Abstract
Objective:
To determine the frequency and correlates of impulse control and related behavior symptoms in patients with de novo, untreated Parkinson disease (PD) and healthy controls (HCs).
Methods:
The Parkinson's Progression Markers Initiative is an international, multisite, case-control clinical study conducted at 21 academic movement disorders centers. Participants were recently diagnosed, untreated PD patients (n = 168) and HCs (n = 143). The outcome measures were presence of current impulse control and related behavior symptoms based on recommended cutoff points for the Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease (QUIP)-Short Form.
Results:
There were 311 participants with complete QUIP data. Frequencies of impulse control and related behavior symptoms for patients with PD vs HCs were as follows: gambling (1.2% vs 0.7%), buying (3.0% vs 2.1%), sexual behavior (4.2% vs 3.5%), eating (7.1% vs 10.5%), punding (4.8% vs 2.1%), hobbyism (5.4% vs 11.9%), walkabout (0.6% vs 0.7%), and any impulse control or related behavior (18.5% vs 20.3%). In multivariable models, a diagnosis of PD was not associated with symptoms of any impulse control or related behavior (p ≥ 0.10 in all cases).
Conclusions:
PD itself does not seem to confer an increased risk for development of impulse control or related behavior symptoms, which further reinforces the reported association between PD medications and impulse control disorders in PD. Given that approximately 20% of patients with newly diagnosed PD report some impulse control or related behavior symptoms, long-term follow-up is needed to determine whether such patients are at increased risk for impulse control disorder development once PD medications are initiated.
Impulse control disorders (ICDs), including compulsive gambling, buying, sexual behavior, and eating, are common and clinically significant in Parkinson disease (PD).1 Other compulsive behaviors reported to occur include punding (excessive repetition of non–goal directed activity),2 hobbyism (excessive repetition of more complex activities),3 and walkabout (aimless wandering).2 In a recent large observational study of patients with treated PD, an ICD was identified in 14% of patients.4 Prevalence rates for punding range from 1% to 14%,5,6 whereas the prevalence of hobbyism and walkabout are not known.
The association between PD medications and ICDs in PD is well established.4,7 However, an important unanswered question is whether PD itself confers an altered risk for ICDs. Preliminary comparison studies suggest that ICDs are more common in treated PD patients than in healthy controls (HCs).8,9 In the only published study assessing newly diagnosed, untreated PD patients, 18% screened positive for impulse control symptoms, a number similar to HCs.10 However, this study used a general impulse control instrument not validated for use in PD, did not query for compulsive eating, and included control data that were published in a separate report.11
Determining the frequency of impulse control symptoms in patients with de novo, untreated PD would help answer the question of whether PD itself confers an increased risk for experiencing such symptoms. Analyzing baseline data from a large observational study of patients with newly diagnosed, untreated PD and an HC group of similar age and education, we hypothesized that the frequency of impulse control symptoms would be similar in the 2 groups.
METHODS
Participants
Patients with newly diagnosed, untreated PD and unmatched HCs were enrolled in the Parkinson's Progression Marker Initiative (PPMI), a study for which the aims and methodology have been published.12 At baseline, PD patients are required to 1) have an asymmetric resting tremor or asymmetric bradykinesia or 2 of bradykinesia, resting tremor, and rigidity, 2) be recently diagnosed (within 2 years), and 3) be untreated. HCs must have no significant neurologic dysfunction, no first-degree family member with PD, and a Montreal Cognitive Assessment (MoCA) score >26.
Standard protocol approvals, registrations, and patient consents
Each participating PPMI site 1) received approval from an ethical standards committee on human experimentation before study initiation, and 2) obtained written informed consent for research from all individuals participating in the study.
Study design
PPMI is an observational, international, multicenter (16 US and 5 European sites) study designed to identify PD progression biomarkers, with a goal to enroll 400 PD patients and 200 HCs. The study was launched in June 2010, and the data used in the preparation of this article were obtained from the PPMI database (www.ppmi-info.org [accessed February 24, 2012]). At this time, 432 people had been screened for the study. Of this total, 329 consented and provided complete baseline data (PD = 186, HC = 143). Of the patients with PD, 18 were identified as having dopamine transporter brain scans without evidence of dopaminergic deficit and were removed from the PD population for the purposes of this study, leaving a total sample size of 311 participants (PD = 168, HC = 143).
Study outcomes
A self-report and self-completed screening instrument, the Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease (QUIP), was recently developed and validated specifically to detect the presence of clinically significant impulse control (compulsive gambling, buying, sexual behavior, and eating) and related behavior (punding, hobbyism, and walkabout) symptoms reported to occur in PD.13 A short-form version of the QUIP was shown to have similar psychometric properties in secondary analyses.13 As a screening instrument, the QUIP is designed to be sensitive for the detection of ICDs and related disorders, but is not highly specific (i.e., it overidentifies patients), so many individuals with a positive QUIP do not meet diagnostic criteria for an ICD or related disorder. A positive QUIP for a particular disorder was based on previous research with the QUIP-Short Form (i.e., a positive response to either of the 2 questions for each the 4 ICDs, and a positive response to the single question each for punding, hobbyism, and walkabout).13
Other measures included basic demographic variables, Unified Parkinson's Disease Rating Scale (UPDRS) motor score14 and Hoehn and Yahr stage15 as measures of disease severity, the MoCA (scores range from 0 to 30, lower scores indicating greater cognitive impairment) for assessment of global cognitive abilities,16 and the 15-item Geriatric Depression Scale (scores range from 0 to 15, higher scores indicating greater depression severity) to assess severity of depressive symptoms.17
Statistical methods
To compare medians, χ2 tests, t tests (with the Levene test for equality of variances), and nonparametric tests were used for between-group comparisons of clinical, demographic, neuropsychological, and imaging variables. Normality assumptions were checked with the Kolmogorov-Smirnov test whenever the tests required normality assumption, and a Mann-Whitney U test was run for variables that were not normally distributed.
Variables either reported to be associated with ICDs in PD (e.g., age4) or for which we found between-group differences (PD patients vs HCs) on bivariate analysis at p value <0.05 (table 1) were entered into stepwise logistic regression models to determine the independent effects of different covariates on the occurrence of impulse control and related behavior symptoms. Because walkabout was uncommon, it was not examined separately, but was included in the category “any ICD or related behavior.”
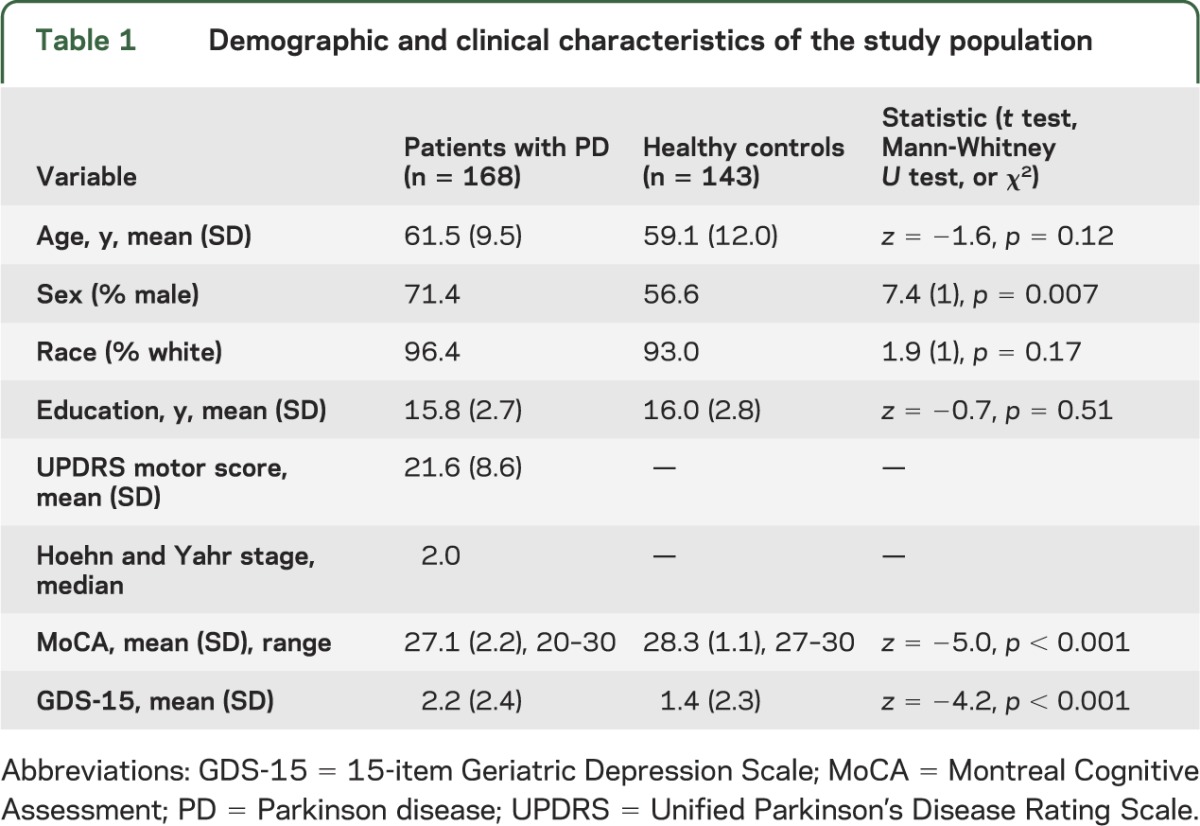
Table 1.
Demographic and clinical characteristics of the study population
All statistical tests were 2-sided. Statistical significance was set at p ≤ 0.05. Analyses were conducted with PASW Statistics (version 20.0) software.18
RESULTS
Participant characteristics
Demographic and clinical information for the 168 PD patients and 143 HC individuals is listed in table 1. On bivariate analysis, there were no significant differences between the 2 groups for age (p = 0.12), race (p = 0.17), or education (p = 0.51), but PD patients were more likely to be male (p = 0.007). PD patients performed statistically worse on the MoCA (p < 0.001), with 19% of PD patients screening positive for a cognitive disorder based on the recommended cutoff score of <26.19 PD patients also demonstrated a higher 15-item Geriatric Depression Scale score (p < 0.001), with 12.5% of PD patients screening positive for depression based on the recommended cutoff score of >4.20
Frequency of ICD and related behavior symptoms
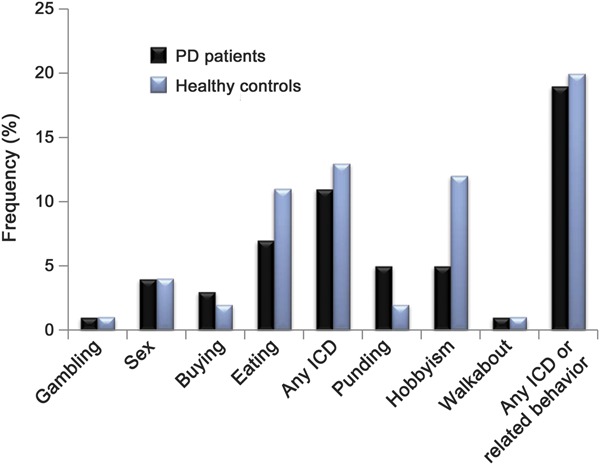
The frequencies of impulse control and related behavior symptoms are illustrated in the figure, and for patients with PD vs HCs were as follows: compulsive gambling (1.2% vs 0.7%), compulsive buying (3.0% vs 2.1%), compulsive sexual behavior (4.2% vs 3.5%), compulsive eating (7.1% vs 10.5%), punding (4.8% vs 2.1%), hobbyism (5.4% vs 11.9%), walkabout (0.6% vs 0.7%), and any impulse control or related behavior symptoms (18.5% vs 20.3%). There were no significant differences between the 2 groups regarding the frequency of symptoms of any of the 4 impulse control behaviors, either individually or as a group. Regarding related behaviors, although there was no significant difference in the frequency of punding or walkabout, HCs were more likely to report symptoms of hobbyism (p = 0.04). There were no significant between-group differences when impulse control and related behavior symptoms were combined into a single group.
Figure. Frequencies of self-reported impulse control disorder (ICD) and related behavior symptoms in patients with Parkinson disease (PD) and in healthy controls.
Correlates of impulse control and related behavior symptoms
Given the significant differences between PD patients and HCs in gender, cognition, and depression severity, as well as research documenting an association between younger age and ICDs in PD, logistic regression models were run including these variables as covariates, with impulse control or related behavior symptoms as the dependent variable (table 2). A diagnosis of PD was not associated with the presence of symptoms of any impulse control or related behavior, either individually or as a group. The only significant correlate in the multivariable model was increasing severity of depressive symptoms. On subanalysis, increasing severity of depression was associated with the presence of compulsive-eating symptoms (β [SE] = 0.17 [0.06], p = 0.007), but not with other impulse control or related behavior symptoms (data not shown).
Table 2.
Logistic regression models examining predictors of impulse control and related behavior symptoms in the entire study population
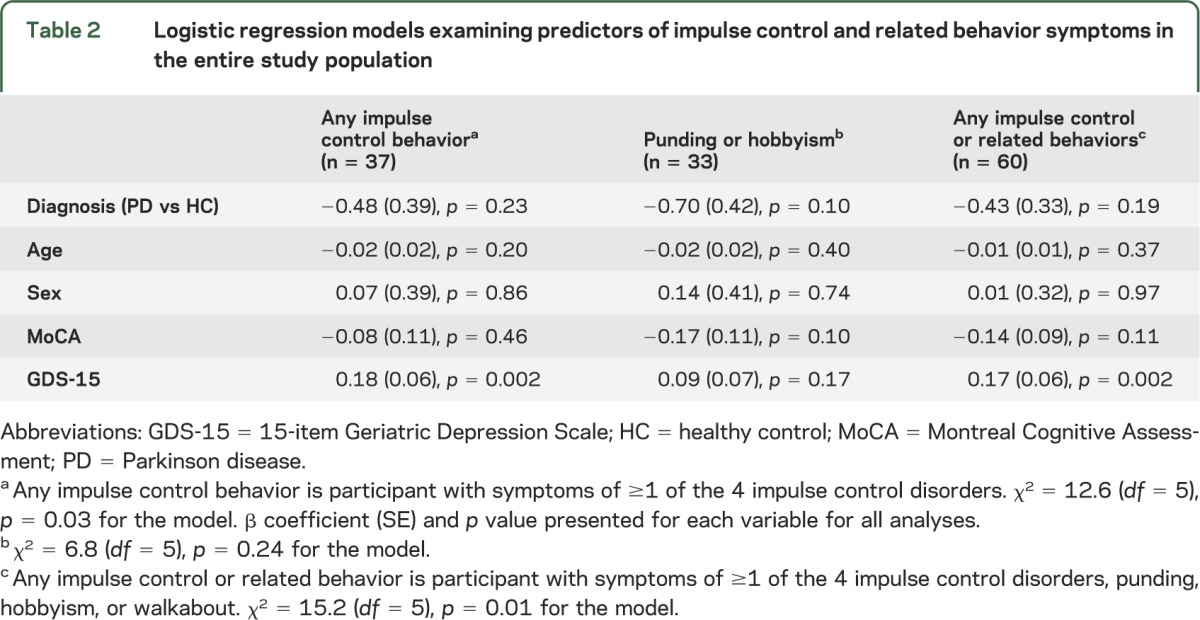
Examining PD patients and HCs separately, increasing severity of depression was associated with presence of any impulse control or related behavior symptoms in HCs (β [SE] = 0.21 [0.09], p = 0.02) and with presence of impulse control symptoms in PD patients (β [SE] = 0.21 [0.09], p = 0.02).
Examining for other correlates in PD patients, there were no significant associations between the presence of any impulse control symptoms or any impulse control or related behavior symptoms and age, sex, education, MoCA score, Hoehn and Yahr stage, and UPDRS motor score (data not shown).
DISCUSSION
The primary finding of this study is that the broad range of impulse control and related behavior symptoms that are reported to occur in patients with established, treated PD is equally common (approximately 20%) in patients with de novo, untreated PD and the general population. Additional findings are that increasing severity of depression is associated with these symptoms in both the entire population and PD patients, but global cognitive abilities are not. The major strengths of our study are that it is the first to use an impulse control assessment tool developed and validated for use in PD, as well to enroll PD patients and HCs concurrently and to have both groups undergo an identical assessment process.
Given that all research to date examining the frequency and correlates of ICDs and similar behaviors in PD has been cross-sectional, an important unanswered question is to what extent the increased frequency reported in PD patients compared with the general population is attributable to treatment with PD medications vs a disease-related effect.
Our results suggest that PD itself does not confer an increased risk for experiencing ICDs and related disorders. Therefore, the excess occurrence of these disorders in PD is likely driven by exposure to PD pharmacotherapy and possibly other PD treatments (e.g., deep brain stimulation surgery21), with certain clinical and demographic variables (e.g., younger age, family or personal history of similar behaviors3,4) moderating an individual's risk for developing an ICD. It also supports recent case reporting of ICDs occurring with dopamine agonist treatment in other clinical populations (e.g., restless legs syndrome22 and fibromyalgia23).
Despite the fact that impulse control and related behavior symptoms were not more common in PD patients compared with HCs, almost 20% of PD patients did screen positive for one or more of these disorders. Perhaps these patients also have a personal or family history of similar behaviors that has been suggested to be a risk factor for ICDs in PD based on results from cross-sectional studies.4 Long-term follow-up of patients newly diagnosed with PD is necessary to determine whether those patients who report impulse control and related behavior symptoms at the time of PD diagnosis are at increased risk of developing an actual ICD after treatment with PD medications is initiated.
The only correlate of impulse control symptoms in PD patients was increasing severity of depression, which has been reported previously for ICDs in PD.24 However, because depression was also associated with impulse control or related behavior symptoms in controls, this finding does not seem to be specific to PD.
We did not find a correlation in PD patients between impulse control and related behavior symptoms and global cognitive abilities as measured by the MoCA, similar to what was reported in a previous case-control study in PD patients with ICDs.24 Administration of more sensitive and specific cognitive tasks, as previously done in PD ICD studies,25–27 may be needed to detect cognitive changes with ICD symptoms.
Several study limitations should be noted. First, we were not able to determine symptom severity or whether participants actually met diagnostic criteria for an ICD or related disorder based on the methodology of the PPMI project, and it is important to note that a significant percentage of patients who screen positive with the QUIP do not meet diagnostic criteria for an actual disorder. Future research studies need to use formal diagnostic criteria for each disorder to extend our findings. Clinically, it is important that all patients with a positive QUIP undergo a detailed clinical interview to determine whether diagnostic criteria for an ICD or related disorder are met. Second, the PPMI cohort is made up of volunteers and is not necessarily representative of the underlying population of newly diagnosed PD patients. This limitation is balanced by the fact that it is a large cohort, very well characterized, and that the same selection factors probably existed for both cases and controls. Third, many of the clinical and demographic correlates of ICDs reported in previous studies were not available in this database. Finally, PD patients and HCs were not matched on some common characteristics (e.g., gender and global cognition), which necessitated the use of multivariable analyses.
The results of this study provide additional support to the idea that factors other than PD itself lead to the development of ICDs and related behaviors in PD. Long-term follow-up of patients newly diagnosed with PD is needed to determine whether self-reported symptoms at the time of PD onset predict, along with other demographic, clinical, cognitive, or neurobiological characteristics, the development of ICDs and related behaviors after initiation of PD pharmacotherapy.
Supplementary Material
ACKNOWLEDGMENT
PPMI, a public-private partnership, is funded by The Michael J. Fox Foundation for Parkinson's Research and funding partners, including Abbott, Biogen Idec, F. Hoffman-La Roche Ltd., GE Healthcare, Genentech, and Pfizer Inc. A list of the Executive Steering Committee contributors is available online.
GLOSSARY
- HC
healthy control
- ICD
impulse control disorder
- MoCA
Montreal Cognitive Assessment
- PD
Parkinson disease
- PPMI
Parkinson's Progression Marker Initiative
- QUIP
Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease
- UPDRS
Unified Parkinson's Disease Rating Scale
Footnotes
Supplemental data at www.neurology.org
Go to Neurology.org for full disclosures. Funding information and disclosures deemed relevant by the authors, if any, are provided at the end of the article.
AUTHOR CONTRIBUTIONS
Drs. Weintraub and Siderowf were involved with study concept and design, analysis and interpretation of the data, and drafting/revising the manuscript for content, including medical writing for content. Miss Papay was involved with analysis and interpretation of data, and drafting/revising the manuscript for content.
STUDY FUNDING
The study was sponsored by the PPMI.
DISCLOSURE
D. Weintraub receives grant support from the NIH (NS-053488), The Michael J. Fox Foundation for Parkinson's Research, and Novartis Pharmaceuticals; consulting or advisory board membership with honoraria from Teva Pharmaceuticals, Eli Lilly and Company, Lundbeck Inc., Biogen, Pfizer, and Avanir Pharmaceuticals, Merck & Co. K. Papay reports no disclosures. A. Siderowf is supported by a Morris K. Udall Parkinson's Disease Research Center of Excellence grant from NINDS (NS-053488), a U01 from the NINDS (NS0444451), the National Parkinson Foundation and The Michael J. Fox Foundation. He is an employee of Avid Radiopharmaceuticals. He has received consulting fees and honoraria from Teva Neuroscience, Ipsen pharmaceuticals, Schering-Plough and Merck Serono, and General Electric. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Weintraub D. Dopamine and impulse control disorders in Parkinson's disease. Ann Neurol 2008;64(suppl):S93–S100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giovannoni G, O'Sullivan JD, Turner K, Manson AJ, Lees AJL. Hedonistic homeostatic dysregulation in patients with Parkinson's disease on dopamine replacement therapies. J Neurol Neurosurg Psychiatry 2000;68:423–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voon V, Fox SH. Medication-related impulse control and repetitive behaviors in Parkinson disease. Arch Neurol 2007;64:1089–1096 [DOI] [PubMed] [Google Scholar]
- 4.Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol 2010;67:589–595 [DOI] [PubMed] [Google Scholar]
- 5.Evans AH, Katzenschlager R, Paviour D, et al. Punding in Parkinson's disease: its relation to the dopamine dysregulation syndrome. Mov Disord 2004;19:397–405 [DOI] [PubMed] [Google Scholar]
- 6.Miyasaki J, Hassan KL, Lang AE, Voon V. Punding prevalence in Parkinson's disease. Mov Disord 2007;22:1179–1181 [DOI] [PubMed] [Google Scholar]
- 7.Weintraub D, Sohr M, Potenza MN, et al. Amantadine use associated with impulse control disorders in Parkinson disease in cross-sectional study. Ann Neurol 2010;68:963–968 [DOI] [PubMed] [Google Scholar]
- 8.Giladi N, Weitzman N, Schreiber S, Shabtai H, Peretz C. New onset heightened interest or drive for gambling, shopping, eating or sexual activity in patients with Parkinson's disease: the role of dopamine agonist treatment and age at motor symptoms onset. J Psychopharmacol (Oxf) 2007;21:501–506 [DOI] [PubMed] [Google Scholar]
- 9.Avanzi M, Baratti M, Cabrini S, Uber E, Brighetti G, Bonfà F. Prevalence of pathological gambling in patients with Parkinson's disease. Mov Disord 2006;21:2068–2072 [DOI] [PubMed] [Google Scholar]
- 10.Antonini A, Siri C, Santangelo G, et al. Impulsivity and compulsivity in drug-naive patients with Parkinson's disease. Mov Disord 2011;26:464–468 [DOI] [PubMed] [Google Scholar]
- 11.Isaias IU, Siri C, Cilia R, De Gaspari D, Pezzoli G, Antonini A. The relationship between impulsivity and impulse control disorders in Parkinson's disease. Mov Disord 2008;23:411–415 [DOI] [PubMed] [Google Scholar]
- 12.Parkinson Progression Marker Initiative The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol 2011;95:629–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weintraub D, Stewart S, Shea JA, et al. Validation of the questionnaire for impulsive-compulsive behaviors in Parkinson's disease (QUIP). Mov Disord 2009;24:1461–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fahn S, Elton RL; the UPDRS Development Committee Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne D, Goldstein M, editors. Recent Developments in Parkinson's disease. Florham Park, NJ: Macmillan Health Care Information; 1987:153–163 [Google Scholar]
- 15.Hoehn MH, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology 1967;17:427–442 [DOI] [PubMed] [Google Scholar]
- 16.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699 [DOI] [PubMed] [Google Scholar]
- 17.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. In: Brink TL, editor. Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Haworth Press; 1986:165–173 [Google Scholar]
- 18.PASW for Windows. [computer program]. Version 20.0. Chicago: IBM; 2011.
- 19.Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology 2010;75:1717–1725 [DOI] [PubMed] [Google Scholar]
- 20.Weintraub D, Oehlberg KA, Katz IR, Stern MB. Test characteristics of the 15-item Geriatric Depression Scale and Hamilton Depression Rating Scale in Parkinson's disease. Am J Geriatr Psychiatry 2006;14:169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broen M, Duits A, Visser-Vandewalle V, Temel Y, Winogrodzka A. Impulse control and related disorders in Parkinson's disease patients treated with bilateral subthalamic nucleus stimulation: a review. Parkinsonism Relat Disord 2011;17:413–417 [DOI] [PubMed] [Google Scholar]
- 22.Ondo W, Lai D. Predictors of impulsivity and reward seeking behavior with dopamine agonists. Parkinsonism Relat Disord 2008;14:28–32 [DOI] [PubMed] [Google Scholar]
- 23.Holman AJ. Impulse control disorder behaviors associated with pramipexole used to treat fibromyalgia. J Gambl Stud 2009;25:425–431 [DOI] [PubMed] [Google Scholar]
- 24.Voon V, Sohr M, Lang AE, et al. Impulse control disorders in Parkinson disease: a multicenter case-control study. Ann Neurol 2011;69:986–996 [DOI] [PubMed] [Google Scholar]
- 25.Housden CR, O'Sullivan SS, Joyce EM, Lees AJ, Roiser JP. Intact reward learning but elevated delay discounting in Parkinson's disease patients with impulsive-compulsive spectrum behaviors. Neuropsychopharmacology 2010;35:2155–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Claassen DO, van den Wildenberg WPM, Ridderinkhof KR, et al. The risky business of dopamine agonists in Parkinson's disease and impulse control disorders. Behav Neurosci 2011;125:492–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voon V, Pessiglione M, Brezing C, et al. Mechanisms underlying dopamine-mediated reward bias in compulsive behaviors. Neuron 2010;65:135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


