Abstract
A 75-year-old woman developed periorbital pain and blurred vision OS. Visual acuity (VA) was 20/40 OD, 20/400 OS with mild left relative afferent pupillary defect (APD). Left optic nerve was swollen and hyperemic with peripapillary flame hemorrhages (figure, A). Erythrocyte sedimentation rate (ESR) was 124 mm/h. She was treated with IV methylprednisolone, 250 mg every 6 h. On day 3, headache and vision improved. ESR was 98 mm/h and C-reactive protein was 1.40 mg/L. Rheumatoid factor, antinuclear antibodies, and antineutrophil cytoplasmic antibodies titers were negative. On day 4, left temporal artery biopsy revealed thickened intima and intact internal elastic lamina (figure, B) but no medial necrosis characteristic of giant cell arteritis (GCA). Sections of the temporal artery were deparaffinized and incubated with 10% normal sheep serum (NSS) in phosphate-buffered saline (PBS) for 1 hour at room temperature, rinsed 3 times in PBS, and incubated overnight at 4°C with polyclonal antibodies raised against the varicella-zoster virus (VZV) open reading frame 63 protein (1:1,000 dilution) or with normal rabbit serum (1:1,000 dilution). The next day, sections were washed 3 times in PBS, incubated with a 1:300 dilution of biotinylated goat antirabbit immunoglobulin G (IgG) in PBS containing 5% NSS, washed 3 times in PBS, incubated for 1 hour at room temperature with alkaline phosphatase–conjugated streptavidin (1:100 dilution), and washed 3 times with PBS. The color reaction was developed for 5–30 minutes with fresh fuchsin substrate system. Levamisole was added to the color reaction to block endogenous phosphatase. Uninfected and VZV-infected human fibroblast lung cells were used as controls (not shown). Steroids were changed to oral prednisone 60 mg daily. On day 7, brain MRI with gadolinium was negative. On day 9, pain and vision worsened. On day 11, orbital CT and head CT angiography were negative. On day 15, VA was 20/400 OS with relative left APD. On day 17, OS became blind without direct pupillary light reaction; fundus was obscured by vitreous hemorrhage. CSF contained 8 leukocytes/mm3, protein 72 mg/L, and glucose 54 mg/L. CSF cultures for bacteria, fungi, acid-fast bacilli, and cytology were negative. Because asymptomatic temporal artery biopsy was GCA-negative, VZV ischemic optic neuropathy (ION) was considered, and she was treated with IV acyclovir, 10 mg/kg every 8 hours for 7 days. On day 31, CSF contained anti-VZV IgG but not anti-herpes simplex virus IgG antibody, and serum-to-CSF ratio of anti-VZV IgG was reduced (14) compared to ratios for total IgG (121) and albumin (81). Immunohistochemistry and pathology revealed VZV antigen and neutrophils in the original left temporal artery specimen (figure, C). On day 31, she was treated with oral valacyclovir, 1 gram TID for 6 weeks; prednisone was reduced to 20 mg daily and tapered 5 mg/week. Six weeks later, pain resolved, and VA improved to finger counting. Left optic nerve was pale with clear margins and resolution of hemorrhage.
A 75-year-old woman developed periorbital pain and blurred vision OS. Visual acuity (VA) was 20/40 OD, 20/400 OS with mild left relative afferent pupillary defect (APD). Left optic nerve was swollen and hyperemic with peripapillary flame hemorrhages (figure, A). Erythrocyte sedimentation rate (ESR) was 124 mm/h. She was treated with IV methylprednisolone, 250 mg every 6 h. On day 3, headache and vision improved. ESR was 98 mm/h and C-reactive protein was 1.40 mg/L. Rheumatoid factor, antinuclear antibodies, and antineutrophil cytoplasmic antibodies titers were negative. On day 4, left temporal artery biopsy revealed thickened intima and intact internal elastic lamina (figure, B) but no medial necrosis characteristic of giant cell arteritis (GCA). Sections of the temporal artery were deparaffinized and incubated with 10% normal sheep serum (NSS) in phosphate-buffered saline (PBS) for 1 hour at room temperature, rinsed 3 times in PBS, and incubated overnight at 4°C with polyclonal antibodies raised against the varicella-zoster virus (VZV) open reading frame 63 protein (1:1,000 dilution) or with normal rabbit serum (1:1,000 dilution). The next day, sections were washed 3 times in PBS, incubated with a 1:300 dilution of biotinylated goat antirabbit immunoglobulin G (IgG) in PBS containing 5% NSS, washed 3 times in PBS, incubated for 1 hour at room temperature with alkaline phosphatase–conjugated streptavidin (1:100 dilution), and washed 3 times with PBS. The color reaction was developed for 5–30 minutes with fresh fuchsin substrate system. Levamisole was added to the color reaction to block endogenous phosphatase. Uninfected and VZV-infected human fibroblast lung cells were used as controls (not shown). Steroids were changed to oral prednisone 60 mg daily. On day 7, brain MRI with gadolinium was negative. On day 9, pain and vision worsened. On day 11, orbital CT and head CT angiography were negative. On day 15, VA was 20/400 OS with relative left APD. On day 17, OS became blind without direct pupillary light reaction; fundus was obscured by vitreous hemorrhage. CSF contained 8 leukocytes/mm3, protein 72 mg/L, and glucose 54 mg/L. CSF cultures for bacteria, fungi, acid-fast bacilli, and cytology were negative. Because asymptomatic temporal artery biopsy was GCA-negative, VZV ischemic optic neuropathy (ION) was considered, and she was treated with IV acyclovir, 10 mg/kg every 8 hours for 7 days. On day 31, CSF contained anti-VZV IgG but not anti-herpes simplex virus IgG antibody, and serum-to-CSF ratio of anti-VZV IgG was reduced (14) compared to ratios for total IgG (121) and albumin (81). Immunohistochemistry and pathology revealed VZV antigen and neutrophils in the original left temporal artery specimen (figure, C). On day 31, she was treated with oral valacyclovir, 1 gram TID for 6 weeks; prednisone was reduced to 20 mg daily and tapered 5 mg/week. Six weeks later, pain resolved, and VA improved to finger counting. Left optic nerve was pale with clear margins and resolution of hemorrhage.
Figure. Fundus examination, histology, and immunohistochemistry on temporal artery biopsy of varicella-zoster virus ischemic optic neuropathy.
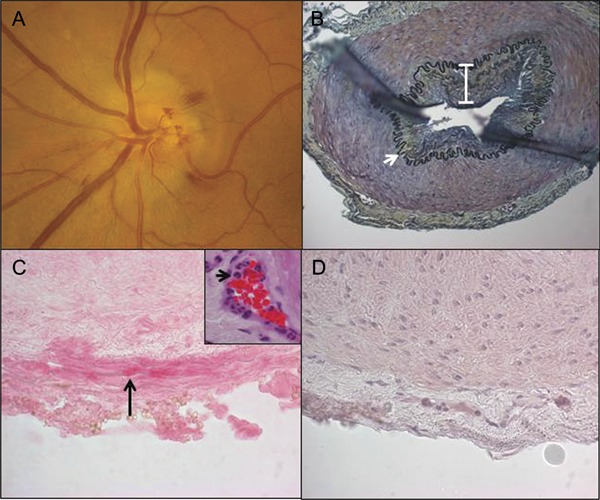
(A) Fundus photograph of the left eye reveals a swollen elevated optic disc with blurred margins and peripapillary flame hemorrhages. (B) Histologic and immunohistochemical analysis of the left temporal artery biopsy obtained 4 days after the patient's onset of loss of vision. Modified Movat pentachrome stain of a formalin-fixed paraffin-embedded section of the temporal artery demonstrates a thickened intima (white bar) and nearly intact internal elastic lamina (white arrow). Original magnification ×200. Note the presence of varicella-zoster virus (VZV) antigen in the adventitia of the temporal artery stained with anti-VZV antibody (C, black arrow, pink, ×600 magnification), but not with normal rabbit serum (D), and an abundance of neutrophils in the adventitia around the vaso vasorum vessels (C, inset, black arrow, hematoxylin & eosin, ×600 magnification).
Discussion
We present an elderly patient with ION caused by VZV without zoster rash. Virologic verification was provided by detection of VZV antigen in adventitia of clinically asymptomatic and pathologically GCA-negative temporal artery ipsilateral to vision loss and anti-VZV IgG antibody in CSF with reduced serum/CSF ratios indicating intrathecal synthesis of anti-VZV IgG antibody. Recently, VZV ION was confirmed by presence of VZV antigen in ipsilateral clinically asymptomatic GCA-negative temporal artery1; the latter patient had ophthalmic-distribution zoster weeks earlier, unlike our patient without history of zoster. Overall, our case expands the spectrum of disease produced by VZV without rash to ION, reveals ipsilateral GCA-negative temporal artery VZV infection useful in diagnosis, and confirms early VZV infection of adventitia in VZV vasculopathy, providing additional evidence that extracranial arteries become infected transaxonally after VZV reactivates from ganglia.
Before diagnosis, the patient was treated with steroids for presumed GCA and noted transient improvement of pain and vision, perhaps due to decreased inflammation. However, only 2 weeks later, she became blind in the affected eye, most likely because she did not receive antiviral treatment for VZV infection that might have been potentiated by steroid treatment. In a prototype case of VZV vasculopathy diagnosed postmortem, the patient's protracted course was aggravated by prolonged treatment with steroids and cyclophosphamide.2 Because both GCA and VZV vasculopathy without rash present with headache, vision loss, and elevated ESR, it is important to consider and diagnose VZV vasculopathy since long-term steroid treatment for presumed GCA may aggravate disease. Virologic confirmation requires either detection of VZV DNA or anti-VZV antibody in CSF or detection of VZV antigen in temporal artery biopsy.
Because VZV reactivates mostly in elderly humans (the same age group in which GCA predominates) and infects arteries with production of multinucleated giant cells, clinicians have questioned whether VZV causes GCA. VZV DNA was initially found in 9 (26%) of 35 pathologically verified cases of GCA.3 A later study showed that prevalence of VZV DNA in GCA-positive patients did not differ significantly from control subjects4; furthermore, none of 37 temporal arteries with histologic evidence of arteritis contained VZV DNA.5 Perhaps more importantly, VZV antigen is not found in temporal arteries of pathologically verified cases of GCA.6 Lack of detection of productive VZV infection in patients with GCA is not surprising since both necrosis and inflammation in media are characteristically seen in acute GCA, while only inflammation is seen in adventitia of early VZV vasculopathy.7 Also, we had previously reported disrupted internal elastic lamina in VZV vasculopathy. However, because this case of VZV vasculopathy was detected early, it would not be expected that internal elastic lamina would yet be disrupted. Finally, because VZV can be found in GCA-negative temporal artery in ION, even without rash, it would be prudent to examine GCA-negative temporal artery biopsies for VZV antigen, particularly since VZV infections respond to antiviral therapy.
Acknowledgments
Acknowledgment: The authors thank Marina Hoffman for reviewing the manuscript for errors in grammar, punctuation, spelling, readability, clarity, and accuracy.
Footnotes
Author contributions: M.A. Nagel: drafting/revising the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data; contribution of vital reagents/tools/patents; acquisition of data; statistical analysis; study supervision or coordination; obtaining funding. A.N. Russman: drafting/revising the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data; acquisition of data. H. Feit: drafting/revising the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data; acquisition of data. I. Traktinskiy: analysis or interpretation of data; acquisition of data. N. Khmeleva: analysis or interpretation of data; acquisition of data. D.S. Schmid: contribution of vital reagents/tools/patents; acquisition of data. B. Skarf: drafting/revising the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data; acquisition of data. D. Gilden: drafting/revising the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data; contribution of vital reagents/tools/patents; acquisition of data; statistical analysis; study supervision or coordination; obtaining funding.
Disclosure: The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
References
- 1.Salazar R, Russman AN, Nagel MA, et al. VZV ischemic optic neuropathy and subclinical temporal artery involvement. Arch Neurol 2011;68:517–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilden D, Kleinschmidt-DeMasters BK, Wellish M, et al. Varicella zoster virus, a cause of waxing and waning vasculitis. NEJM case 5-1995 revisited. Neurology 1996;47:1441–1446 [DOI] [PubMed] [Google Scholar]
- 3.Mitchell BM, Font RL. Detection of varicella zoster virus DNA in some patients with giant cell arteritis. Invest Ophthalmol Vis Sci 2001;42:2572–2577 [PubMed] [Google Scholar]
- 4.Alvarez-Lafuente R, Fernández-Gutiérrez B, Jover JA, et al. Human parvovirus B19, varicella zoster virus, and human herpes virus 6 in temporal artery biopsy specimens of patients with giant cell arteritis: analysis with quantitative real time polymerase chain reaction. Ann Rheum Dis 2005;64:780–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper RJ, D'Arcy S, Kirby M, et al. Infection and temporal arteritis: a PCR-based study to detect pathogens in temporal artery biopsy specimens. J Med Virol 2008;80:501–505 [DOI] [PubMed] [Google Scholar]
- 6.Nordborg C, Nordborg E, Petursdottir V, et al. Search for varicella zoster virus in giant cell arteritis. Ann Neurol 1998;44:413–414 [DOI] [PubMed] [Google Scholar]
- 7.Nagel MA, Traktinskiy I, Azarkh Y, et al. Varicella zoster virus vasculopathy: analysis of virus-infected arteries. Neurology 2011;77:364–370 [DOI] [PMC free article] [PubMed] [Google Scholar]


