Abstract
Objective:
To evaluate the efficacy and safety of minocycline in the management of HIV-associated cognitive impairment.
Methods:
We enrolled HIV-positive participants with a CD4 count of 250 to 500 cells/μL in a randomized, double-blind, placebo-controlled study. They received 100 mg of minocycline or matching placebo orally every 12 hours for 24 weeks. Cognitive function was measured using the Uganda Neuropsychological Test Battery Summary Measure (U NP Sum) and the Memorial Sloan-Kettering (MSK) scale. The primary efficacy measure was the 24-week change in an average of 9 standardized U NP Sum z scores.
Results:
Seventy-three participants were enrolled. Of these, 90% were female, 49% were between the ages 30 and 39 years, and 74% had 6 or more years of education. One participant had MSK score of stage 1 (i.e., mild HIV dementia), and 72 participants had MSK stage 0.5 (i.e., equivocal or subclinical dementia) at the baseline evaluation. The minocycline effect on the 24-week change of the U NP Sum compared with placebo was 0.03 (95% confidence interval −0.51, 0.46; p = 0.37).
Conclusion:
Minocycline was safe and well tolerated in HIV-positive individuals. However, it did not improve HIV-associated cognitive impairment.
Classification of evidence:
This study provides Class II evidence that 100 mg of minocycline given orally every 12 hours for 24 weeks had no significant effect compared with placebo in the improvement of cognitive function in antiretroviral therapy–naive, HIV-positive patients.
Although combination antiretroviral therapy (cART) improves cognitive impairment,1 the treatment response may be unsatisfactory or short-lived, or the agents may be poorly tolerated in doses adequate for CNS penetration.2–4 In Uganda as well as some other African countries, almost 50% of cART-eligible patients, even those with HIV cognitive impairment, remain untreated.5–8
Sequestration of the HIV virus in brain tissue may require an adjunct potent drug that can cross the blood-brain barrier with the ability to interfere with the inflammatory events of HIV infection and improve the management of HIV cognitive impairment. Minocycline would offer a unique therapeutic strategy for HIV-associated neurocognitive disorders because of its antiinflammatory and neuroprotective effects as has been noted in in vivo studies.9–14 To validate these findings, we hypothesized that minocycline treatment for 24 weeks would improve HIV-associated cognitive impairment and would be safe and well tolerated in individuals with HIV.
We thus conducted a phase I/II, randomized, double-blind, placebo-controlled study to assess the efficacy, tolerability, and safety of minocycline for the treatment of HIV-associated cognitive impairment in Uganda. In contrast to a prior study15 in the United States in which all HIV-positive individuals were receiving cART, this study was conducted in HIV-positive individuals who were not eligible for ART in the Uganda setting. Thus, the direct effect of minocycline and placebo in the absence of ART was examined in this study.
METHODS
Recruitment/enrollment
During the screening process from April 2008 to September 2009, we selected every fifth patient out of a list of ART-naive, HIV-positive patients with a CD4 count in the range of 250 to 500 cells/μL who presented on each clinic day at the Infectious Diseases Clinic, Kampala, Uganda. This specialized outpatient HIV health unit offers free care and serves as a national referral center, providing specialist consultations for infected patients including those who are not responding to treatment from other health facilities. More than 9,000 HIV/AIDS clients have been registered since its establishment in 2004.
The screening included a measure for cognitive impairment using the 0- to 12-point International HIV Dementia Scale.16 Any person scoring ≤10 points was suspected of having HIV dementia as previously determined during the validation of the instrument. Two research assistants administered a detailed neuropsychological test battery that included the Timed Gait, Grooved Pegboard Dominant Hand, Grooved Pegboard Nondominant Hand, Color Trails 1, Color Trails 2, Symbol Digit, World Health Organization–University of California at Los Angeles (WHO-UCLA) Verbal Learning Test trial 5, WHO-UCLA Verbal Learning Test delayed recall, and Digit Span forward and Digit Span backward.
The selection criteria for the study participants are shown in table 1. The study had 2 steps: step 1 was the double-blind phase in which individuals received minocycline or placebo for 24 weeks; step 2, an optional open phase, was available for those who completed step 1 and every participant who agreed to enroll received minocycline for 24 additional weeks.
Table 1.
Eligibility criteria

Standard protocol approvals, registrations, and patient consents
The study clinical trial identifier number in ClinicalTrials.gov was NCT00855062.
The Makerere University School of Medicine Research and Ethics Committee as well as the Uganda National Council for Science and Technology approved the use of human subjects. All participants gave informed consent before enrollment into the study.
Intervention
In this double-blind, placebo-controlled study, we randomized 73 participants to receive either 100 mg of minocycline orally every 12 hours or matching placebo orally every 12 hours daily for 24 weeks. We performed a neurologic examination and safety laboratory tests including serum chemistry profiles (electrolytes, liver function tests), hematology, and CD4 lymphocyte counts at screening and weeks 4, 12, and 24. A neuropsychological test battery was performed at screening, entry, 12 weeks, and 24 weeks. The presence of depression symptomatology was assessed with the Center for Epidemiologic Studies Depression Scale.17 We measured the participants' functional performance with the Karnofsky scale and Instrumental Activities of Daily Living (IADL). Individuals who had higher scores on the Karnofsky scale at subsequent visits compared with the baseline score were considered to be “better” in their functional performance. The level of cognitive function was scored using the Memorial Sloan-Kettering scale.
The participants were randomly assigned to a treatment group based on a preassigned randomization list. The list was kept by the study pharmacist who had no access to the patients' clinical evaluations both at baseline and through follow-up. Once a patient had been screened and found fit for the study, the research assistant would notify the pharmacist of the participant. The pharmacist would then assign a number in the randomization list as appropriate.
Outcome measures
The primary objective of the study was the change in neurocognitive performance from baseline to 24 weeks as measured by the Uganda Neuropsychological Test Battery Summary Measure (U NP Sum). Each test score was standardized to normal, 1, or 2 SDs from the mean in comparison to normative values of an age-, sex-, and education-matched general non-HIV population.18 The U NP Sum was defined as the mean of the z scores of the following tests: Grooved Pegboard Dominant Hand, Grooved Pegboard Nondominant Hand, Color Trails 1, Color Trails 2, Symbol Digit, WHO-UCLA Verbal Learning Test trial 5 total, WHO-UCLA Verbal Learning Test delayed recall, Digit Span forward, and Digit Span backward. The secondary objective of the study was the measure of safety including the frequency of adverse events and abnormal results on laboratory tests, and changes over time in vital signs and symptoms.
Statistical methods
The primary analysis followed the intention-to-treat principle, and all 73 randomized participants were included. The number needed to treat was 25. Because the primary end point was a continuous variable, a new dichotomous variable (0 = did not reach a clinically meaningful cutoff of 0.5; 1 = reached the cutoff) was created and the proportions of participants who reached the clinically meaningful cutoff from the minocycline and control groups were used to calculate the number needed to treat.
The study sample size was arrived at by assuming that both arms (minocycline and placebo) would have an SD of 0.7 in the 24-week change in U NP Sum, a 17% loss-to-follow-up rate, 10% noncompliance, and Pitman efficiency of 0.864. To be able to detect a difference of 0.5 (a minimum clinically relevant difference) in the 24-week U NP Sum changes with 85% power and a type I error rate of 0.05 required 100 subjects (50 per group).
Because 19% of participants had missing 24-week U NP Sum for various dropout reasons, the missing values were imputed by a multiple regression imputation method. Once all values were imputed, a regression model was fit to estimate the efficacy of minocycline on the 24-week change of U NP Sum, adjusting for the baseline U NP Sum. Two different sensitivity analyses were also conducted: 1) a linear regression model based on observed outcomes, and 2) a mixed model with all observed and repeatedly measured outcomes. All secondary analyses were based on observed data. For continuous outcomes, linear regression models adjusting for a baseline score were used, and for categorical outcomes, logistic regression models were used. For both primary and secondary analyses, the Bonferroni correction was applied on type I error to adjust for multiplicity.
We also examined the primary objective using the reliable change index (RCI). The RCIs between the minocycline and placebo groups were compared using a z test to determine the effect on the 24-week change of U NP Sum.
Classification of evidence
The primary research question for this study was: Does minocycline improve cognitive function among HIV-positive individuals? This intervention study provides Class II evidence that minocycline treatment of 100 mg given orally every 12 hours for 24 weeks does not improve HIV-associated cognitive impairment among HIV-positive individuals with a CD4 count of 250 to 500 cells/μL (minocycline effect on the 24-week change of cognitive function compared with placebo was −0.03, 95% confidence interval [CI] −0.51 to 0.46; p = 0.37).
RESULTS
The study was initially planned to enroll 100 participants. After half the participants completed the double-blind phase, an interim analysis of primary and secondary outcome measures was performed and reviewed by the Neurological AIDS Research Consortium Data Safety and Monitoring Board (DSMB). The DSMB recommended early termination of the study because of outcome futility, and participants were informed and discharged after completing the final study visits. The overall accrual and study step discontinuation in each study arm are summarized in the figure. Of 73 randomized participants, 52 (71%) completed the 24-week protocol, and 28 (38%) completed the 48-week protocol. As shown in table 2, 90% of the participants were women. The modal age group was 30 to 39 years (49%) and most (53%) had received 6 to 10 years of education. The treatment arms were similar in baseline characteristics. Only 1 participant in the minocycline arm had stage-1 dementia (i.e., mild HIV dementia), and the remainder of the 72 participants had stage 0.5 (i.e., equivocal or subclinical dementia). The minocycline effect on the 24-week change of the U NP Sum compared with placebo was −0.03 (95% CI −0.51, 0.46; p = 0.37) (table 3). The upper CI did not reach the prespecified clinically meaningful cutoff point of 0.5, and this result rules out a significant minocycline effect compared with placebo. The effect of minocycline on the 24-week change in the CD4 cell count after adjusting for the baseline CD4 count was 8.11 (95% CI −27.23, 43.45; p value = 0.65). The mean CD4 count decreased by 25 cells/μL in the minocycline arm and by 28 cells/μL in the placebo arm; however, this was not significantly different. Two participants (6%) in the placebo arm and 1 participant (3%) in the minocycline arm had improved score on the Karnofsky functional performance scale at week 24 compared with baseline (95% CI 0.04, 0.06; p = 0.61). Four participants (14%) in the minocycline arm and 3 participants (12%) in the placebo arm had an improved IADL score at week 24 compared with their baseline. The treatment effect on the IADL (estimated by odds ratio) was 1.28 (95% CI 0.26, 6.34; p = 0.76). The 95% CI of being “better" for the minocycline arm was 0.05, 0.37 and 0.03, 0.34 for the placebo arm.
Figure. Participant flow chart.
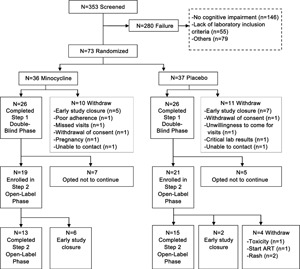
ART = antiretroviral therapy.
Table 2.
Baseline characteristics of study participants
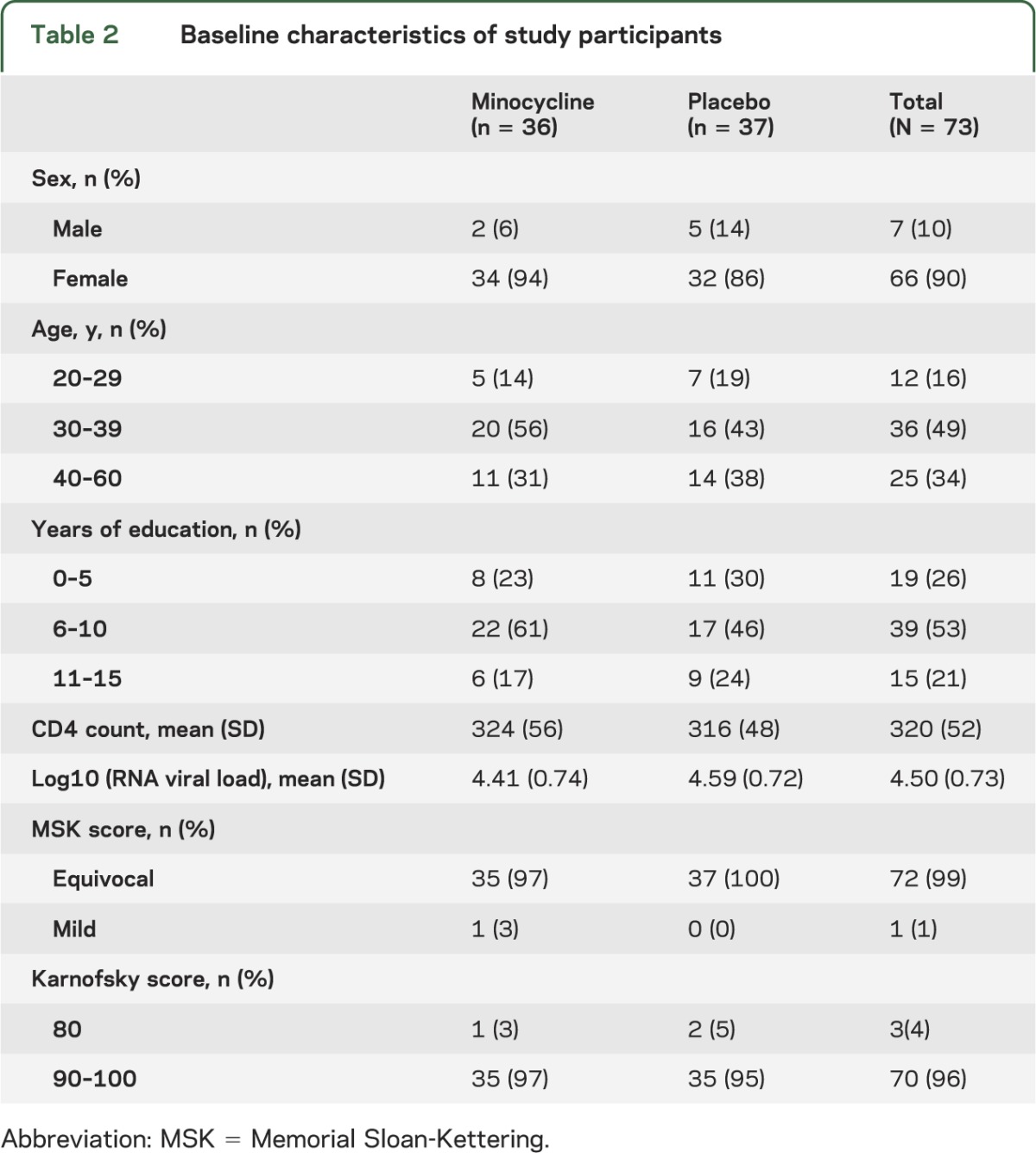
Table 3.
Neuropsychological standardized z-score change and minocycline effect at 24 weeks
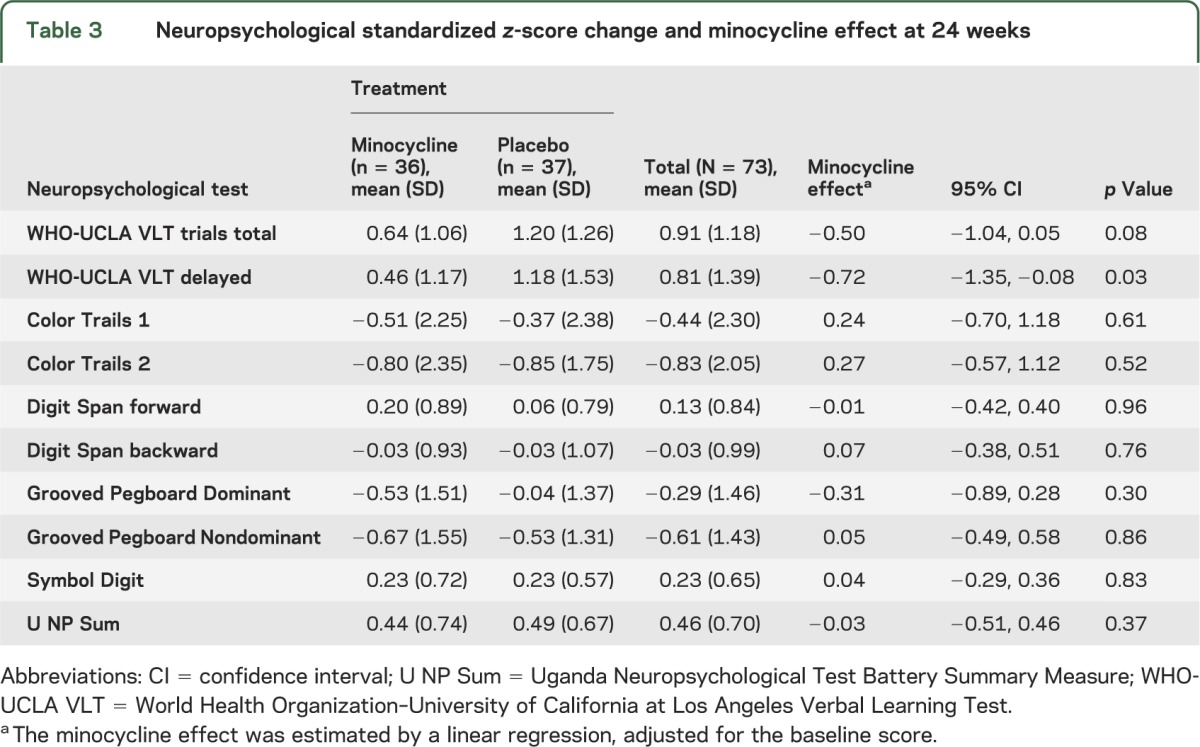
None of the secondary cognitive outcome measures showed a difference between the 2 treatment arms with one exception. The 24-week change of the delayed verbal memory recall in a general linear model adjusting for baseline neuropsychological test score showed a benefit for the placebo arm of −0.718 (95% CI −1.35, −0.08; p value = 0.03).
The mean (SD) RCI for the minocycline and placebo groups was 0.84 (1.43) and 0.95 (1.28), respectively. The minocycline effect on the 24-week change of U NP Sum compared with the placebo effect was not statistically significant (p = 0.76). There were no significant differences between the minocycline and placebo arms on the subjective and performance-based functional measures, measures of mood, or viral load assessments.
Safety
Of note were 7 cases (19.4%) of grade-2 hyperpigmentation that occurred among patients taking minocycline. This usually happened by week 12; however, the condition would resolve by the subsequent clinic visits. Two patients experienced a grade-4 rash related to the study drug. The probability of not experiencing any signs and symptoms by week 24 was higher for the minocycline group but was not significantly different from the placebo arm using the log-rank test (p = 0.58).
There was 1 death that was unrelated to study drug. This patient had only 11% adherence to study drug at the end of the fourth week after randomization. In the sixth week, the study drug was withdrawn after continuing adherence problems. She was later diagnosed to be coinfected with pulmonary tuberculosis, to which she later succumbed.
DISCUSSION
In this randomized, double-blind, placebo-controlled study, 100 mg of minocycline given orally every 12 hours had no significant effect over placebo in the improvement of cognitive function in ART-naive, HIV-positive patients. The drug, however, was safe and well tolerated. Apart from the delayed verbal recall test, there were no differences detected among the 2 arms in the change scores for the secondary outcome measures of neuropsychological test performance. The level of functional improvement between the 2 study arms was similar. These results are consistent with a second trial of minocycline for the treatment of HIV-associated cognitive impairment recently performed in HIV-positive individuals with neurocognitive impairment on cART.15
Cognitive impairment as seen in the study population continues to be an important manifestation of HIV infection, affecting 40% to 60% of HIV seropositive individuals even after the initiation of highly active antiretroviral therapy.19 The cognitive impairment observed in this particular study group could also have been due to non-HIV comorbid infections or conditions. Hepatitis C infection is one such condition but it is rare among HIV-positive individuals in Uganda, with a prevalence of 3%,20,21 and thus an unlikely cause for the observations made. Tuberculosis with CNS involvement can affect the level of consciousness and consequently the level of cognitive function.22 However, the screening criteria for this particular study excluded all subjects who had evidence of an active tuberculous infection. Nutritional deficiencies including micronutrients can be a primary causative factor in HIV disease progression, thereby contributing to cognitive dysfunction.23 The population in this study, however, was mostly observed to be adequately nourished.
There are usually differences in response to pharmacologic agents among males and females. Our study population was mostly female and thus the low number of males may have obscured sex effects of performance on tests as well as the effects of the investigational drug.
Other factors such as depression or side effects from medications have also been associated with cognitive impairment.24 We screened for depression with the Center for Epidemiologic Studies Depression Scale, and the study sample had equal distribution of individuals with the same range of symptoms within the placebo and minocycline arms. All study participants were prescribed trimethoprim-sulfamethoxazole for prophylaxis in compliance with Uganda treatment guidelines. This drug has no known effects on cognitive function.
The early penetration of the HIV virus into brain tissue leads to sequestration of the virus,25 and therefore the minocycline dose of 200 mg/d used in the study may have been inadequate to change inflammatory events or provide neuroprotective properties within the CNS even though minocycline has relatively good brain penetration.26,27 In addition, neuronal injury or dysfunction occurs early in HIV infection, and may be unresponsive to potential antiinflammatory or neuroprotective effects of minocycline started during late-stage HIV infection. Surrogate markers such as neuroimaging or CSF biomarkers may be needed to evaluate early evidence of neuroprotection within the CNS rather than the clinical neuropsychological tests and functional outcomes used in this evaluation. The duration of observation for cognitive change was relatively short; evaluations conducted for a longer period of time could possibly yield different results. The neurocognitive testing was conducted 3 times in the 24 weeks, and this may have resulted in practice effects that may have contributed to the improvement in both groups and thus may have obscured differences between the minocycline and placebo groups. Unfortunately, this kind of evaluation was not designed for this study. Interestingly, the probability of individuals not experiencing signs and symptoms of “any cause” was higher in the minocycline arm compared with placebo by week 24. The signs and symptoms included any clinical event such as cough, fever, and nausea, among others, and the patients seemed to fare better on the treatment. This may be secondary to the antiinflammatory properties offered by the drug.28
Minocycline has been shown to have a neuroprotective effect in animal models for a variety of neurologic conditions.13,29 In humans, the drug has been shown to have therapeutic benefit in clinical trials of multiple sclerosis and stroke.10,30 However, in a study of 412 individuals with amyotrophic lateral sclerosis, participants taking minocycline deteriorated more quickly as measured by the amyotrophic lateral sclerosis functional rating scale of gross and fine motor tasks, bulbar function, and respiratory function compared with those receiving placebo.31
Supplementary Material
ACKNOWLEDGMENT
The authors thank Alice Namudde, Hope Mackline, and Charles Ssebunya for the data collection. The authors extend their gratitude to the study participants for their willingness to participate in the study.
GLOSSARY
- cART
combination antiretroviral therapy
- CI
confidence interval
- DSMB
Data Safety and Monitoring Board
- IADL
Instrumental Activities of Daily Living
- RCI
reliable change index
- U NP Sum
Uganda Neuropsychological Test Battery Summary Measure
- WHO-UCLA
World Health Organization–University of California at Los Angeles
Footnotes
Go to Neurology.org for full disclosures. Funding information and disclosures deemed relevant by the authors, if any, are provided at the end of the article.
AUTHOR CONTRIBUTIONS
N. Nakasujja contributed to the design, conduction of the study, interpretation of data, and manuscript preparation. S. Miyahara, S. Evans, and A. Lee contributed to the data analysis, interpretation of the data and manuscript writing. S. Musisi and E. Katabira contributed to the conduction of the study, interpretation of data, and manuscript writing. K. Robertson and A. Ronald contributed to the design of the study, interpretation of data, and manuscript writing. D.B. Clifford contributed to the conduction of the study, interpretation of data, and manuscript writing. N. Sacktor contributed to the design and conduction of the study, interpretation of data, and manuscript writing.
STUDY FUNDING
The study was supported by the Neurologic AIDS Research Consortium, NS32228.
DISCLOSURE
N. Nakasujja receives support from the NIH (NIMH, NICHD). S. Miyahara reports no disclosures. S. Evans receives support from the NIH and has had consultancies (e.g., DSMB, educational presentations, advisory roles) with Millenium, Pfizer, Averion, Massachusetts General Hospital, Roche, Harvard Clinical Research Institute, IMPAACT, InterMune, HANC, Genentech, HNRC, CIS Biotech, Affymax, FzioMed, Novartis, Alcon, Sunovion, Boehringer-Ingelheim, ASA, ANA, BIDMC, Osaka University, Statistical Society of Australia, Interfarma, BASS, Society for Clinical Trials, FDA, PERI, Harvard Medical School, the Graybill Conference, the Deming Conference on Applied Statistics, MBSW, New Jersey Chapter of ASA, NIH, Merck, Schering-Plough, and the Center for Novel Therapeutics in HIV-Associated Cognitive Disorders (Johns Hopkins). A. Lee receives support from NIH (NIAID). S. Musisi, E. Katabira, and K. Robertson receive support from NIH (NIMH). A. Ronald reports no disclosures. D.B. Clifford serves on Data Safety Boards for Biogen, GSK, Millennium, Genzyme, Genentech, and Pfizer. He has been a consultant to Amgen, Brinker Biddle and Reath (PML Consortium), Genentech, Genzyme, Bristol-Myers Squibb, Johns Hopkins University, Millennium, Biogen Idec, Janssen, and Pfizer. He has received research support from Biogen Idec, NeurogesX, Tibotec, and Pfizer. He has received speaking fees from University of Kentucky and CMSC/ACTRIMS. N. Sacktor receives support from the NIH (NIMH, NINDS, NIA, NIAID). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Sacktor N, Nakasujja N, Skolasky R, et al. Antiretroviral therapy improves cognitive impairment in HIV+ individuals in sub-Saharan Africa. Neurology 2006;67:311–314 [DOI] [PubMed] [Google Scholar]
- 2.Gendelman HE, Zheng J, Coulter CL, et al. Suppression of inflammatory neurotoxins by highly active antiretroviral therapy in human immunodeficiency virus-associated dementia. J Infect Dis 1998;178:1000–1007 [DOI] [PubMed] [Google Scholar]
- 3.Sacktor N, Lyles RH, Skolasky RL, et al. Combination antiretroviral therapy improves psychomotor speed performance in HIV+ homosexual men. Neurology 1999;52:1640–1647 [DOI] [PubMed] [Google Scholar]
- 4.Marra CM, Zhao Y, Clifford DB, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS 2009;23:1359–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uganda AIDS Commission Moving Towards Universal Access: National HIV & AIDS Strategic Plan 2007/8-2011/12. Kampala: Uganda AIDS Commission; 2007 [Google Scholar]
- 6.Wong MH, Robertson K, Nakasujja N, et al. Frequency of and risk factors for HIV dementia in an HIV clinic in sub-Saharan Africa. Neurology 2007;68:350–355 [DOI] [PubMed] [Google Scholar]
- 7.Ministry of Finance Millennium Development Goals Report for Uganda 2010. Kampala: Ministry of Finance Planning and Economic Development; 2010 [Google Scholar]
- 8.UNAIDS WHO AIDS Epidemic Update. Geneva: UNAIDS; 2010 [Google Scholar]
- 9.Zink MC, Uhrlaub J, DeWitt J, et al. Neuroprotective and anti-human immunodeficiency virus activity of minocycline. JAMA 2005;293:2003–2011 [DOI] [PubMed] [Google Scholar]
- 10.Lampl Y, Boaz M, Gilad R, et al. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology 2007;69:1404–1410 [DOI] [PubMed] [Google Scholar]
- 11.Kloppenburg M, Brinkman BM, de Rooij-Dijk HH, et al. The tetracycline derivative minocycline differentially affects cytokine production by monocytes and T lymphocytes. Antimicrob Agents Chemother 1996;40:934–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA 1998;95:15769–15774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Y, Ma Z, Lin S, et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc Natl Acad Sci USA 2001;98:14669–14674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin S, Zhang Y, Dodel R, Farlow MR, Paul SM, Du Y. Minocycline blocks nitric oxide-induced neurotoxicity by inhibition p38 MAP kinase in rat cerebellar granule neurons. Neurosci Lett 2001;315:61–64 [DOI] [PubMed] [Google Scholar]
- 15.Sacktor N, Miyahara S, Deng L, et al. Minocycline treatment for HIV-associated neurocognitive disorders: results from a randomized trial. Neurology 2011;77:1135–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sacktor NC, Wong M, Nakasujja N, et al. The International HIV Dementia Scale: a new rapid screening test for HIV dementia. AIDS 2005;19:1367–1374 [PubMed] [Google Scholar]
- 17.Radloff LS. The CES-D scale: a self report depression scale for research in the general population. Appl Psychol Meas 1977;3:385–401 [Google Scholar]
- 18.Robertson KR, Nakasujja N, Wong M, et al. Pattern of neuropsychological performance among HIV positive patients in Uganda. BMC Neurol 2007;7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robertson K, Smurzynski M, Parsons TD, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 2007;21:1915–1921 [DOI] [PubMed] [Google Scholar]
- 20.Farag N, Rashed H, Hassan M, et al. Hepatitis C infection, cognition, and inflammation in an Egyptian sample. J Med Virol 2011;83:261–266 [DOI] [PubMed] [Google Scholar]
- 21.Walusansa V, Kagimu M. Screening for hepatitis C among HIV positive patients at Mulago Hospital in Uganda. Afr Health Sci 2009;9:143–146 [PMC free article] [PubMed] [Google Scholar]
- 22.Vinnard C, Macgregor RR. Tuberculous meningitis in HIV-infected individuals. Curr HIV/AIDS Rep 2009;6:139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldewicz T, Brouwers P, Goodkin K, Kumar A, Kumar M. Nutritional contributions to the CNS pathophysiology of HIV-1 infection and implications for treatment. CNS Spectr 2000;5:61–72 [DOI] [PubMed] [Google Scholar]
- 24.Nakasujja N, Skolasky RL, Musisi S, et al. Depression symptoms and cognitive function among individuals with advanced HIV infection initiating HAART in Uganda. BMC Psychiatry 2009;10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Destache CJ. Brain as an HIV sequestered site: use of nanoparticles as a therapeutic option. In: Sharma HS, editor. Progress in Brain Research, Volume 180. New York: Elsevier; 2009:225–233 [DOI] [PubMed] [Google Scholar]
- 26.Brogden R, Speight T, Avery G. Minocycline: a review of its antibacterial and pharmacokinetic properties and therapeutic use. Drugs 1975;9:251–291 [DOI] [PubMed] [Google Scholar]
- 27.Si Q, Cosenza M, Kim MO, et al. A novel action of minocycline: inhibition of human immunodeficiency virus type 1 infection in microglia. J Neurovirol 2004;10:284–292 [DOI] [PubMed] [Google Scholar]
- 28.Szeto GL, Brice AK, Yang HC, Barber SA, Siliciano RF, Clements JE. Minocycline attenuates HIV infection and reactivation by suppressing cellular activation in human CD4+ T cells. J Infect Dis 2010;201:1132–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yong VW, Wells J, Giuliani F, Casha S, Power C, Metz LM. The promise of minocycline in neurology. Lancet Neurol 2004;3:744–751 [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Metz LM, Yong VW, et al. Pilot study of minocycline in relapsing-remitting multiple sclerosis. Can J Neurol Sci 2008;35:185–191 [DOI] [PubMed] [Google Scholar]
- 31.Gordon PH, Moore DH, Miller RG, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol 2007;6:1045–1053 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


