Abstract
Objective:
The effectiveness of anthelminthic and corticosteroid drug therapy in parenchymal neurocysticercosis is well established. The treatment of parenchymal solitary cysticercus granuloma (SCG), however, remains controversial. We attempted to obtain a consistent estimate of the efficacy of anthelminthic and corticosteroid drug treatment in SCG.
Methods:
Randomized-controlled trials (RCTs) comparing rates of seizure freedom, granuloma resolution, and residual calcification in individuals with SCG treated with anthelminthic or corticosteroid drugs with those treated with antiepileptic drugs (AEDs) alone were systematically reviewed and quantified using fixed- or random-effects meta-analysis.
Results:
Fifteen RCTs were identified for inclusion. Ten RCTs assigned 765 people with SCG to AED treatment with or without anthelminthic drug (albendazole) treatment. A further 5 RCTs assigned 457 people with SCG to AED treatment with or without corticosteroid drugs. Anthelminthic treatment was associated with significantly increased rates of seizure freedom (nonevent odds ratio: 2.45; 95% confidence interval: 1.49–4.03; p = 0.0004) and significantly higher rates of granuloma resolution (odds ratio: 2.09; 95% confidence interval: 1.41–3.00; p = 0.0003), but did not alter the risk of residual calcification. Corticosteroid treatment was not significantly associated with any outcome.
Conclusions:
Anthelminthic treatment with albendazole provides improved rates of seizure freedom and hastens resolution of the granuloma. The role of corticosteroid treatment remains uncertain. The benefits (or lack thereof in the case of corticosteroids) are consistent when measured across different time points after treatment.
Neurocysticercosis is a common parasitic infestation of the CNS and is possibly the most common risk factor for acquired epilepsy worldwide.1 The infestation is widespread in most resource-poor countries.2 It is also being diagnosed with increasing frequency in high-income countries, especially in those with a large number of immigrants, such as the United States.2–7
Seizures are the most common manifestation of neurocysticercosis and are caused by the helminthic larvae which, on entering the brain, form cysts. These cysticerci subsequently degenerate, triggering a local host inflammatory response, and eventually resolve with or without calcification. The severity of brain infestation ranges from a single lesion to hundreds.8,9 Treatment differs for multiple and solitary parenchymal cysticercosis as well as for different stages of the cysticerci in the parenchyma. There is evidence to support the use of anthelminthic treatment together with corticosteroids in individuals presenting with multiple viable cysts.10–13 The roles of both anthelminthics and corticosteroids in the treatment of solitary cysticercus granuloma (SCG), however, remain uncertain.8 Several randomized-controlled trials (RCTs) of anthelminthic drugs and corticosteroids have been conducted in SCG, but most were small. We systematically reviewed all available RCTs of anthelminthic drugs (either albendazole or praziquantel, the only 2 anthelminthics available; alone or with corticosteroids) and corticosteroids alone (without anthelminthic treatment) in SCG. We sought to identify the best strategy for treatment of SCG.
METHODS
Information sources and search
RCTs were identified by searching electronic databases and reference lists of articles with no time or language restriction. Detailed information on the databases used and the search strategies is provided in appendix e-1 on the Neurology® Web site at www.neurology.org. The eligibility criteria for the RCT inclusion are provided in appendix e-2.14,15
Methods of analysis
All data were entered in RevMan version 5.1.6 for analysis (http://ims.cochrane.org/revman). Significant heterogeneity between studies was defined by p value <0.1 using the χ2 test or an I2 >50%. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using the Mantel-Haenszel method, followed by fitting fixed- and random-effects models. We used the random-effects model results if there was evidence for heterogeneity between RCTs, because this provides a more conservative effect than the fixed-effects model.
RESULTS
RCT selection
The search identified 235 citations (figure 1). After adjusting for duplicates, 200 remained. We excluded 169 because they were reviews, case reports, letters to editor, editorials, case series, retrospective studies, nonparenchymal neurocysticercosis studies, or active comparator trials (comparing 2 agents, e.g., albendazole with praziquantel). Full texts of the remaining 31 were reviewed; 16 were excluded because these contained data previously published,16–18 4 RCTs studied agents other than anthelminthics or corticosteroids,19–22 5 included patients with multiple enhancing or cystic lesions,13,23–26 and 4 had no control group for comparison.27–30 Eventually, 10 RCTs, involving 765 subjects with SCG, were included in the meta-analysis of anthelminthic treatment.31–40 In one of these, some subjects had 2 rather than a single enhancing lesion.36 We chose to include this trial because the proportion of subjects with 2 lesions was small (18%). Five RCTs, involving 457 people with SCG, were included in the meta-analysis of corticosteroid treatment.e1-e5
Figure 1. Flow chart of the literature search.
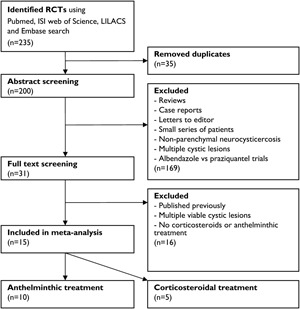
Flow chart of the literature search and identification of randomized-controlled trials (RCTs) included in this study.
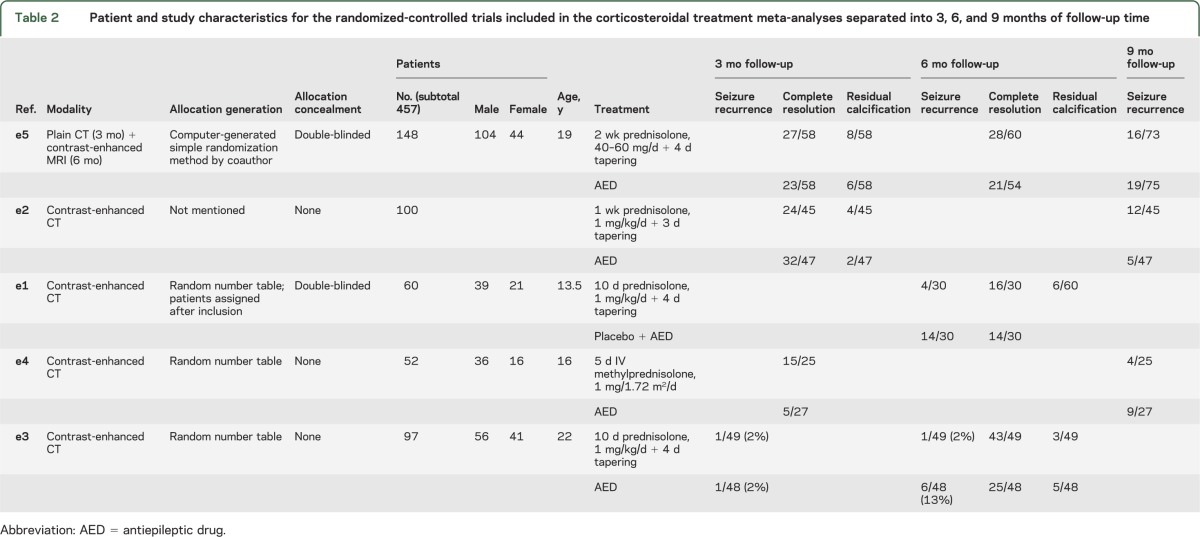
Because the outcome assessment was performed after different periods of follow-up (mostly 3, 6, 9, and 12 months after intervention) in the various trials and resolution rates for SCG are directly related to the duration of follow-up,21 the meta-analyses for each of the outcome parameters (including seizure freedom during follow-up, complete granuloma resolution, and residual calcification) were undertaken separately for 3, 6, 9, and 12 months of follow-up. A combined analysis regardless of the time point of outcome assessment was also undertaken for both agents and all outcomes. Features of the included RCTs are provided in tables 1 and 2.
Table 1.
Patient and study characteristics for the randomized-controlled trials included in the anthelminthic treatment meta-analyses separated into 3, 6, and 12 months of follow-up time
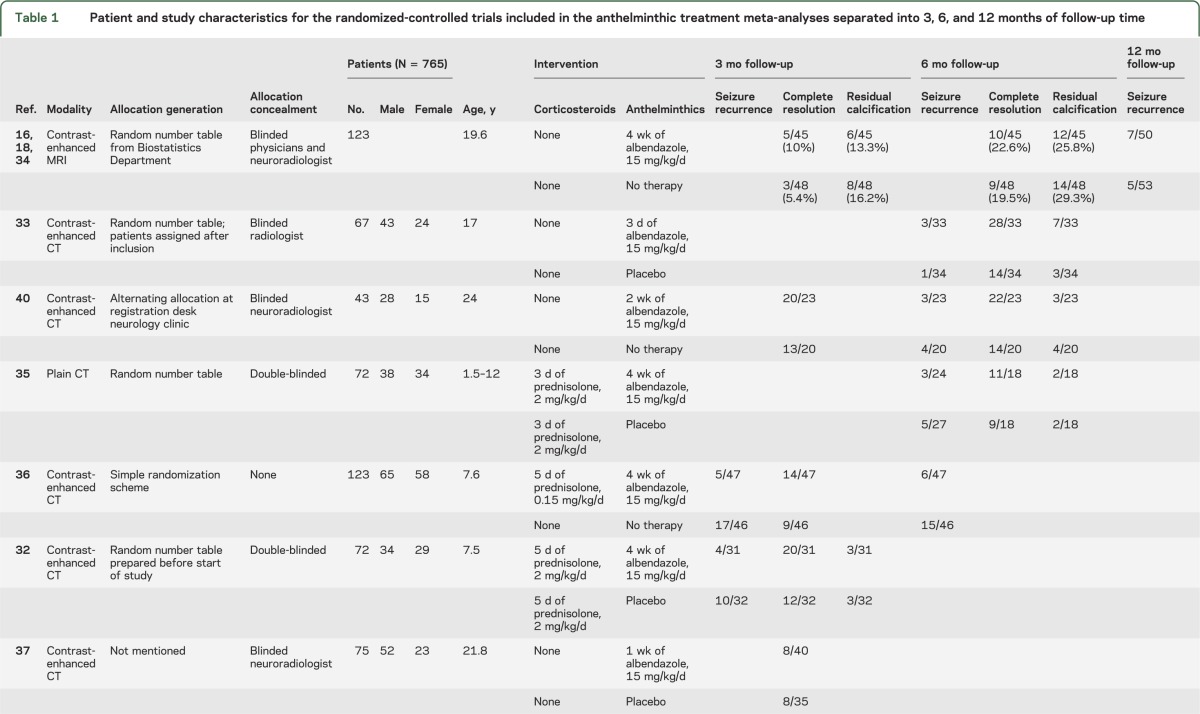
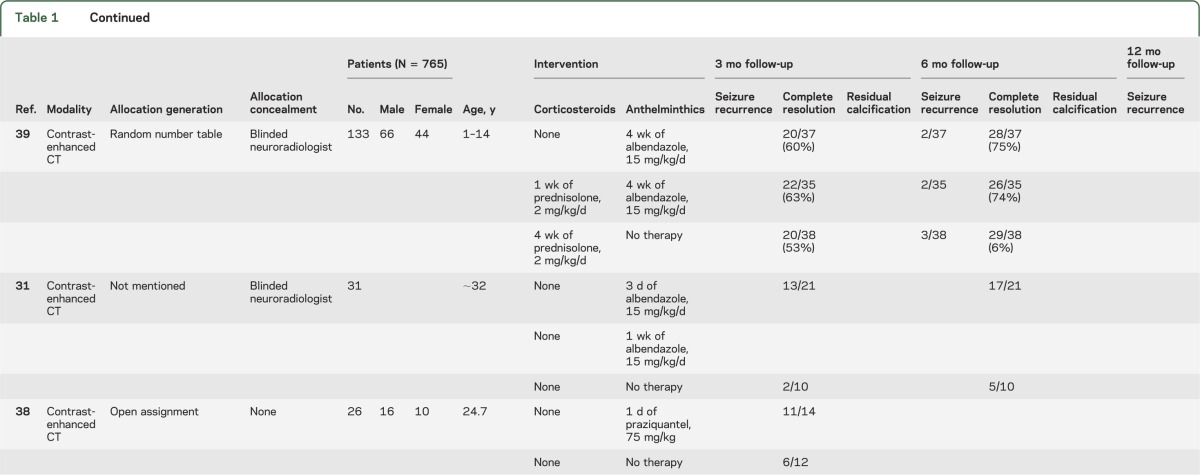
Table 2.
Patient and study characteristics for the randomized-controlled trials included in the corticosteroidal treatment meta-analyses separated into 3, 6, and 9 months of follow-up time
Seizure freedom with anthelminthic treatment
The proportion of subjects with seizure recurrence on antiepileptic drugs (AEDs) after therapeutic intervention with anthelminthic drugs was reported for 3-month follow-up in 2 RCTs,32,36 6-month follow-up in 5 RCTs,33,35,36,39,40 and 12-month follow-up in 1 RCT.34 This information could not be extracted from one of the reports but was obtained after contacting the authors.34
The proportion of subjects who remained seizure-free was significantly higher in the anthelminthic-treated group compared with controls for 3 months follow-up (nonevent OR [i.e., 1/OR for seizure recurrence]: 4.05; 95% CI: 1.76–9.33; p = 0.001; I2 = 0%), but not for 6 months (nonevent OR: 1.79; 95% CI: 0.95–3.38; p = 0.45; I2 = 0%) or 12 months (nonevent OR: 0.64; 95% CI: 0.19–2.17; p = 0.47; heterogeneity not applicable) (figure 2A).
Figure 2. Forest plots of anthelminthic therapy.
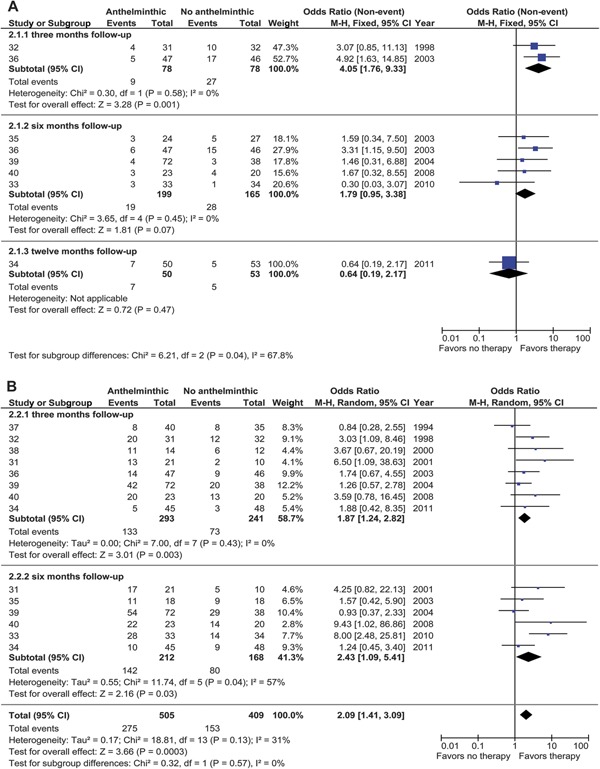
(Nonevent) odds ratios (ORs) of seizure freedom (A) and granuloma resolution (B) in patients with parenchymal solitary cysticercus granuloma treated with anthelminthics. The vertical bar indicates no difference between anthelminthic and no anthelminthic therapy. Point estimated ORs are given as squares and scaled proportional to the study's contribution to the (sub)total. Horizontal lines indicate the 95% OR (95% confidence interval [CI]) obtained by fixed- and random-effects analysis. Diamonds indicate the pooled OR 95% CI. df = degrees of freedom; M-H = Mantel-Haenszel.
Pooled analysis of the data from various RCTs reporting outcome at 3, 6, and 12 months of follow-up revealed significant heterogeneity (I2 = 68%); no heterogeneity was found among trials restricted to 3 and 6 months (I2 = 1%). Meta-analysis of the pooled data from trials reporting outcome at 3 and 6 months revealed significantly higher seizure-freedom rates in the pooled anthelminthic-treated group compared with the pooled controls (nonevent OR: 2.45; 95% CI: 1.49–4.03; p = 0.0004). One RCT in this pooled analysis reported seizure outcome both at 3 and 6 months,36 but removal of this did not substantially affect the pooled nonevent OR (2.00, 95% CI 1.14–3.52, p = 0.02, I2 = 0%; and 2.24, 95% CI 1.27–3.95, p = 0.005, I2 = 12% at 3 and 6 months, respectively).
Granuloma resolution with anthelminthic treatment
Seven RCTs reported rates of granuloma resolution based on contrast-enhanced CT scan and 1 on MRI performed at 3 months after intervention.31,32,34,36–40 Five RCTs reported resolution at 6 months follow-up time using CT31,33,35,39,40 or MRI acquisition.34 All CT studies but one35 used contrast-enhanced acquisition. The MRI study included gadolinium-enhanced images.34
We found significant heterogeneity among RCTs at the 6-month follow-up time point (χ2 p = 0.04; I2 = 57%); therefore, random-effects analysis was used to pool the rates of granuloma resolution in the RCTs (figure 2B).
Separate analysis at 3 and 6 months follow-up time showed a significantly higher rate of granuloma resolution in the anthelminthic group both at 3 months (OR: 1.87; 95% CI: 1.24–2.82; p = 0.003; I2 = 0%) and 6 months (OR: 2.43; 95% CI: 1.09–5.41; p = 0.03; I2 = 57%). Four of the RCTs reported granuloma resolution rates at multiple time points of follow-up and hence had a repeated-measures design.31,34,39,40
A significantly higher rate of granuloma resolution was observed with anthelminthic treatment in the pooled analysis of data from 3 and 6 months (OR: 2.09; 95% CI: 1.41–3.09; p = 0.0003; I2 = 31%).
When trials with repeated-measures design were excluded from analysis, the ORs for granuloma resolution remained increased in favor of anthelminthic treatment both at 3 months (OR: 2.25; 95% CI: 1.43–3.52; p = 0.0004; I2 = 12% (data removed from 6 months) and 6 months (OR: 2.10; 95% CI: 1.28–3.47; p = 0.004; I2 = 41%).
Residual calcification with anthelminthic treatment
Five RCTs reported residual calcification on follow-up imaging as one of the outcomes.32–35,40 Two RCTs reported residual calcification at 3 months follow-up,32,34 and 4 reported the outcome parameter at 6 months follow-up.33–35,40
No significant heterogeneity among the RCTs was detected (I2 = 0%). We found no significant difference between the treated and control subjects regarding the proportion of subjects with residual calcifications in the pooled analysis using fixed-effects analysis (OR: 1.00; 95% CI: 0.59–1.70; p = 0.99).
Seizure freedom with corticosteroid treatment
One corticosteroid treatment RCT reported rates of seizure recurrence over 3 months of follow-up,e3 2 over 6 months,e1,e3 and 3 over 9 months follow-up time.e2,e4,e5 We used random-effects analysis to test differences between the pooled treated and controls because significant heterogeneity was present in the overall analysis (I2 = 64%). One RCT appeared twice in the pooled analysis because of its repeated-measures design.e3
The single RCT reporting seizure recurrence over 3 months of follow-up time showed no significant difference in the rates of seizure recurrence between corticosteroid-treated and control subjects (nonevent OR: 1.02; 95% CI: 0.06–16.81; I2 not applicable) (figure 3A). At 6 months, a significantly lower rate of seizure recurrence was found in the corticosteroid-treated group (nonevent OR: 5.97; 95% CI: 1.99–17.88; p = 0.001; I2 = 0%). No significant differences between the treated and control subjects were found in the 3 RCTs that followed subjects up to 9 months (nonevent OR: 0.99; 95% CI: 0.34–2.85; I2 = 66%). We did not pool subgroups because of between-trials subgroup heterogeneity (I2 = 64.7%).
Figure 3. Forest plots of corticosteroids.
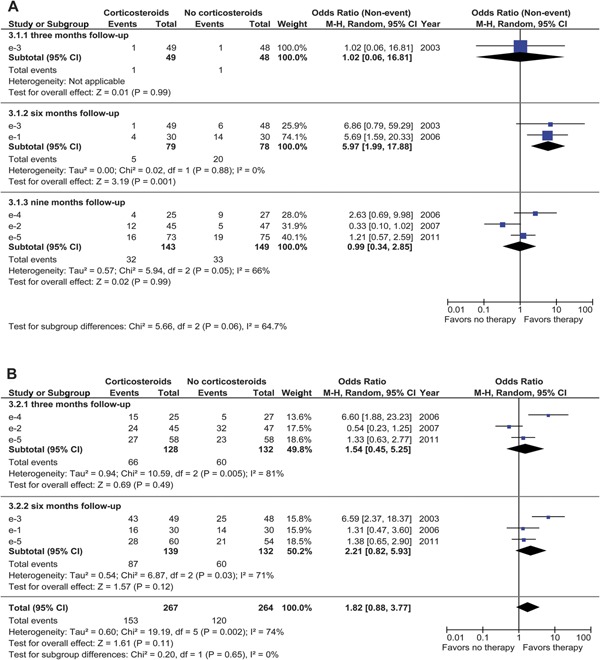
(Nonevent) odds ratios (ORs) of seizure freedom (A) and granuloma resolution (B) in patients with parenchymal solitary cysticercus granuloma treated with corticosteroids. The vertical bar indicates no difference between corticosteroids and no corticosteroids. Point estimated ORs are given as squares and scaled proportional to the study's contribution to the (sub)total. Horizontal lines indicate the 95% confidence interval (CI) obtained by random-effects analysis. Diamonds indicate the pooled OR 95% CI. df = degrees of freedom; M-H = Mantel-Haenszel.
Granuloma resolution with corticosteroid treatment
Five RCTs measured the effect of treatment with a short course of corticosteroids alone (standard AED treatment) on granuloma resolution.e1–e5 Three RCTs reported rates of granuloma resolution at 3 monthse2,e4,e5 and 3 at 6 monthse1,e3,e5 of follow-up time. One RCT used a repeated-measures design with different modalities, CT at 3 months and MRI at 6 months.e5
A random-effects analysis (performed because of significant heterogeneity; I2 = 74%) did not reveal any significant difference in the rates of granuloma resolution over 3 and 6 months as well as in the overall combined analysis (OR: 1.82; 95% CI: 0.88–3.77; p = 0.11) (figure 3B). This nonsignificant pooled effect remained using a sensitivity analysis when the repeated-measures data were excluded from the 3-month analysis (OR: 1.99; 95% CI: 0.79–5.04; p = 0.15) and the 6-month analysis (OR: 1.98; 95% CI: 0.78–5.00; p = 0.15).
Residual calcification with corticosteroid treatment
Three RCTs of corticosteroid in SCG reported rates of residual calcification on follow-up imaging studies.e2,e3,e5 The proportions of subjects with residual calcification on follow-up imaging were similar in the 2 pooled groups using fixed-effects analysis (OR: 1.18; 95% CI: 0.54–2.56; p = 0.68; I2 = 0%). Subgroup heterogeneity was not significant (p = 0.25; I2 = 25.7%).
DISCUSSION
The treatment of SCG mainly comprises the use of 3 different classes of drugs, i.e., AEDs, anthelminthic drugs (albendazole and praziquantel), and corticosteroids. Using AEDs to prevent seizures is standard practice. The use of anthelminthic agents and corticosteroids (alone and in combination with anthelminthic agents) is based on sound rationale of the biology, natural history, and symptomatology of the cysticercus located in the brain parenchyma and previous experience of their use in live, active cysticerci.13
The efficacy of anthelminthic drugs in live, vesicular, and active parenchymal cysticercosis has been established.10–12 These drugs were originally used to treat live, active cysticercosis but their use was later extended to treatment of SCG based on several small, uncontrolled trials.e6–e8 The earliest RCT of anthelminthic treatment (albendazole) in SCG found no difference in the frequency of resolution of the granuloma at 3 months among those who were treated with albendazole compared with those who were treated with nonspecific therapy (i.e., AEDs).37 Since this initial RCT, several RCTs of anthelminthic treatment in SCG have been undertaken, providing conflicting results.32,35,36 A meta-analysis of RCTs of albendazole in people with 1–2 parenchymal granuloma was reported in 2006.11 It failed to demonstrate a better resolution rate with the use of albendazole (OR: 1.18; 95% CI: 0.82–1.71; p = 0.38). A recalculation after excluding 1 trial with an outlier OR, however, suggested that albendazole improved resolution of the granuloma (OR: 1.93; 95% CI: 1.21–3.08; p = 0.006). The meta-analysis pooled various RCTs regardless of follow-up period and the time period of outcome assessment, which varied from 3 to 15 months. Since this meta-analysis, more RCTs have been undertaken.18,31,33,38,40 This prompted us to undertake the current study. It is also different from the previous study because we analyzed pooled data according to the time of outcome assessment in the component RCTs (i.e., 3, 6, and 12 months). The rationale for analyzing resolution rates in the pooled data according to the follow-up period after which outcome assessment was performed is that rates of natural resolution (without intervention) vary as a function of time period of follow-up. One prospective study of 210 people with an SCG followed-up after their first seizure estimated resolution rates on follow-up CT of 19% at 3 months, 36% at 6 months, and 63% at 1 year.21 Thus, the chances of resolution of the SCG increase with longer follow-up. Compared with the earlier meta-analysis, our study conclusively established the benefit of anthelminthic treatment with albendazole both at 3 and 6 months and in the overall pooled analysis.
The benefits of anthelminthic treatment were also evident when seizure freedom was used as an outcome because the proportions of subjects who remained seizure-free over 3 and 6 months of follow-up were significantly higher in the albendazole-treated group (figure 2A). Because SCGs might resolve with or without calcification and the presence of residual calcification is associated with the occurrence of seizures, especially in the long-term,e9,e10 we also analyzed the effect of albendazole treatment on the frequency of residual calcification on follow-up imaging. There was no evidence, however, for either an increased or decreased risk of residual calcification associated with anthelminthic treatment in the pooled analysis.
It may be concluded that although the treatment of degenerating cysticercus granulomas is counterintuitive from a theoretical (biological) point of view because the cysts are already being destroyed by the host immune system, the significantly improved resolution rate of the granuloma and better seizure-freedom rates with albendazole treatment suggest that anthelminthics might hasten the involution of the granuloma as well as offer the clinical benefit of improved possibility of being seizure-free.
This is the first meta-analysis of corticosteroid treatment in SCG. The administration of a short course of corticosteroids in conjunction with anthelminthic treatment to control edema and symptoms due to the host inflammatory response to neurocysticercosis is common practice. Likewise, anecdotal observations of the benefits of corticosteroid treatment regarding amelioration of certain manifestations (including seizures and headache) attributable to the inflammatory degeneration of SCG prompted several RCTs of a short course of corticosteroids (typically 2 weeks) alone (i.e., with AEDs but without anthelminthic therapy) in people with this type of neurocysticercosis.e1–e4 We identified 5 RCTs of a short course of corticosteroids in subjects with SCG for this meta-analysis. Nearly all of the RCTs included small numbers of people. The trials also differed in treatment protocols used (e.g., the corticosteroid agent used, route of administration, and timing of treatment) and design (times of follow-up and outcome assessment). Some, but not all, RCTs suggested benefits of corticosteroid administration. We found no overall significant difference in the rates of seizure freedom, granuloma resolution, or residual calcification between the corticosteroid-treated and control subjects. This lack of benefit of corticosteroid treatment on various outcomes is an important finding in guiding treatment policies for SCG. Theoretically, administration of corticosteroids alone, which is purported to suppress host inflammatory response to the degenerating SCG, is counterintuitive and in conflict with the practice of using albendazole in SCG. The latter hastens resolution by promoting degeneration of the SCG and has clearly been shown to improve outcome. Corticosteroids are useful in the management of inflammatory symptoms in multiple neurocysticercosis, but the lack of benefit in solitary lesions as we have shown might be due to a milder inflammatory response triggered by a single degenerating cysticercus as opposed to many. Whether using a short (2-week) course of corticosteroids would be expected to have any effect on seizure recurrence and granuloma resolution in the long-term (i.e., over several months) is a matter of speculation.
Our study has limitations. Many of the RCTs involved small numbers. There were also considerable differences among trials in the population sampled (differing selection criteria [some included only children] and differing time between first seizure and treatment onset), treatment protocols (choice, dose, timing, and duration of treatment), and study design (timing and methods of outcome assessment and duration of follow-up, AED protocols, and documentation of seizures). Hence, the results were prone to heterogeneity because of selection of participants from different study populations and the variable delay between infection and treatment across various studies.27 The period of follow-up in the trials was also variable, which increased heterogeneity in the pooled analysis, although we tried to separate the early from the later follow-up time points. The quality of some of the RCTs regarding reporting of randomization techniques, outcome assessment, and blinding was poor. Most trials used CT for outcome assessment and its limitations must be considered. A single enhancing lesion on CT might encompass different stages of an involuting cysticercus including a cystic (or late vesicular or colloidal) or nodular (granulomatous) stage. There is evidence from trials of multiple neurocysticercosis that cystic lesions respond better to albendazole than granular-nodular stages. MRI offers the advantage of being able to differentiate better between cystic and granular cysticerci and therefore provide insights into effects of treatment on subgroups divided according to the stage of SCG. To our knowledge, outcome assessment stratified according to the stage of the single parenchymal cysticercus has not been undertaken so far. An additional source of variability among studies could be the imaging modality, because 2 RCTs used MRI,34,e5 which has a different contrast-to-noise ratio from the CT scans used in the majority of the RCTs.
Despite limitations, our study provides support for the use of albendazole (with or without corticosteroids) in the treatment of SCG because it offers increased possibility of seizure freedom and improves rates of granuloma resolution. However, the pooled evidence does not confirm a beneficial effect of the administration of corticosteroids alone in the treatment of SCG. We recommend the use of albendazole (with or without corticosteroids) in the treatment of SCG.
Ideally, however, large, multicenter, multi-arm RCTs comparing the efficacy of albendazole (with and without corticosteroids) and corticosteroids with placebo are required to confirm our findings and to clarify the exact contributions of anthelminthic therapy, corticosteroids, or their combined regimen in long-term seizure recurrence, granuloma resolution, and residual calcification.
Supplementary Material
GLOSSARY
- AED
antiepileptic drug
- CI
confidence interval
- OR
odds ratio
- RCT
randomized-controlled trial
- SCG
solitary cysticercus granuloma
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
All authors contributed to the study design, data analysis, and data interpretation. W.M.O., M.S., and G.S. collected data. W.M.O., G.S., and J.W.S. drafted the report, tables, and figures. All authors critically revised the manuscript and agreed on the final version.
STUDY FUNDING
J.W.S. is supported by the Department of Health's NIHR Biomedical Research Centres funding scheme, the Epilepsy Society, and the Dr. Marvin Weil Epilepsy Research Fund, UK.
DISCLOSURE
W.M. Otte and M. Singla report no disclosures. J.W. Sander is based at UCL/UCLH, which is supported by the Department of Health's NIHR Biomedical Research Centres funding scheme, the Epilepsy Society, and the Dr. Marvin Weil Epilepsy Research Fund, UK. He has received research support, honoraria for consultancy, and travel compensation from UCB, Janssen-Cilag, GSK, and Eisai, and has served on advisory boards to GSK, UCB, and Viropharma. G. Singh reports no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Garcia HH, Gonzalez AE, Evans CA, Gilman RH. Taenia solium cysticercosis. Lancet 2003;362:547–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pal DK, Carpio A, Sander JW. Neurocysticercosis and epilepsy in developing countries. J Neurol Neurosurg Psychiatry 2000;68:137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croker C, Redelings M, Reporter R, Sorvillo F, Mascola L, Wilkins P. The impact of neurocysticercosis in California: a review of hospitalized cases. PLoS Negl Trop Dis 2012;6:e1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ong S, Talan DA, Moran GJ, et al. Neurocysticercosis in radiographically imaged seizure patients in U.S. emergency departments. Emerg Infect Dis 2002;8:608–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeGiorgio CM, Sorvillo F, Escueta SP. Neurocysticercosis in the United States: review of an important emerging infection. Neurology 2005;64:1486. [DOI] [PubMed] [Google Scholar]
- 6.Earnest MP, Reller LB, Filley CM, Grek AJ. Neurocysticercosis in the United States: 35 cases and a review. Rev Infect Dis 1987;9:961–979 [DOI] [PubMed] [Google Scholar]
- 7.Guerra LG, Cuetter AC. Neurocysticercosis in the southwestern United States. Am Fam Physician 1993;48:1223. [PubMed] [Google Scholar]
- 8.Singh G, Rajshekhar V, Murthy JM, et al. A diagnostic and therapeutic scheme for a solitary cysticercus granuloma. Neurology 2010;75:2236–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallin MT, Kurtzke JF. Neurocysticercosis in the United States: review of an important emerging infection. Neurology 2004;63:1559–1564 [DOI] [PubMed] [Google Scholar]
- 10.Abba K, Ramaratnam S, Ranganathan LN. Anthelmintics for people with neurocysticercosis. Cochrane Database Syst Rev 2010;1:CD000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Brutto OH, Roos KL, Coffey CS, Garcia HH. Meta-analysis: cysticidal drugs for neurocysticercosis: albendazole and praziquantel. Ann Intern Med 2006;145:43–51 [DOI] [PubMed] [Google Scholar]
- 12.Mazumdar M, Pandharipande P, Poduri A. Does albendazole affect seizure remission and computed tomography response in children with neurocysticercosis? A Systematic review and meta-analysis. J Child Neurol 2007;22:135–142 [DOI] [PubMed] [Google Scholar]
- 13.Garcia HH, Pretell EJ, Gilman RH, et al. A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. N Engl J Med 2004;350:249–258 [DOI] [PubMed] [Google Scholar]
- 14.Rajshekhar V. Etiology and management of single small CT lesions in patients with seizures: understanding a controversy. Acta Neurol Scand 1991;84:465–470 [DOI] [PubMed] [Google Scholar]
- 15.Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323:42–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Souza A, Nalini A, Kovoor JM, Yeshraj G, Siddalingaiah HS, Thennarasu K. Natural history of solitary cerebral cysticercosis on serial magnetic resonance imaging and the effect of albendazole therapy on its evolution. J Neurol Sci 2010;288:135–141 [DOI] [PubMed] [Google Scholar]
- 17.Baranwal AK, Singhi PD, Khandelwal N, Singhi SC. Morphometry of single small enhancing computed tomographic lesions: outcome and effect of albendazole therapy. J Trop Pediatr 2002;48:219–224 [DOI] [PubMed] [Google Scholar]
- 18.de Souza A, Thennarasu K, Yeshraj G, Kovoor JME, Nalini A. Randomized controlled trial of albendazole in new onset epilepsy and MRI confirmed solitary cerebral cysticercal lesion: effect on long-term seizure outcome. J Neurol Sci 2009;276:108–114 [DOI] [PubMed] [Google Scholar]
- 19.Gupta M, Agarwal P, Khwaja GA, et al. Randomized prospective study of outcome of short term antiepileptic treatment in small single enhancing CT lesion in brain. Neurol India 2002;50:145–147 [PubMed] [Google Scholar]
- 20.Kaushal S, Rani A, Chopra S, Singh G. Safety and efficacy of clobazam versus phenytoin-sodium in the antiepileptic drug treatment of solitary cysticercus granulomas. Neurol India 2006;54:157–160 [PubMed] [Google Scholar]
- 21.Rajshekhar V, Jeyaseelan L. Seizure outcome in patients with a solitary cerebral cysticercus granuloma. Neurology 2004;62:2236–2240 [DOI] [PubMed] [Google Scholar]
- 22.Verma A, Misra S. Outcome of short-term antiepileptic treatment in patients with solitary cerebral cysticercus granuloma. Acta Neurol Scand 2006;113:174–177 [DOI] [PubMed] [Google Scholar]
- 23.Carpio A, Kelvin EA, Bagiella E, et al. Effects of albendazole treatment on neurocysticercosis: a randomised controlled trial. J Neurol Neurosurg Psychiatry 2008;79:1050–1055 [DOI] [PubMed] [Google Scholar]
- 24.Das K, Mondal GP, Banerjee M, Mukherjee BB, Singh OP. Role of antiparasitic therapy for seizures and resolution of lesions in neurocysticercosis patients: an 8 year randomised study. J Clin Neurosci 2007;14:1172–1177 [DOI] [PubMed] [Google Scholar]
- 25.Garcia HH, Gilman RH, Horton J, et al. Albendazole therapy for neurocysticercosis: a prospective double-blind trial comparing 7 versus 14 days of treatment. Neurology 1997;48:1421–1427 [DOI] [PubMed] [Google Scholar]
- 26.Padma MV, Behari M, Misra NK, Ahuja GK. Albendazole in neurocysticercosis. Natl Med J India 1995;8:255–258 [PubMed] [Google Scholar]
- 27.Goel D, Mittal M, Bansal KK, Singhal A. Natural history of solitary cerebral cysticercosis cases after albendazole therapy: a longitudinal follow-up study from India. Acta Neurol Scand 2010;121:204–208 [DOI] [PubMed] [Google Scholar]
- 28.Kaur P, Dhiman P, Dhawan N, Nijhawan R, Pandit S. Comparison of 1 week versus 4 weeks of albendazole therapy in single small enhancing computed tomography lesion. Neurol India 2010;58:560–564 [DOI] [PubMed] [Google Scholar]
- 29.Medina MT, Genton P, Montoya MC, Cordova S, Dravet C, Sotelo J. Effect of anticysticercal treatment on the prognosis of epilepsy in neurocysticercosis: a pilot trial. Epilepsia 1993;34:1024–1027 [DOI] [PubMed] [Google Scholar]
- 30.Thussu A, Arora A, Lal V, Prabhakar S, Sawhney IM. Albendazole therapy for solitary persistent cysticercus granuloma. Neurol India 2001;49:95–97 [PubMed] [Google Scholar]
- 31.Alarcón F, Dueñas G, Diaz M, Cevallos N, Estrada G. Short course of albendazole therapy for neurocysticercosis: a prospective randomized trial comparing three days, eight days, and a control group without albendazole. Rev Ecuat Neurol 2001;10:1–2 [Google Scholar]
- 32.Baranwal AK, Singhi PD, Khandelwal N, Singhi SC. Albendazole therapy in children with focal seizures and single small enhancing computerized tomographic lesions: a randomized, placebo-controlled, double blind trial. Pediatr Infect Dis J 1998;17:696–700 [DOI] [PubMed] [Google Scholar]
- 33.Chaurasia RN, Garg RK, Agarwall A, et al. Three day albendazole therapy in patients with a solitary cysticercus granuloma: a randomized double blind placebo controlled study. Southeast Asian J Trop Med Public Health 2010;41:517–525 [PubMed] [Google Scholar]
- 34.de Souza A, Nalini A, Kovoor JM, Yeshraj G, Siddalingaiah HS, Thennarasu K. Perilesional gliosis around solitary cerebral parenchymal cysticerci and long-term seizure outcome: a prospective study using serial magnetization transfer imaging. Epilepsia 2011;52:1918–1927 [DOI] [PubMed] [Google Scholar]
- 35.Gogia S, Talukdar B, Choudhury V, Arora BS. Neurocysticercosis in children: clinical findings and response to albendazole therapy in a randomized, double-blind, placebo-controlled trial in newly diagnosed cases. Trans R Soc Trop Med Hyg 2003;97:416–421 [DOI] [PubMed] [Google Scholar]
- 36.Kalra V, Dua T, Kumar V. Efficacy of albendazole and short-course dexamethasone treatment in children with 1 or 2 ring-enhancing lesions of neurocysticercosis: a randomized controlled trial. J Pediatr 2003;143:111–114 [DOI] [PubMed] [Google Scholar]
- 37.Padma MV, Behari M, Misra NK, Ahuja GK. Albendazole in single CT ring lesions in epilepsy. Neurology 1994;44:1344–1346 [DOI] [PubMed] [Google Scholar]
- 38.Pretell EJ, Garcia HH, Custodio N, et al. Short regimen of praziquantel in the treatment of single brain enhancing lesions. Clin Neurol Neurosurg 2000;102:215–218 [DOI] [PubMed] [Google Scholar]
- 39.Singhi P, Jain V, Khandelwal N. Corticosteroids versus albendazole for treatment of single small enhancing computed tomographic lesions in children with neurocysticercosis. J Child Neurol 2004;19:323–327 [DOI] [PubMed] [Google Scholar]
- 40.Thussu A, Chattopadhyay A, Sawhney IMS, Khandelwal N. Albendazole therapy for single small enhancing CT lesions (SSECTL) in the brain in epilepsy. J Neurol Neurosurg Psychiatry 2008;79:272–275 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


