Abstract
Objectives:
We conducted a systematic review of the literature with meta-analysis to determine whether complex regional pain syndrome (CRPS) is associated with a specific inflammatory profile and whether this is dependent on the duration of the condition.
Methods:
Comprehensive searches of the literature using MEDLINE, Embase, Scopus, Web of Science, and reference lists from published reviews identified articles that measured inflammatory factors in CRPS. Two independent investigators screened titles and abstracts, and performed data extraction and risk of bias assessments. Studies were subgrouped by medium (blood, blister fluid, and CSF) and duration (acute and chronic CRPS). Where possible, meta-analyses of inflammatory factor concentrations were performed and pooled effect sizes were calculated using random-effects models.
Results:
Twenty-two studies were included in the systematic review and 15 in the meta-analysis. In acute CRPS, the concentrations of interleukin (IL)-8 and soluble tumor necrosis factor receptors I (sTNF-RI) and II (sTNF-RII) were significantly increased in blood. In chronic CRPS, significant increases were found in 1) TNFα, bradykinin, sIL-1RI, IL-1Ra, IL-2, sIL-2Ra, IL-4, IL-7, interferon-γ, monocyte chemoattractant protein-1 (MCP-1), and sRAGE (soluble receptor for advanced glycation end products) in blood; 2) IL-1Ra, MCP-1, MIP-1β, and IL-6 in blister fluid; and 3) IL-1β and IL-6 in CSF. Chronic CRPS was also associated with significantly decreased 1) substance P, sE-selectin, sL-selectin, sP-selectin, and sGP130 in blood; and 2) soluble intercellular adhesion molecule-1 (sICAM-1) in CSF. Most studies failed to meet 3 or more of our quality criteria.
Conclusion:
CRPS is associated with the presence of a proinflammatory state in the blood, blister fluid, and CSF. Different inflammatory profiles were found for acute and chronic cases.
Complex regional pain syndrome (CRPS) is characterized by severe pain, allodynia, hyperalgesia, and motor and autonomic signs and symptoms.1 Although the precise etiology of CRPS is unknown, persistent inflammatory activity has been demonstrated in a number of methodologically diverse studies.2–6 Although narrative reviews have attempted to synthesize these results, no systematic review including a rigorous quality assessment of original studies has been conducted. As such, no definitive conclusions can be drawn about the presence of inflammatory activity in CRPS, which limits the identification of potential targets for therapy. Therefore, we conducted a systematic review and meta-analysis of the literature. We aimed firstly to determine whether, in adults, CRPS is associated with a specific inflammatory profile; and secondly, to determine whether the inflammatory profile is dependent on the duration of the condition.
METHODS
Study selection and quality appraisal
We conducted the study according to a protocol that we developed in adherence with the PRISMA statement.7 We performed a sensitive search of accepted and published studies up to January 30, 2012 in MEDLINE, Embase, Scopus, and Web of Science using MeSH (Medical Subject Heading) terms and free text terms that were collated with the assistance of a research librarian at the University of New South Wales, Sydney, Australia (appendix). We also manually searched the article titles in the reference lists of key reviews and editorials published since 2005 for additional studies.
We included all studies that measured biomarkers of inflammation in human CRPS subjects and controls; that obtained samples from blood, CSF, or blister fluid; and were published or in press/accepted. No restrictions were imposed on the duration of CRPS, the age of participants, the language of the article, or the year of publication.
Two investigators independently selected studies from abstracts and full-text articles, and performed data extraction and quality assessment. Disagreements were resolved by discussion. If consensus was not reached, the opinion of a third investigator was sought. We calculated the interrater agreement (κ) for the pre-consensus stage.
To assess study quality, we adapted the STROBE statement8 to focus on research methodology and reporting of outcomes, and included sources of bias relevant to case-control study designs. Each quality criterion (table e-1 on the Neurology® Web site at www.neurology.org) was assessed and scored as “yes,” “no,” or “unclear” based on whether it was clearly reported. The quality appraisal form was piloted on 2 studies. We evaluated evidence of publication bias by inspecting funnel plots.
Data extraction
Data extracted from the studies included study design; inclusion and exclusion criteria; sample type including plasma, serum, blister, or CSF; source of the sample including affected or unaffected extremity; collection method including which container was used for blood samples; participants' age and gender; which criteria were used to diagnose CRPS; pain intensity; duration of symptoms; which measurement technology was used to measure concentrations in samples; and the main study findings. Concentrations of all measured cytokines were recorded. Details about collection and analysis methods were extracted as they are known to influence cytokine measurements.9,10 When possible, we calculated summary statistics from individual case data. G.L.M. contacted study authors up to 3 times to request missing data.
Quantitative analysis: Pooling of results
We subgrouped data by whether the sample was obtained from blood, blister, or CSF. Results from studies measuring concentrations in serum and plasma were pooled into a single “blood” category. Results from studies using different assays were also pooled. Where possible, data were further divided into an acute subgroup if CRPS symptoms were present for less than 6 months and a chronic subgroup if they were present for 6 or more months.
We pooled the subgrouped data when results for an inflammatory factor were available from at least 2 studies. Studies were excluded from the quantitative analysis if sufficient data were not reported, could not be obtained from the study authors, or we were unable to calculate the mean and SD values from group-level statistical results or from provided median and range values.11
Quantitative analysis: statistical procedures
We calculated standardized mean differences (Hedges' adjusted g) from the absolute concentrations of inflammatory markers. Homogeneity of effect sizes was tested using the χ2 and the I2 test and by visual inspection of forest plots. We decided whether statistically significant heterogeneity was present on the basis of χ2 p < 0.10, and substantial heterogeneity on the basis of I2 >60%, or significant irregularity in the point estimates and 95% confidence interval (CI) of the individual studies.12 If significant heterogeneity was detected, we planned to select the most conservative estimate from the fixed- or random-effects models.
We used SPSS 18.0 software (SPSS Inc., Chicago, IL) and RevMan 5.1.6 (Cochrane IMS, Baltimore, MD) to conduct the statistical analysis of extracted data. The variables of interest included the concentrations of inflammatory mediators obtained from blood (plasma or serum), CSF, experimentally induced blister fluid, or any other suitable body fluid. We interpreted effect sizes as small (0.20–0.49), medium (0.50–0.79), or large (≥0.80).13
RESULTS
Figure 1 outlines the process of record screening and article selection. We did not identify any additional relevant studies by a reference search of key reviews and editorials (list available on request). Investigator agreement for the record screening, before consensus, was κ = 0.65. All data results are presented as standardized mean difference and its 95% CI.
Figure 1. Flow diagram of study selection.
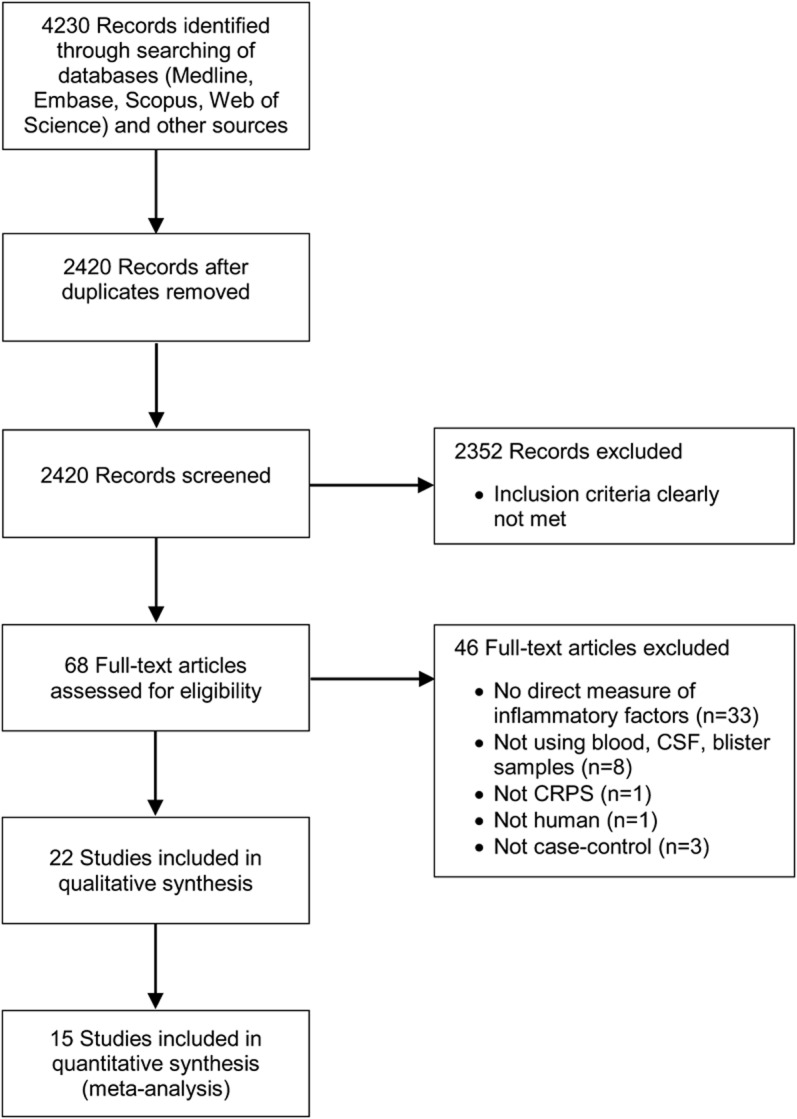
CRPS = complex regional pain syndrome.
Quality assessment
The 15 studies included in the quantitative analysis were published in English and included 467 cases of CRPS/reflex sympathetic dystrophy and 340 controls (including studies with an intrasubject comparison design). The outcome of study quality assessment is provided in table 1.
Table 1.
Summary of the characteristics and quality assessment of studies included in the systematic review
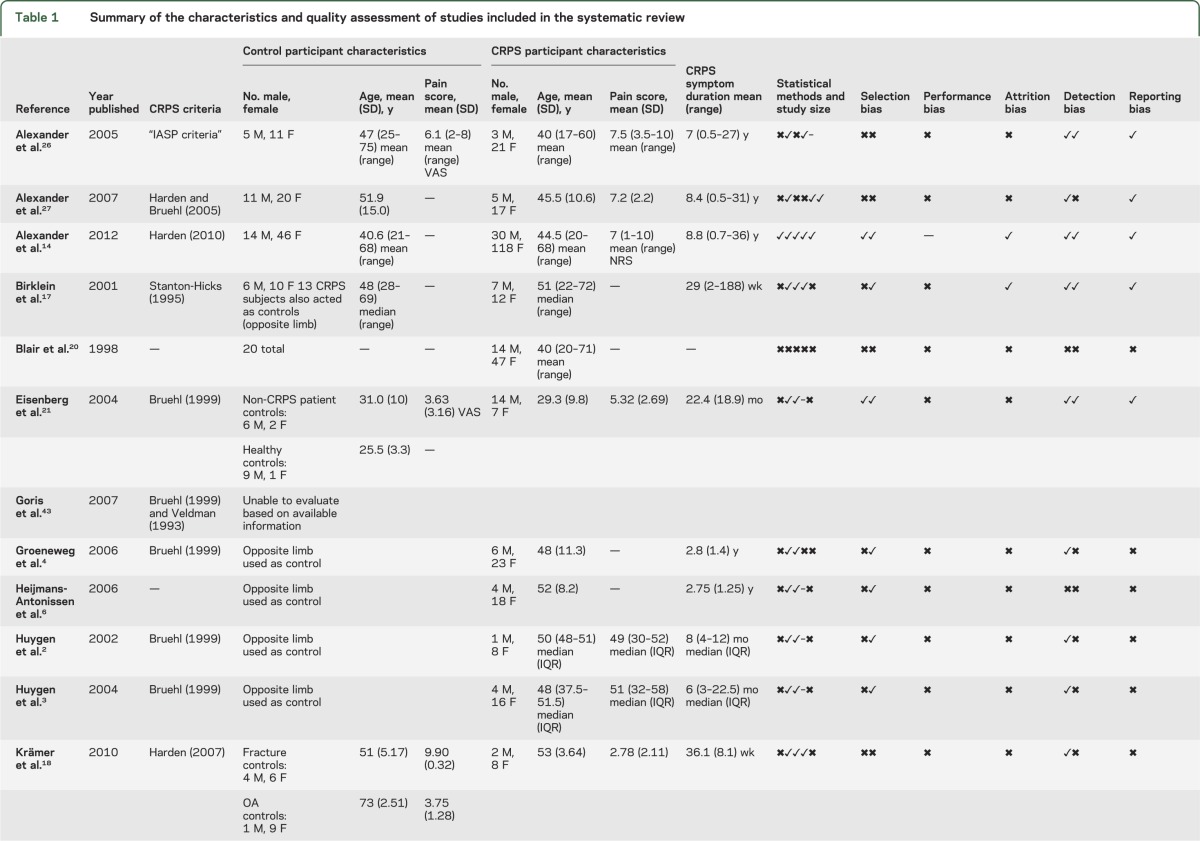
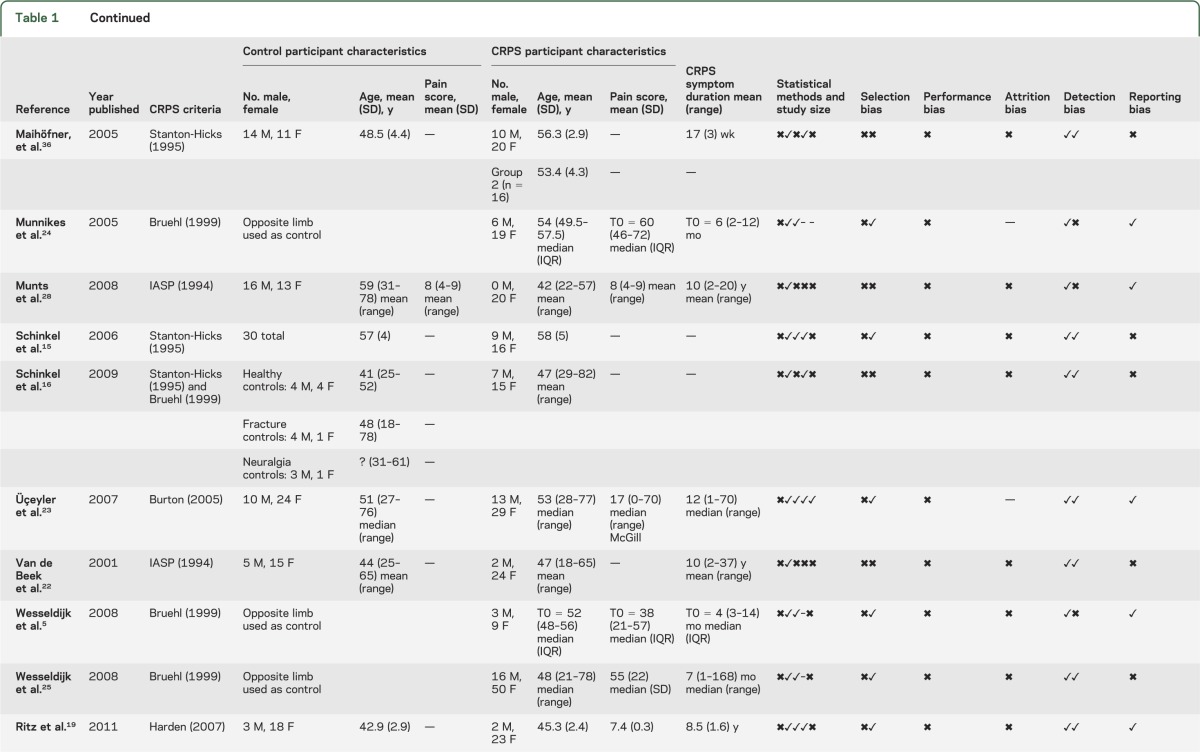
Investigator agreement for study quality was κ = 0.71; disagreement usually concerned the presence of adequate descriptions of the handling of assay sensitivities. We did not perform formal assessments of publication/small study bias because of the small number of studies in each comparison.
Only 1 study met all of the applicable criteria on our quality assessment tool14 and the remainder failed to successfully meet 3 or more of these criteria. The diagnostic criteria used to identify CRPS were found to vary substantially across the included studies. Authors also generally did not clearly report how they managed samples with concentrations measured below the lowest detectable threshold of the test (for instance, whether they imputed a zero or threshold value). Authors frequently did not explicitly state whether a control limb was diagnosed as unaffected by CRPS using recognized CRPS diagnostic criteria. Lastly, authors did not explicitly report which, if any, study investigators were blinded to the status of the participants.
Quantitative analysis
Blood
Inflammatory factor concentrations in blood were reported in 4 acute CRPS studies,15–18 9 chronic CRPS studies,2,14,16–22 and 1 mixed-duration study.23 Healthy controls were utilized by most studies,14–17,19–22 with the exception of 2 that used subjects with fractures or osteoarthritis,18 and the unaffected arm2 as controls. The results are presented in figures 2 and 3.
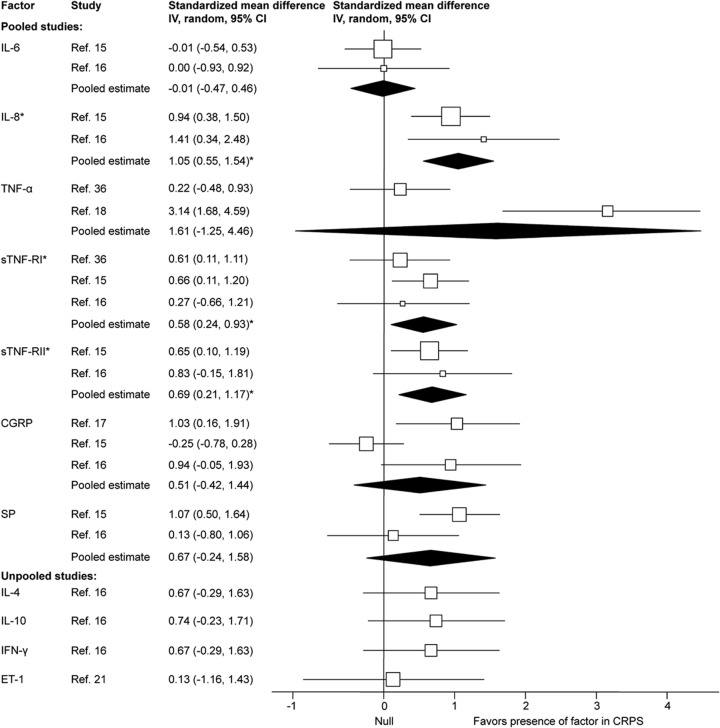
Figure 2. Forest plot of inflammatory factor effect sizes for blood samples from acute complex regional pain syndrome cases compared with healthy controls.
Each study effect size (standardized mean difference) and weight is indicated by an open box and its 95% confidence interval (CI) are indicated by a horizontal line. The filled diamonds represent the overall effect size and CIs for each factor. Statistically significant outcomes are indicated by an asterisk. CGRP = calcitonin gene-relaxed peptide; ET-1 = endothelin-1; IL = interleukin; SP = substance P; sTNF-R = soluble tumor necrosis factor receptor.
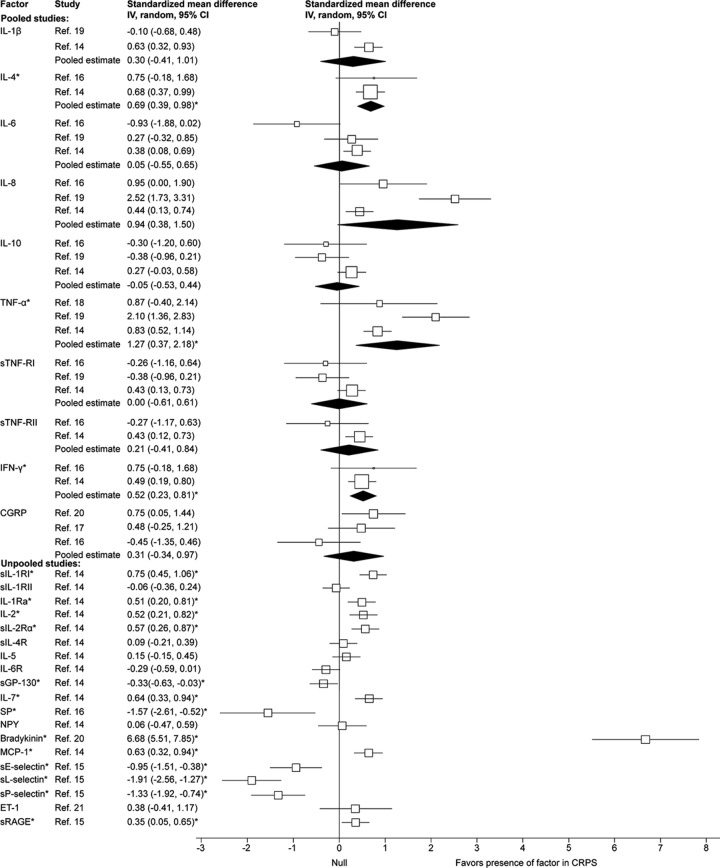
Figure 3. Forest plot of inflammatory factor effect sizes for blood samples from chronic complex regional pain syndrome cases compared with healthy controls.
Each study effect size (standardized mean difference) and weight is indicated by an open box and its 95% confidence intervals (CIs) are indicated by a horizontal line. The filled diamonds represent the overall effect size and CIs for each factor. Statistically significant outcomes are indicated by an asterisk. CGRP = calcitonin gene-relaxed peptide; ET-1 = endothelin-1; GP = glycoprotein; IFN = interferon; IL = interleukin; MCP-1 = monocyte chemoattractant protein-1; NPY = neuropeptide Y; SP = substance P; sRAGE = soluble receptor for advanced glycation end products; sTNF-R = soluble tumor necrosis factor receptor.
Acute CRPS
The pooled effect estimates indicated significantly increased concentrations with a large effect size for interleukin (IL)-8, and small effect sizes for soluble tumor necrosis factor receptors I (sTNF-RI) and II (sTNF-RII) in CRPS cases compared with controls. The pooled effect estimates of IL-6, IL-4, IL-10, interferon-γ, tumor necrosis factor (TNF)α, calcitonin gene-relaxed peptide (CGRP), substance P (SP), and endothelin-1 (ET-1) were not significantly different in CRPS cases compared with controls. Significant heterogeneity was detected for SP (χ2 = 2.85, p = 0.09, I2 = 65%).
Studies that compared samples from the affected and the unaffected limb found no significant differences in the levels of IL-6,15 IL-8,15 sTNF-RI,15 sTNF-RII,15 sE-selectin,15 sL-selectin,15 sP-selectin,15 SP,15 neuropeptide Y (NPY),15 and CGRP.15,17
Chronic CRPS
The pooled effect estimates indicated significantly increased concentrations with large effect sizes for TNFα and bradykinin, moderate effect sizes for sIL-1RI, IL-1Ra, IL-2, sIL-2Ra, IL-4, IL-7, interferon-γ, and monocyte chemoattractant protein-1, and a small effect size for soluble receptor for advanced glycation end products in CRPS cases compared with controls. The pooled effect estimates also indicated significantly decreased concentrations with large effect sizes for SP, sE-selectin, sL-selectin, and sP-selectin, and a small effect size for sGP130 in CRPS cases. The concentrations of IL-1β, sIL-1RII, sIL-4R, IL-5, IL-6, IL-6R, IL-8, IL-10, sTNF-RI, sTNF-RII, CGRP, NPY, and ET-1 were not significantly different in CRPS cases compared with controls. Significant heterogeneity was detected for IL-1β (χ2 = 4.75, p = 0.03, I2 = 79%), IL-6 (χ2 = 6.61, p = 0.04, I2 = 70%), IL-8 (χ2 = 23.40, p < 0.001, I2 = 91%), and TNFα (χ2 = 9.73, p = 0.008, I2 = 79%).
In agreement with the pooled results, the concentrations of IL-1β,2,22 IL-6,2,22 IL-8,22 IL-10,22 CGRP,2 and NPY2 were not significantly different in CRPS cases compared with controls in the 2 studies for which we could not obtain suitable summary data. However, contrary to the pooled results, the levels of IL-1Ra22, prostaglandin E2 (PGE2),2 and TNFα2,22 were also not significantly different in CRPS cases.
We reported 1 study separately because the sample was almost evenly composed of acute and chronic CRPS cases (mean = 19 ± 17 weeks) and suitable summary data for the meta-analysis was not available.23 The authors found that, in blood, cytokine mRNA and protein concentrations in CRPS cases were higher for TNFα and IL-2, and lower for IL-4 and IL-10 than in controls. Additionally, transforming growth factor-β1 mRNA levels were not different between groups but serum protein levels were found to be higher in CRPS. IL-8 mRNA was lower in CRPS but the IL-8 protein levels were not different between groups.
Five studies did not specify whether the blood sample was taken from the affected or the unaffected arm.14,19,20,22,23 Only 2 studies compared the levels of inflammatory factors in the affected and the unaffected limbs. These found that the levels of IL-6,2 IL-1β,2 TNFα,2 NPY,2 PGE2,2 and CGRP2,17 were not significantly different between the affected and the unaffected limb.
Blister
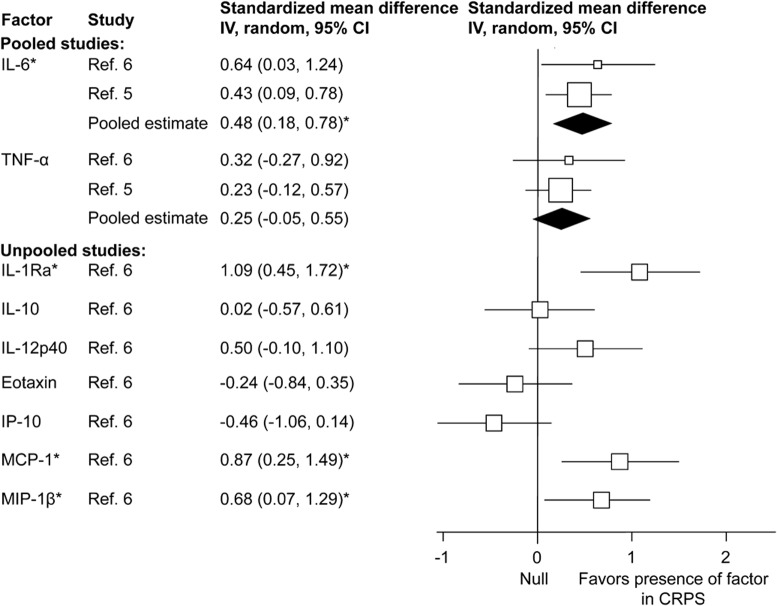
Inflammatory factor concentrations in blister fluid were reported in 5 chronic CRPS studies that compared samples obtained from the CRPS-affected arm with the unaffected arm.2,3,6,24,25 The results are presented in figure 4.
Figure 4. Forest plot of inflammatory factor effect sizes for suction blister fluid samples from chronic complex regional pain syndrome (CRPS) cases (CRPS-affected compared with the unaffected arm).
Each study effect size (standardized mean difference) and weight is indicated by an open box and its 95% confidence intervals (CIs) are indicated by a horizontal line. The filled diamonds represent the overall effect size and CIs for each factor. Statistically significant outcomes are indicated by an asterisk. IL = interleukin; IP-10 = interferon-inducible protein-10; MCP-1 = monocyte chemoattractant protein-1; MIP-1β = macrophage inflammatory protein 1 beta; TNF = tumor necrosis factor.
The pooled estimates demonstrated significantly increased concentrations with large effect sizes for IL-1RA and monocyte chemoattractant protein-1, a moderate effect size for MIP-1β, and a small effect size for IL-6 in the CRPS-affected arm. The levels of IL-10, IL-12p40, TNFα, eotaxin, and interferon-inducible protein-10 (IP-10) were not significantly different in the CRPS-affected arm.
Mixed results were seen for TNFα and IL-6 in studies for which we could not obtain suitable summary data. Specifically, whereas the levels of TNFα were not significantly different in 1 study,2 others found an increase in the CRPS-affected compared with the unaffected arm.3,4,24 Similarly, the levels of IL-6 were not significantly different in 1 study2 but significantly increased in the CRPS-affected compared with the unaffected arm in other studies.3,4,24 Finally, the concentrations of tryptase2 and ET-14 were found to be significantly increased in the CRPS-affected arm, but the levels of IL-1β,2 CGRP,2 NPY,2 and PGE22 were not.
Cerebrospinal fluid
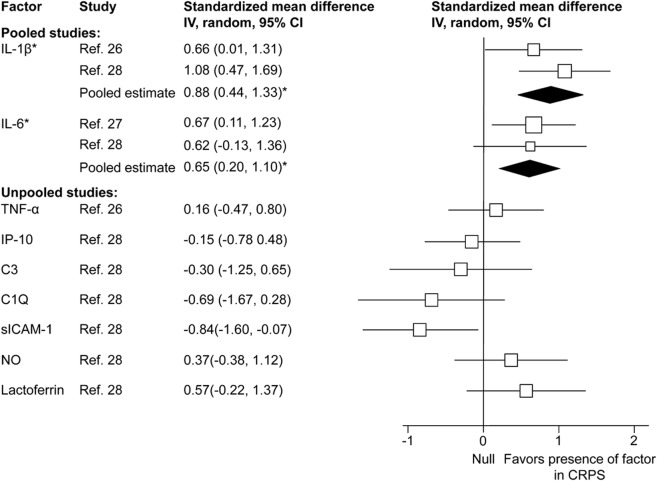
Inflammatory factor concentrations in CSF were reported in 3 chronic CRPS studies comparing CRPS cases to predominantly hospital controls.26–28 The results are presented in figure 5.
Figure 5. Forest plot of inflammatory factor effect sizes for CSF samples from chronic complex regional pain syndrome (CRPS) cases (CRPS cases compared with controls).
Each study effect size (standardized mean difference) and weight is indicated by an open box and its 95% confidence intervals (CIs) are indicated by a horizontal line. The filled diamonds represent the overall effect size and CIs for each factor. Statistically significant outcomes are indicated by an asterisk. IL = interleukin; IP-10 = interferon-inducible protein-10; MCP-1 = monocyte chemoattractant protein-1; MIP-1β = macrophage inflammatory protein 1 beta; NO = nitric oxide; sICAM-1 = soluble intercellular adhesion molecule-1; TNF = tumor necrosis factor.
The pooled effect estimates indicated significantly increased concentrations with a large effect size for IL-1β and a moderate effect size for IL-6 in the CSF of CRPS compared with controls. A significantly decreased concentration with a large effect size was found for intercellular adhesion molecule-1 (ICAM). The concentrations of TNFα, IP-10, C3, C1Q, nitric oxide, and lactoferrin were not significantly different in the CSF of CRPS cases.
DISCUSSION
Our results indicate that, firstly, CRPS is associated with a predominantly proinflammatory state; secondly, that inflammatory profiles differ between acute CRPS and chronic CRPS.
Acute CRPS
We found that higher levels of IL-8 and the TNFα receptors (sTNF-RI, and sTNF-RII) were expressed in the blood of acute CRPS cases than in controls. The levels of TNFα were not increased, but this cytokine is known to have a short half-life29 and can be difficult to measure in the circulation. Consequently, its longer-lasting receptors are considered to provide a more sensitive measure of TNFα30 and other cytokine activity.31,32 Although these and other cytokines can cause sensitization and hyperalgesia,33–35 relationships between cytokine levels and CRPS severity have not been clearly demonstrated. Whereas 1 study found that higher levels of sTNF-RI in CRPS were correlated with mechanical hyperalgesia,36 others found no correlations between the clinical symptoms of CRPS and the levels of TNFα18,25 and IL-6.25
Given the increases in proinflammatory factors and the lack of an attendant increase in the levels of IL-4 and IL-10, it seems that acute CRPS may be associated with an increased Th1-to-Th2 ratio.
Chronic CRPS
We found that the inflammatory factor profile in chronic CRPS, shown in figures 3 to 5, suggests a proinflammatory drive. In particular, the presence of cytokines such as IL-1 and IL-6 in the CSF suggests that the highly proinflammatory Th17 mechanisms may be active. However, Th17 activity has not been adequately tested in CRPS, except for 1 suction blister fluid study that found no increase in IL-17.6 However, unlike in acute CRPS, there also seems to be an antiinflammatory response from IL-4.
Studies that were not pooled because of the unavailability of suitable summary data were broadly consistent with this interpretation. Although 1 study22 found no increase in the blood levels of IL-1Ra and TNFα in chronic CRPS, the subjects recruited in this study had very long-term CRPS (disease duration of 2–37 years) and most had symptoms that had spread to multiple extremities. It is possible that the inflammatory profile keeps changing or that more extensive and prolonged CRPS is more dependent on other factors than inflammation.
Although it is possible that an inflammatory profile characteristic of CRPS exists, our findings may reflect a more generic chronic pain state response. Previous work has shown that chronic pain is associated with higher levels of proinflammatory37 and lower antiinflammatory activity.38 Because very few studies in this review used control subjects who had other causes of chronic pain (e.g., osteoarthritis18), we could not find adequate data to test for a specific inflammatory signature in CRPS that is independent of such generic responses.
We found that acute and chronic cases of CRPS exhibited different inflammatory profiles but we did not find any studies that tested whether acute abnormal inflammatory responses are a characteristic feature of patients with acute trauma who subsequently develop CRPS. Consequently, although this study supports the presence of inflammation in established CRPS, whether a heightened inflammatory response has a causal role in generating the condition remains unknown.
We did not include studies that investigated neurogenic inflammation by using methods to activate C-nociceptor fibers, microdialysis to measure plasma protein extravasation, or skin biopsy. It has been proposed that sensitization of primary afferents by cytokines may contribute to the symptoms of CRPS through a local release of neuropeptides.39 However, in our study, we found that the levels of neuropeptides in the blood and blister fluid of CRPS cases were not higher than in controls.
Finally, our study found little empirical support for the position that the CRPS-affected limb expresses higher inflammatory factors than the unaffected limb. We note that this may be attributable in part to methodologic and/or reporting shortcomings because many studies failed to clearly report from which side samples were obtained, and only 3 directly compared the levels in samples obtained from both sides.2,15,17 Importantly, those studies that performed these side-to-side comparisons concluded that the levels of inflammatory factors in blood are not significantly different between the affected and the unaffected limb in either acute or chronic CRPS.
Study strengths and limitations
The main strength of this study is the systematic approach to the identification and analysis of CRPS research data and study quality assessment. However, our findings should be interpreted in light of some of the limitations of the included evidence.
We found considerable variation in the characteristics of the source studies including diagnostic criteria and the methods of collection and analysis of samples. We also found that the quality of reporting was inconsistent. In particular, studies frequently failed to specify assay sensitivities and how sample concentrations below these sensitivities were handled during analysis. We pooled the results from all reported assay methods and blood tests, but we recognize that it is possible that these variables may influence effect sizes. At times, we were not able to extract data in the required format and used a published method to estimate the required data.11 Finally, although we performed separate analyses on acute and chronic CRPS, we found significant variability in the concentrations of individual inflammatory factors. This may be attributable to heterogeneity in study methodology and quality that is highlighted by 95% of studies failing to meet more than 2 of our 12 quality criteria. Unfortunately, the small number of studies available for each analysis (figures 2–5) did not permit a sensitivity analysis by study quality. The variability in our results may also indicate that CRPS is not a distinct entity but rather a collection of disorders that manifest in a similar manner clinically; that the inflammatory response is not a consistent feature of CRPS14; or that clinically important subgroups of CRPS exist.40 For instance, perhaps the presence of substantial autonomic nervous system involvement or cold vs hot CRPS may be associated with different levels of inflammatory factors. In support of our overall approach to pooling, individual study effect sizes were relatively congruent and no extreme effect sizes were noted. Also, after subgrouping studies by duration of CRPS, only 4 analyses were found to display significant signs of heterogeneity.
Recommendations
These results suggest that appropriate antiinflammatory therapies may be useful in managing some cases of CRPS. A recent review of pharmacologic treatments of CRPS found limited evidence from a small collection of trials that oral prednisolone improves the symptoms of CRPS and conflicting evidence for the efficacy of free-radical scavengers (dimethyl sulfoxide and N-acetylcysteine).41 The trials were generally small and of mixed methodologic quality.41 Formal, large, high-quality studies are required to identify clinical subgroups of CRPS and to assess the clinical efficacy of specific cytokine inhibitors (e.g., TNFα inhibitors) or immunosuppressive medication in this condition. Furthermore, we recommend further in vivo research on the immunomodulatory effects of common analgesic medications. For instance, morphine has been shown, in vitro, to exert proinflammatory effects by decreasing IL-10 and increasing IL-12 levels42; these effects should be considered in conditions such as CRPS where adverse inflammation may be present.
Finally, we recommend that future studies consider examining the possible role of inflammation in the developmental stages of CRPS, relationships between inflammatory factors and clinical symptoms, the role of Th17 inflammatory factors in CRPS, and differences between the levels of inflammatory factors in CRPS vs other chronic pain conditions. Furthermore, we would strongly recommend that published studies include clear details about the source, collection, and processing of samples, including assay sensitivity and the proportion and management of samples that fall below the lowest detectable threshold values.
Supplementary Material
GLOSSARY
- CI
confidence interval
- CGRP
calcitonin gene-relaxed peptide
- CRPS
complex regional pain syndrome
- ET-1
endothelin-1
- IL
interleukin
- IP-10
interferon-inducible protein-10
- MCP-1
monocyte chemoattractant protein-1
- NPY
neuropeptide Y
- PGE2
prostaglandin E2
- SP
substance P
- sTNF-R
soluble tumor necrosis factor receptor
- TNF
tumor necrosis factor
APPENDIX
Search terms: 1) the condition: complex regional pain syndrome, CRPS, algoneurodystrophy, algodystrophy, shoulder-hand syndrome, reflex sympathetic dystrophy, RSD, Sudeck, Sudek, causalgia, sympathetically maintained pain, SMP, post*traumatic dystrophy; and 2) inflammation: inflam*, immune*, cytokine*, mediator, serum, plasma, blood, CSF, cerebro*, blister, biopsy.
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
L. Parkitny and J.H. McAuley were responsible for drafting/revising the manuscript for content, study concept and design, and the analysis and interpretation of data. F. Di Pietro was responsible for drafting/revising the manuscript for content, and study concept and design. T.R. Stanton was responsible for drafting/revising the manuscript for content, study concept and design, and analysis and interpretation of data. N.E. O'Connell, J. Marinus, and J.J. van Hilten were responsible for drafting/revising the manuscript for content, and analysis and interpretation of data. G.L. Moseley was responsible for drafting/revising the manuscript for content, study concept and design, and analysis and interpretation of data.
STUDY FUNDING
This work was supported by the Australian National Health and Medical Research Council (NHMRC) project grant 630431; L. Parkitny is supported by an NHMRC scholarship; F. Di Pietro is supported by an Australian Postgraduate Award scholarship; T.R. Stanton is supported by a Canadian Institutes of Health Research postdoctoral training award ID 223354; the work of J. Marinus and J.J. van Hilten from the Dutch consortium on CRPS (TREND) is supported by a grant of the Dutch Ministry of Economic Affairs (BSIK03016); G.L. Moseley is supported by fellowship ID 571090.
DISCLOSURE
L. Parkitny receives an NHMRC (Australia) scholarship; has received travel funding from several professional bodies; and derived income from teaching pain management. J.H. McAuley reports no disclosures. F. Di Pietro receives an Australian postgraduate award scholarship. T.R. Stanton receives a Canadian Institutes of Health Research postdoctoral training award (ID 223354). N.E. O’Connell reports no disclosures. J. Marinus and J.J. van Hilten are supported by a grant of the Dutch Ministry of Economic Affairs (BSIK03016). G.L. Moseley is funded by an NHMRC (Australia) fellowship ID 571090. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Marinus J, Moseley GL, Birklein F, et al. Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurol 2011;10:637–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huygen F, de Bruijn AGJ, de Bruin MT, Groeneweg JG, Klein J, Zijlstra FJ. Evidence for local inflammation in complex regional pain syndrome type 1. Mediators Inflamm 2002;11:47–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huygen FJ, Ramdhani N, van Toorenenbergen A, Klein J, Zijlstra FJ. Mast cells are involved in inflammatory reactions during complex regional pain syndrome type 1. Immunol Lett 2004;91:147–154 [DOI] [PubMed] [Google Scholar]
- 4.Groeneweg JG, Huygen FJ, Heijmans-Antonissen C, Niehof S, Zijlstra FJ. Increased endothelin-1 and diminished nitric oxide levels in blister fluids of patients with intermediate cold type complex regional pain syndrome type 1. BMC Musculoskelet Disord 2006;7:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wesseldijk F, Huygen FJ, Heijmans-Antonissen C, Niehof SP, Zijlstra FJ. Six years follow-up of the levels of TNF-alpha and IL-6 in patients with complex regional pain syndrome type 1. Mediators Inflamm 2008;2008:469439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heijmans-Antonissen C, Wesseldijk F, Munnikes RJ, et al. Multiplex bead array assay for detection of 25 soluble cytokines in blister fluid of patients with complex regional pain syndrome type 1. Mediators Inflamm 2006;2006:28398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–1012 [DOI] [PubMed] [Google Scholar]
- 8.Gallo V, Egger M, McCormack V, et al. STrengthening the Reporting of OBservational studies in Epidemiology–Molecular Epidemiology STROBE-ME: an extension of the STROBE statement. J Clin Epidemiol 2011;64:1350–1363 [DOI] [PubMed] [Google Scholar]
- 9.Flower L, Ahuja RH, Humphries SE, Mohamed-Ali V. Effects of sample handling on the stability of interleukin 6, tumour necrosis factor-alpha and leptin. Cytokine 2000;12:1712–1716 [DOI] [PubMed] [Google Scholar]
- 10.Skogstrand K, Ekelund CK, Thorsen P, et al. Effects of blood sample handling procedures on measurable inflammatory markers in plasma, serum and dried blood spot samples. J Immunol Methods 2008;336:78–84 [DOI] [PubMed] [Google Scholar]
- 11.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Baltimore: The Cochrane Collaboration; 2011 [Google Scholar]
- 13.Cohen J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed Hillsdale, NJ: Lawrence Erlbaum Associates; 1998 [Google Scholar]
- 14.Alexander GM, Peterlin BL, Perreault MJ, Grothusen JR, Schwartzman RJ. Changes in plasma cytokines and their soluble receptors in complex regional pain syndrome. J Pain 2012;13:10–20 [DOI] [PubMed] [Google Scholar]
- 15.Schinkel C, Gaertner A, Zaspel J, Zedler S, Faist E, Schuermann M. Inflammatory mediators are altered in the acute phase of posttraumatic complex regional pain syndrome. Clin J Pain 2006;22:235–239 [DOI] [PubMed] [Google Scholar]
- 16.Schinkel C, Scherens A, Koller M, Roellecke G, Muhr G, Maier C. Systemic inflammatory mediators in post-traumatic complex regional pain syndrome (CRPS I): longitudinal investigations and differences to control groups. Eur J Med Res 2009;14:130–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birklein F, Schmelz M, Schifter S, Weber M. The important role of neuropeptides in complex regional pain syndrome. Neurology 2001;57:2179–2184 [DOI] [PubMed] [Google Scholar]
- 18.Krämer HH, Eberle T, Uceyler N, et al. TNF-alpha in CRPS and ‘normal' trauma: significant differences between tissue and serum. Pain 2011;152:285–290 [DOI] [PubMed] [Google Scholar]
- 19.Ritz BW, Alexander GM, Nogusa S, et al. Elevated blood levels of inflammatory monocytes (CD14+ CD16+) in patients with complex regional pain syndrome. Clin Exp Immunol 2011;164:108–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blair SJ, Chinthagada M, Hoppenstehdt D, Kijowski R, Fareed J. Role of neuropeptides in pathogenesis of reflex sympathetic dystrophy. Acta Orthop Belg 1998;64:448–451 [PubMed] [Google Scholar]
- 21.Eisenberg E, Erlich T, Zinder O, et al. Plasma endothelin-1 levels in patients with complex regional pain syndrome. Eur J Pain 2004;8:533–538 [DOI] [PubMed] [Google Scholar]
- 22.van de Beek WJ, Remarque EJ, Westendorp RG, van Hilten JJ. Innate cytokine profile in patients with complex regional pain syndrome is normal. Pain 2001;91:259–261 [DOI] [PubMed] [Google Scholar]
- 23.Üçeyler N, Eberle T, Rolke R, Birklein F, Sommer C. Differential expression patterns of cytokines in complex regional pain syndrome. Pain 2007;132:195–205 [DOI] [PubMed] [Google Scholar]
- 24.Munnikes RJ, Muis C, Boersma M, Heijmans-Antonissen C, Zijlstra FJ, Huygen FJ. Intermediate stage complex regional pain syndrome type 1 is unrelated to proinflammatory cytokines. Mediators Inflamm 2005;2005:366–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wesseldijk F, Huygen FJ, Heijmans-Antonissen C, Niehof SP, Zijlstra FJ. Tumor necrosis factor-alpha and interleukin-6 are not correlated with the characteristics of complex regional pain syndrome type 1 in 66 patients. Eur J Pain 2008;12:716–721 [DOI] [PubMed] [Google Scholar]
- 26.Alexander GM, van Rijn MA, van Hilten JJ, Perreault MJ, Schwartzman RJ. Changes in cerebrospinal fluid levels of pro-inflammatory cytokines in CRPS. Pain 2005;116:213–219 [DOI] [PubMed] [Google Scholar]
- 27.Alexander GM, Perreault MJ, Reichenberger ER, Schwartzman RJ. Changes in immune and glial markers in the CSF of patients with Complex Regional Pain Syndrome. Brain Behav Immun 2007;21:668–676 [DOI] [PubMed] [Google Scholar]
- 28.Munts AG, Zijlstra FJ, Nibbering PH, et al. Analysis of cerebrospinal fluid inflammatory mediators in chronic complex regional pain syndrome related dystonia. Clin J Pain 2008;24:30–34 [DOI] [PubMed] [Google Scholar]
- 29.Beutler BA, Milsark IW, Cerami A. Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J Immunol 1985;135:3972–3977 [PubMed] [Google Scholar]
- 30.Lantz M, Malik S, Slevin ML, Olsson I. Infusion of tumor necrosis factor (TNF) causes an increase in circulating TNF-binding protein in humans. Cytokine 1990;2:402–406 [DOI] [PubMed] [Google Scholar]
- 31.Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood 1994;83:113–118 [PubMed] [Google Scholar]
- 32.Bargetzi MJ, Lantz M, Smith CG, et al. Interleukin-1 beta induces interleukin-1 receptor antagonist and tumor necrosis factor binding protein in humans. Cancer Res 1993;53:4010–4013 [PubMed] [Google Scholar]
- 33.Cunha FQ, Lorenzetti BB, Poole S, Ferreira SH. Interleukin-8 as a mediator of sympathetic pain. Br J Pharmacol 1991;104:765–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Czeschik JC, Hagenacker T, Schafers M, Busselberg D. TNF-alpha differentially modulates ion channels of nociceptive neurons. Neurosci Lett 2008;434:293–298 [DOI] [PubMed] [Google Scholar]
- 35.Jin X, Gereau RW., 4th Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci 2006;26:246–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maihöfner C, Handwerker HO, Neundorfer B, Birklein F. Mechanical hyperalgesia in complex regional pain syndrome: a role for TNF-alpha? Neurology 2005;65:311–313 [DOI] [PubMed] [Google Scholar]
- 37.Koch A, Zacharowski K, Boehm O, et al. Nitric oxide and pro-inflammatory cytokines correlate with pain intensity in chronic pain patients. Inflamm Res 2007;56:32–37 [DOI] [PubMed] [Google Scholar]
- 38.Uceyler N, Valenza R, Stock M, Schedel R, Sprotte G, Sommer C. Reduced levels of antiinflammatory cytokines in patients with chronic widespread pain. Arthritis Rheum 2006;54:2656–2664 [DOI] [PubMed] [Google Scholar]
- 39.Birklein F, Schmelz M. Neuropeptides, neurogenic inflammation and complex regional pain syndrome (CRPS). Neurosci Lett 2008;437:199–202 [DOI] [PubMed] [Google Scholar]
- 40.Bruehl S, Harden RN, Galer BS, Saltz S, Backonja M, Stanton-Hicks M. Complex regional pain syndrome: are there distinct subtypes and sequential stages of the syndrome? Pain 2002;95:119–124 [DOI] [PubMed] [Google Scholar]
- 41.Fischer SG, Zuurmond WW, Birklein F, Loer SA, Perez RS. Anti-inflammatory treatment of complex regional pain syndrome. Pain 2010;151:251–256 [DOI] [PubMed] [Google Scholar]
- 42.Messmer D, Hatsukari I, Hitosugi N, Schmidt-Wolf IG, Singhal PC. Morphine reciprocally regulates IL-10 and IL-12 production by monocyte-derived human dendritic cells and enhances T cell activation. Mol Med 2006;12:284–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goris RJ, Leixnering M, Huber W, Figl M, Jaindl M, Redl H. Delayed recovery and late development of complex regional pain syndrome in patients with an isolated fracture of the distal radius: prediction of a regional inflammatory response by early signs. J Bone Joint Surg Br 2007;89:1069–1076 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.