DOI] [PubMed] [Google Scholar]
- 8.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke 2003;34:1126–1129 [DOI] [PubMed] [Google Scholar]
- 9.Leys D, Henon H, Mackowiak-Cordoliani MA, Pasquier F. Poststroke dementia. Lancet Neurol 2005;4:752–759 [DOI] [PubMed] [Google Scholar]
- 10.Andreason PJ, Zametkin AJ, Guo AC, Baldwin P, Cohen RM. Gender-related differences in regional cerebral glucose metabolism in normal volunteers. Psychiatry Res 1994;51:175–183 [DOI] [PubMed] [Google Scholar]
- 11.Gur RC, Mozley LH, Mozley PD, et al. Sex differences in regional cerebral glucose metabolism during a resting state. Science 1995;267:528–531 [DOI] [PubMed] [Google Scholar]
- 12.Fure B, Bruun Wyller T, Engedal K, et al. Cognitive impairments in acute lacunar stroke. Acta Neurol Scand 2006;114:17–22 [DOI] [PubMed] [Google Scholar]
- 13.O'Sullivan M, Morris RG, Markus HS. Brief cognitive assessment for patients with cerebral small vessel disease. J Neurol Neurosurg Psychiatry 2005;76:1140–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Zandvoort MJ, Aleman A, Kappelle LJ, et al. Cognitive functioning before and after a lacunar infarct. Cerebrovasc Dis 2000;10:478–479 [DOI] [PubMed] [Google Scholar]
- 15.Anderson JF, Saling MM, Donnan GA. Chronic information-processing changes in individuals with a first-ever clinical lacunar syndrome. Cogn Behav Neurol 2008;21:236–241 [DOI] [PubMed] [Google Scholar]
- 16.Centre for Dissemination and Reviews Systematic Reviews. York: University of York; 2008 [Google Scholar]
- 17.Roberts C. Modelling patterns of agreement for nominal scales. Stat Med 2008;27:810–830 [DOI] [PubMed] [Google Scholar]
- 18.Johnson B. Software for the Meta-Analytic Review of Research Literatures. Hillsdale, NJ: Lawrence Erlbaum Associates; 1993 [Google Scholar]
- 19.Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. New York: Academic Press; 1985 [Google Scholar]
- 20.Borenstein M, Higgins JH. Comprehensive Meta-Analysis. Englewood, NJ: Biostat; 2005 [Google Scholar]
- 21.Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101–129 [Google Scholar]
- 22.Babikian VL, Wolfe N, Linn R, et al. Cognitive changes in patients with multiple cerebral infarcts. Stroke 1990;21:1013–1018 [DOI] [PubMed] [Google Scholar]
- 23.Grau-Olivares M, Arboix A, Bartres-Faz D, et al. Neuropsychological abnormalities associated with lacunar infarction. J Neurol Sci 2007;257:160–165 [DOI] [PubMed] [Google Scholar]
- 24.McMurtray AM, Liao A, Haider J, et al. Cognitive performance after lacunar stroke correlates with leukoaraiosis severity. Cerebrovasc Dis 2007;24:271–276 [DOI] [PubMed] [Google Scholar]
- 25.O'Sullivan M, Morris RG, Huckstep B, Jones DK, Williams SC, Markus HS. Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. J Neurol Neurosurg Psychiatry 2004;75:441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabri O, Hellwig D, Schreckenberger M, et al. Correlation of neuropsychological, morphological and functional (regional cerebral blood flow and glucose utilization) findings in cerebral microangiopathy. J Nucl Med 1998;39:147–154 [PubMed] [Google Scholar]
- 27.Van der Werf YD, Scheltens P, Lindeboom J, Witter MP, Uylings HB, Jolles J. Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia 2003;41:1330–1344 [DOI] [PubMed] [Google Scholar]
- 28.Van Zandvoort MJ, Kappelle LJ, Algra A, et al. Decreased capacity for mental effort after single supratentorial lacunar infarct may affect performance in everyday life. J Neurol Neurosurg Psychiatry 1998;65:697–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Zandvoort M, de Haan E, van Gijn J, et al. Cognitive functioning in patients with a small infarct in the brainstem. J Int Neuropsychol Soc 2003;9:490–494 [DOI] [PubMed] [Google Scholar]
- 30.van Zandvoort MJ, van der Grond J, Kappelle LJ, et al. Cognitive deficits and changes in neurometabolites after a lacunar infarct. J Neurol 2005;252:183–190 [DOI] [PubMed] [Google Scholar]
- 31.Anderson JF, Saling MM, Srikanth VK, et al. Individuals with first-ever clinical presentation of a lacunar infarction syndrome: Is there an increased likelihood of developing mild cognitive impairment in the first 12 months after stroke? J Neuropsychol 2008;2:373–385 [DOI] [PubMed] [Google Scholar]
- 32.Mok VC, Wong A, Lam WW, et al. A case-controlled study of cognitive progression in Chinese lacunar stroke patients. Clin Neurol Neurosurg 2008;110:649–656 [DOI] [PubMed] [Google Scholar]
- 33.Nitkunan A, Barrick TR, Charlton RA, Clark CA, Markus HS. Multimodal MRI in cerebral small vessel disease: its relationship with cognition and sensitivity to change over time. Stroke 2008;39:1999–2005 [DOI] [PubMed] [Google Scholar]
- 34.Rasquin SM, van Oostenbrugge RJ, Verhey FR, et al. Vascular mild cognitive impairment is highly prevalent after lacunar stroke but does not increase over time: a 2-year follow-up study. Dement Geriatr Cogn Disord 2007;24:396–401 [DOI] [PubMed] [Google Scholar]
- 35.Van Zandvoort MJ, De Haan EH, Kappelle LJ. Chronic cognitive disturbances after a single supratentorial lacunar infarct. Neuropsychiatry Neuropsychol Behav Neurol 2001;14:98–102 [PubMed] [Google Scholar]
- 36.Ramos-Estebanez C, Moral-Arce I, Gonzalez-Mandly A, et al. Vascular cognitive impairment in small vessel disease: clinical and neuropsychological features of lacunar state and Binswanger's disease. Age Ageing 2011;40:175–180 [DOI] [PubMed] [Google Scholar]
- 37.McMurtray AM, Sultzer DL, Monserratt L, et al. Content-specific delusions from right caudate lacunar stroke: association with prefrontal hypometabolism. J Neuropsychiatry Clin Neurosci 2008;20:62–67 [DOI] [PubMed] [Google Scholar]
- 38.Mok VC, Liu T, Lam WW, et al. Neuroimaging predictors of cognitive impairment in confluent white matter lesion: volumetric analyses of 99 brain regions. Dement Geriatr Cogn Disord 2008;25:67–73 [DOI] [PubMed] [Google Scholar]
- 39.O'Sullivan M, Barrick TR, Morris RG, Clark CA, Markus HS. Damage within a network of white matter regions underlies executive dysfunction in CADASIL. Neurology 2005;65:1584–1590 [DOI] [PubMed] [Google Scholar]
- 40.Della Nave R, Foresti S, Pratesi A, et al. Whole-brain histogram and voxel-based analyses of diffusion tensor imaging in patients with leukoaraiosis: correlation with motor and cognitive impairment. AJNR Am J Neuroradiol 2007;28:1313–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tatemichi TK, Desmond DW, Prohovnik I, et al. Confusion and memory loss from capsular genu infarction: a thalamocortical disconnection syndrome? Neurology 1992;42:1966–1979 [DOI] [PubMed] [Google Scholar]
- 42.Madureira S, Guerreiro M, Ferro JM. A follow-up study of cognitive impairment due to inferior capsular genu infarction. J Neurol 1999;246:764–769 [DOI] [PubMed] [Google Scholar]
- 43.Pantoni L, Basile AM, Romanelli M, et al. Abulia and cognitive impairment in two patients with capsular genu infarct. Acta Neurol Scand 2001;104:185–190 [DOI] [PubMed] [Google Scholar]
- 44.Chukwudelunzu FE, Meschia JF, Graff-Radford NR, Lucas JA. Extensive metabolic and neuropsychological abnormalities associated with discrete infarction of the genu of the internal capsule. J Neurol Neurosurg Psychiatry 2001;71:658–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Brien JT. Vascular cognitive impairment. Am J Geriatr Psychiatry 2006;14:724–733 [DOI] [PubMed] [Google Scholar]
- 46.Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron 2004;44:195–208 [DOI] [PubMed] [Google Scholar]
- 47.Moscovitch M, Winocur G. Frontal lobes, memory, and aging. Ann NY Acad Sci 1995;769:119–150 [DOI] [PubMed] [Google Scholar]
- 48.Appelros P. Characteristics of Mini-Mental State Examination 1 year after stroke. Acta Neurol Scand 2005;112:88–92 [DOI] [PubMed] [Google Scholar]
- 49.Appelros P, Andersson AG. Changes in Mini Mental State Examination score after stroke: lacunar infarction predicts cognitive decline. Eur J Neurol 2006;13:491–495 [DOI] [PubMed] [Google Scholar]
- 50.Arciniegas DB, Kellermeyer GF, Bonifer NM, Anderson-Salvi KM, Anderson CA. Screening for cognitive decline following single known stroke using the Mini-Mental State Examination. Neuropsychiatr Dis Treat 2011;7:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diener HC, Sacco RL, Yusuf S, et al. Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: a double-blind, active and placebo-controlled study. Lancet Neurol 2008;7:875–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.PROGRESS Collaborative Group Randomised trial of a perindopril-based blood- pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358:1033–1041 [DOI] [PubMed] [Google Scholar]
- 53.Zhu L, Fratiglioni L, Guo Z, Aguero-Torres H, Winblad B, Viitanen M. Association of stroke with dementia, cognitive impairment, and functional disability in the very old: a population-based study. Stroke 1998;29:2094–2099 [DOI] [PubMed] [Google Scholar]
- 54.Enzinger C, Fazekas F, Ropele S, Schmidt R. Progression of cerebral white matter lesions: clinical and radiological considerations. J Neurol Sci 2007;257:5–10 [DOI] [PubMed] [Google Scholar]
- 55.Benavente OR, White C, Pearce L, et al. ; for the SPS3 Investigators The Secondary Prevention of Small Subcortical Stroke (SPS3) study. Int J Stroke 2011;6:164–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Srikanth V, Saling MM, Thrift AG, Donnan GA. Cerebrovascular disease and dementia. Timely Top Med Cardiovasc Dis 2006;10:E9. [PubMed] [Google Scholar]
- 57.Srikanth V, Thrift AG, Fryer JL, et al. The validity of brief screening cognitive instruments in the diagnosis of cognitive impairment and dementia after first-ever stroke. Int Psychogeriatr 2006;18:295–305 [DOI] [PubMed] [Google Scholar]
- 58.Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke–Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 2006;37:2220–2241 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


