ABSTRACT
Objectives:
Age and stroke severity are major determinants of stroke outcomes, but systematically incorporating these prognosticators in the routine practice of acute ischemic stroke can be challenging. We evaluated the effect of an index combining age and stroke severity on response to IV tissue plasminogen activator (tPA) among patients in the National Institute of Neurological Disorders and Stroke (NINDS) tPA stroke trials.
Methods:
We created the Stroke Prognostication using Age and NIH Stroke Scale (SPAN) index by combining age in years plus NIH Stroke Scale (NIHSS) ≥100. We applied the SPAN-100 index to patients in the NINDS tPA stroke trials (parts I and II) to evaluate its ability to predict clinical response and risk of intracerebral hemorrhage (ICH) after thrombolysis. The main outcome measures included ICH (any type) and a composite favorable outcome (defined as a modified Rankin Scale score of 0 or 1, NIHSS ≤1, Barthel index ≥95, and Glasgow Outcome Scale score of 1) at 3 months. Bivariate and multivariable logistic regression analyses were used to determine the association between SPAN-100 and outcomes of interest.
Results:
Among 624 patients in the NINDS trials, 62 (9.9%) participants were SPAN-100 positive. Among those receiving tPA, ICH rates were higher for SPAN-100–positive patients (42% vs 12% in SPAN-100–negative patients; p < 0.001); similarly, ICH rates were higher in SPAN-100–positive patients (19% vs 5%; p = 0.005) among those not receiving tPA. SPAN-100 was associated with worse outcomes. The benefit of tPA, defined as favorable composite outcome at 3 months, was present in SPAN-100–negative patients (55.4% vs 40.2%; p < 0.001), but not in SPAN-100–positive patients (5.6% tPA vs 3.9%; p = 0.76). Similar trends were found for secondary outcomes (e.g., symptomatic ICH, catastrophic outcome, discharge home).
Conclusion:
The SPAN-100 index could be a simple method for estimating the clinical response and risk of hemorrhagic complications after tPA for acute ischemic stroke. These results need further confirmation in larger contemporary datasets.
Age and stroke severity are the 2 most important determinants of stroke outcomes.1–3 The decision to treat with IV thrombolysis (tissue plasminogen activator [tPA]) may be challenging in elderly patients with high NIH Stroke Scale (NIHSS) score. Previous clinical trials of IV thrombolysis excluded patients who were older or had higher NIHSS scores. For example, the European Cooperative Acute Stroke Study (ECASS) and Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke trials excluded patients older than 80 years4–6 and ECASS 3 excluded patients with a baseline NIHSS >25.7 There were no upper limits for age or exclusion criteria based on NIHSS in the National Institute of Neurological Disorders and Stroke (NINDS) tPA stroke trial.8
Although age is not a formal contraindication for thrombolysis, observational studies and randomized trials have shown poorer outcomes and higher risk of bleeding in the elderly.9,10 Stroke patients and their families request information about the likelihood of a good outcome and risk of intracranial bleeding if tPA is given, and clinicians have to weigh multiple factors when deciding to treat with thrombolysis, especially in patients excluded from clinical trials.4–6 Despite an improvement on physicians' estimations, risk calculators are not widely used among clinicians, even when available online and free.11
The objective of this study was to create a simple and practical index that can be systematically and consistently applied in routine clinical practice, and assess its role as a prognosticator among tPA-treated acute ischemic stroke patients.
METHODS
We created the Stroke Prognostication using Age and NIHSS (SPAN) index. Individuals whose age in years plus NIHSS score was greater than or equal to 100 are designated as SPAN-100–positive patients, while those with a score <100 are designated as SPAN-100–negative. The rationale for the creation of this index was 1) age and stroke severity are the 2 most important prognostic factors for acute ischemic stroke,1–3 2) patients aged 80 and older and with high NIHSS (e.g., ≥20) have poorer prognosis,10,12 and 3) a simple and easy index is needed considering the limited use of currently available scores that require more complex estimations.13–15 We then applied the SPAN-100 index to patients encountered in both NINDS tPA trials. The NINDS tPA stroke trials were multicenter, double-blind, placebo-controlled, randomized trials of IV tPA for acute ischemic stroke performed from January 1991 through October 1994.8 A noncontrast CT scan of the brain was mandatory before enrollment in the study to rule out intracerebral hemorrhage (ICH). All the baseline CT scans were obtained with 10-mm slice thickness. Further details and methodology of the trial have been previously published.8,16 We used the NINDS tPA trial to compare clinical outcomes and the risk of hemorrhagic complication between SPAN-100–positive and SPAN-100–negative patients randomized to tPA and placebo.
Outcome measures
Main outcome measures included ICH (symptomatic and any type) and a composite favorable outcome. ICH was defined as the presence of any hemorrhagic transformation that occurred within 36 hours after treatment. Symptomatic ICH (sICH) was defined if a decline in the neurologic status was documented. Composite favorable outcome was defined as in the NINDS tPA trial: a modified Rankin Scale (mRS) score of 0 or 1, NIHSS ≤1, Barthel index ≥95, and Glasgow Outcome Scale score of 1 at 3 months.8
Secondary outcomes included 1) fatal ICH, 2) catastrophic outcome defined as a mRS of 4 to 6 at 3 months, 3) discharge home, 4) death at 3 months, and 5) lack of neurologic improvement defined as less than 3-point difference between baseline and 24-hour NIHSS.17
Standard protocol approvals, registrations, and patient consents
In the NINDS trial, informed consent was obtained for all patients.8 Approvals from the St. Michael's Hospital review board were obtained. We described our findings in accordance with the CONSORT 2010 Statement.
Statistical analysis
To compare categorical variables, χ2 tests were used; analysis of variance or Kruskal-Wallis tests were used to compare mean and median differences for continuous variables. The primary analysis was conducted to evaluate the association between SPAN-100 with the outcomes of interest. Secondary analyses were conducted using logistic regression with adjustment for age, NIHSS, tPA, and SPAN-100 to determine whether there was an interaction between SPAN-100 and tPA. The attributable risk (AR) was estimated as the difference between the event rate in the exposed and nonexposed groups. The population attributable risk (PAR) estimates the proportion of the outcome in the study population that is attributable to the exposure (SPAN-100). We reported the PAR by applying the following formula: [PAR = AR × prevalence of SPAN-100]. The number needed to treat (NNT) and the number needed to harm (NNH) were calculated as the inverse of the absolute risk difference between tPA and placebo, and 95% confidence intervals (CIs) for each are reported.18 Composite favorable outcome, catastrophic functional outcome, discharge home, and lack of improvement were available for all patients at 3 months. Twelve-month data for the estimation of catastrophic outcomes were available for 598 patients.
A sensitivity analysis was conducted to compare different cutoff points of the SPAN index (e.g., 90, 95, 100, 105, 110) expressed as the area under the curve (AUC). We reported p value with adjustment for multiple tests for the comparison of AUCs of different cutoff points with the AUC for SPAN-100. We also analyzed the effect of SPAN-100 status by onset to treatment. We used the same categories (≤90 [n = 302] vs >91 minutes [n = 322]) as defined in the NINDS tPA trial.8
Statistical analysis was performed using STATA version 9 (StataCorp LP, College Station, TX). Rocgold command was used to compare AUCs for different SPAN cutoff points. All tests were 2-tailed, and p values <0.05 were considered significant.
RESULTS
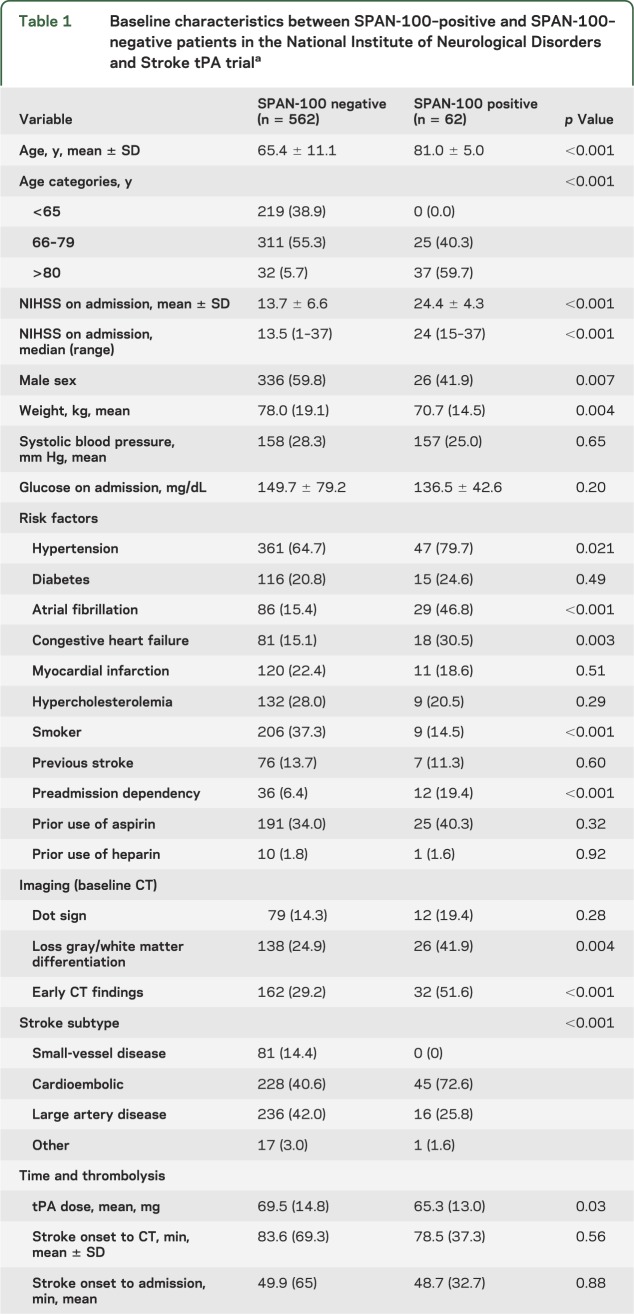
The SPAN-100 index was applied to all 624 patients enrolled in the trials. There were 62 (9.9%) participants who met criteria for the SPAN-100–positive group. SPAN-100–positive patients were more likely to be women, and to have atrial fibrillation, congestive heart failure, early ischemic changes, and a cardioembolic stroke compared to SPAN-100–negative patients (table 1). There were no differences in time from stroke onset to admission or CT between groups. The mean baseline NIHSS was 14 (range 1–37) in the SPAN-100–negative and 24 (15–37) in the SPAN-100–positive patients. The mean age was 65 years (range 26–89) and 81 years (range 65–89) for SPAN-100–negative and SPAN-100–positive groups, respectively. Other differences in baseline characteristics are summarized in table 1. The AR of SPAN-100 for ICH was 23.7% and for sICH was 9.2%, whereas the AR of SPAN-100 for catastrophic outcome (mRS 4–6) at 3 months was 23.3%. The PARs were 2.4 for ICH, 0.9% for sICH, and 2.3% and 5.5% for catastrophic outcome at 3 and 12 months, respectively.
Table 1.
Baseline characteristics between SPAN-100–positive and SPAN-100–negative patients in the National Institute of Neurological Disorders and Stroke tPA triala
There were 276 SPAN-100–negative patients (49.1%) and 36 SPAN-100–positive patients (58.1%) who received thrombolysis (p = 0.18). The final mean infarct volume based on CT at 3 months was 56.1 ± 88 mL for the SPAN-100–negative group and 143.7 ±128 mL for the SPAN-100–positive group (p < 0.0001).
Outcome measures
Intracerebral hemorrhage
Overall, ICH was seen in 20 (32.3%) SPAN-100–positive patients and 48 (8.5%) SPAN-100–negative patients (p < 0.0001). SPAN-100–positive patients had a higher ICH rate compared to SPAN-100–negative patients receiving tPA (41.7% vs 12.0%; p < 0.0001) or placebo (19.2% vs 5.2%; p < 0.01). Figure 1A represents the incidence of ICH by treatment effect in each group. tPA administration doubled the risk of ICH of any type in both SPAN-100–positive and SPAN-100–negative groups. Symptomatic and fatal ICH were also more common in the SPAN-100–positive patients (table 2). The NNH for ICH in the SPAN-100–negative and SPAN-100–positive groups were 14 and 4, respectively.
Figure 1. Main outcomes by the SPAN-100 group in tPA and placebo.
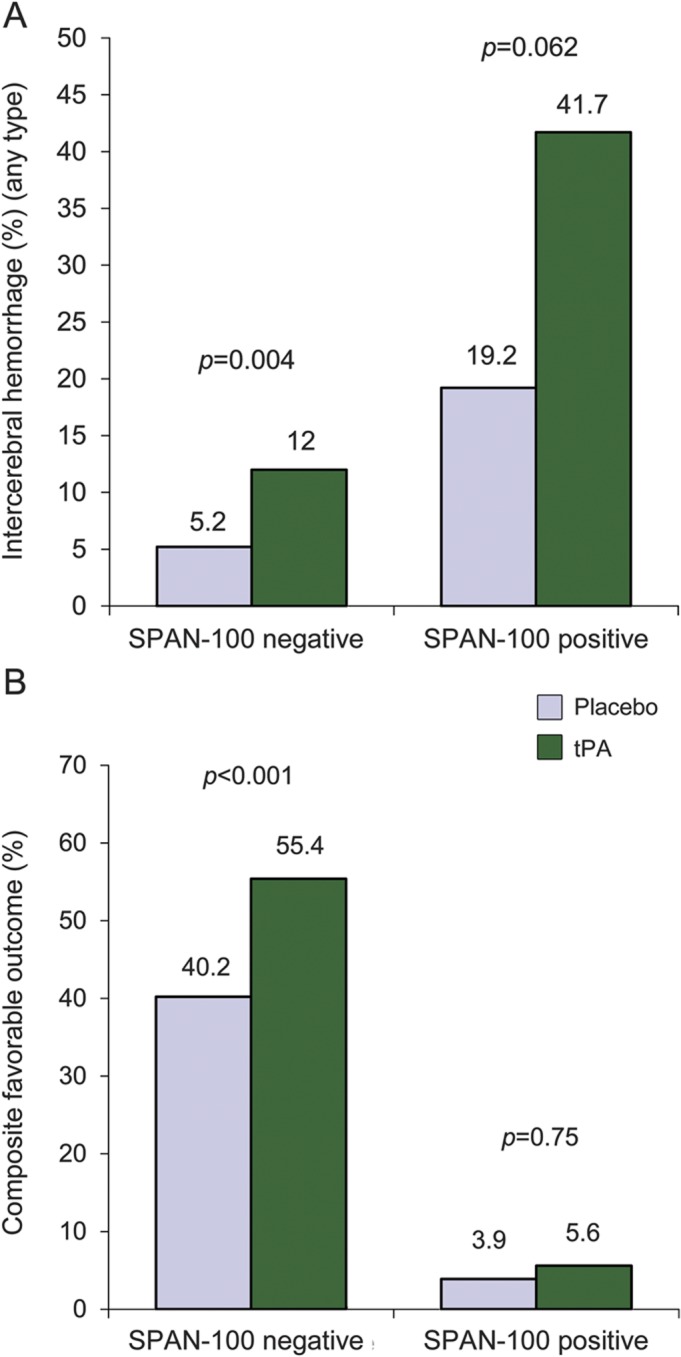
(A) Intracerebral hemorrhage (any type). (B) Composite favorable outcome, defined as a modified Rankin scale score of 0 or 1, NIH Stroke Scale score ≤1, Barthel index ≥95, or Glasgow Outcome Scale score <1. Further details are explained in the text. SPAN = Stroke Prognostication using Age and NIH Stroke Scale; tPA = tissue plasminogen activator.
Table 2.
Outcome measures according to the SPAN-100 index by treatment assignmenta
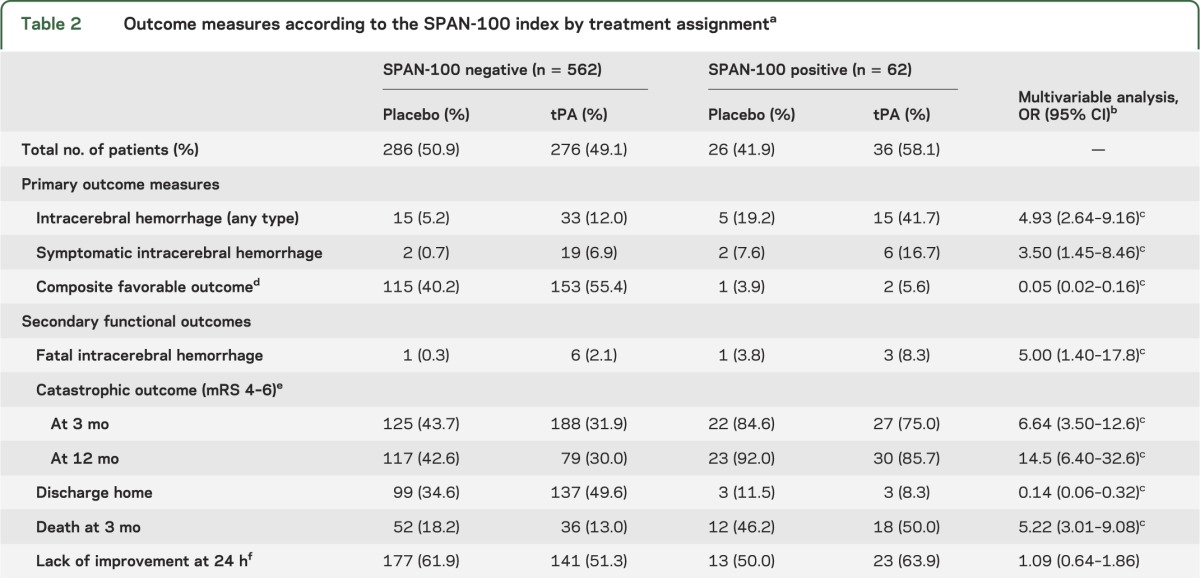
In the logistic regression analysis after adjusting for tPA, SPAN-100 patients had a 3.5-fold increase in the odds of sICH (c statistic 0.733; correctly classified 95.4%). Similarly, SPAN-100 patients had a nearly 5-fold increased odds of ICH of any type (c statistic 0.682; correctly classified 89.1%) (table 2). There was no statistically significant interaction between SPAN-100 status and tPA for sICH (p = 0.20) or ICH (p = 0.77).
Composite favorable outcome
Among SPAN-100–negative patients, outcomes with tPA were more favorable compared to placebo (for a composite favorable outcome at 3 months: 55.4% vs 40.2%; p < 0.001, NNT 7). However, only a small number of SPAN-100–positive patients (n = 2) achieved a favorable outcome at 3 months (5.6% tPA vs 3.9%; p = 0.76) (figure 1B).
In the logistic regression analysis after adjusting for tPA, SPAN-100 status was associated with a significantly lower odds of favorable outcome (c statistic 0.641). No significant interaction was found between SPAN-100 status and tPA (p = 0.86).
Secondary functional outcomes
Secondary functional outcomes are detailed in figure 2.
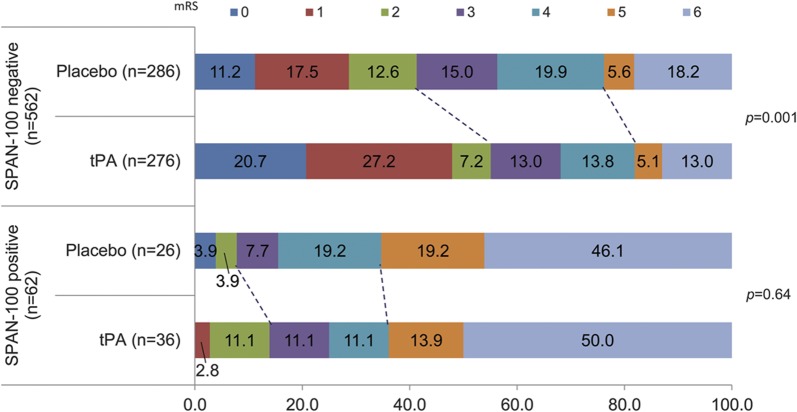
Figure 2. Relationship between functional outcome at 3 months according to the modified Rankin Scale and SPAN-100 by treatment assignment.
This figure illustrates the disability at 3 months according to the modified Rankin Scale (mRS) (0 = no symptoms; 6 = death) between tissue plasminogen activator (tPA) and placebo for patients stratified by Stroke Prognostication using Age and NIH Stroke Scale (SPAN)–100. The dotted lines show the corresponding category (mRS 0–2 and mRS 5–6) between tPA and placebo in each strata. For participants in the SPAN-100–negative group, tPA administration was associated with a significant chance of a favorable functional outcome (mRS 0–2) at 3 months (p < 0.001). Contrarily, there was no benefit among those in the SPAN-100–positive group with the administration of tPA (p = 0.64).
Among SPAN-100–negative patients, tPA administration was associated with lower risk of a catastrophic outcome (major disability or death as defined by mRS 4–6) at 3 months (tPA 31.9% vs 43.7% with placebo; p < 0.004, NNT 8). Contrarily, no significant reduction in death or major disability was observed with the administration of tPA among SPAN-100 patients (tPA 75.0% vs 84.6% placebo; p = 0.36) (table 2). Similar results were observed for catastrophic outcome at 12 months.
Compared to placebo, SPAN-100–negative patients receiving tPA were more likely to be discharged home (49.6% vs 34.6%; p < 0.001, NNT 7). Contrarily, no benefit was observed with tPA for SPAN-100–positive patients (8.3% vs 11.5%; p = 0.67). SPAN-100–positive patients receiving tPA had a significantly higher mortality at 3 months than SPAN-100–negative patients (50.0% vs 13.0%; p < 0.001, NNH 3). Other secondary functional outcomes are reported in table 2.
Sensitivity analysis
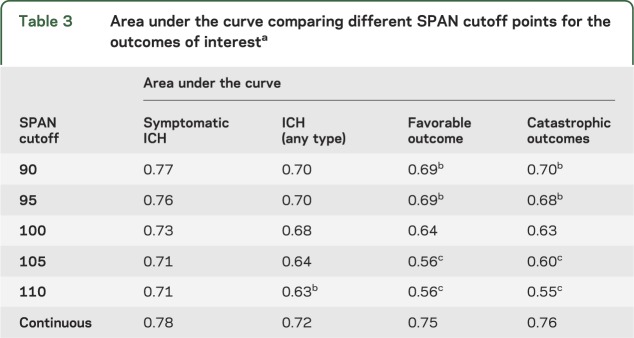
The sensitivity analysis showed a slightly better performance (2–5 points) for lower cutoff points (e.g., SPAN 90 or 95) for the primary outcomes (table 3). There was no significant difference between AUCs (SPAN-90 or SPAN-95 vs SPAN-100) for the primary outcome (ICH of any type or symptomatic). There were no patients with a favorable outcome at 3 months with SPAN cutoff of 105 or 110.
Table 3.
Area under the curve comparing different SPAN cutoff points for the outcomes of interesta
Time to treatment
SPAN-100 was associated with ICH in patients treated within 90 minutes (odds ratio [OR] 6.07, 95% CI 2.52–14.6; p < 0.0001) and beyond 90 minutes (OR 4.02, 95% CI 1.66–9.76; p = 0.002). Similarly, SPAN-100 was associated with a lower chance of a favorable outcome in both groups (patients treated within 90 minutes: OR 0.039, 95% CI 0.005–90.29; p < 0.0001; patients treated after 90 minutes: OR 0.032, 95% CI 0.0043–90.24; p = 0.002). There was no significant interaction between time to treatment and SPAN-100 status for any of the primary or secondary outcomes (p value for all interactions >0.05).
DISCUSSION
Clinicians need simple and practical tools when discussing prognosis with stroke patients and their families. The prediction of a favorable outcome and risk of ICH after thrombolysis represent a challenge in elderly stroke patients with high NIHSS. The available tools (e.g., iScore, TPI, DRAGON, ASTRAL, Hemorrhage after Thrombolysis [HAT]) are complex; require interpretation of stroke subtype, imaging, or visual fields; or lack validation in a control group (non-tPA), and as a result may not be extensively used.13,15,19–21
In the present study, we analyzed short- and long-term clinical outcomes and risk of hemorrhagic complication by combining 2 stronger predictors (age and NIHSS score) of outcome after stroke in the NINDS tPA stroke trial. Together, the addition of age plus NIHSS ≥100 (SPAN-100) was associated with 3-fold higher risk of ICH (absolute risk after tPA: 41.7% in the SPAN-100–positive vs 12.0% in SPAN-100–negative patients), and no improvement in favorable outcomes (table 2) irrespective of time from stroke onset to treatment. At 1 year, 85.7% of SPAN-100–positive patients receiving tPA had a catastrophic outcome (mRS 4–6) compared to 30% in the SPAN-100–negative group.
SPAN-100 patients had 2.6 times larger final infarct volumes (determined by CT at 3 months) and 5-fold higher mortality at 3 months compared to the SPAN-100–negative patients.
At the population level (prevalence 10%), there would be one extra ICH out of 42 stroke patients attributed to SPAN-100. Similarly, there would be one extra patient with a catastrophic outcome at 12 months for every 18 stroke patients due to SPAN-100.
In a recently presented study, outcomes in 257 subjects from the University of California, San Diego (UCSD) stroke registry were analyzed using a similar definition: age + NIHSS ≥100 (n = 53), coining the term “Stroke 100 Club.”22 In contrast to our study, they found no difference in the sICH rate between tPA-treated (3.8%) and non-tPA–treated (3.4%) patients (p = 0.99). Differences in sample size (smaller in the UCSD cohort), inclusion criteria, adjustment, and stroke care practices (NINDS includes patients enrolled in 1994) may explain the observed disparity.22 Our group, using a more complex index in the Canadian Stroke Registry, found that patients with an iScore greater than 200 had no apparent benefit from IV tPA and a 3-fold higher risk of hemorrhagic complications (20% vs 6%; p < 0.001).21 The iScore is a risk score that estimates functional outcomes in patients with an ischemic stroke early after hospitalization using clinical parameters and comorbid conditions.14,21,23 When the iScore was applied to the NINDS tPA trial, an iScore >200 was associated with 3-fold higher risk of ICH (OR 3.08, 95% CI 1.41–6.74; 30.8% tPA vs 11.5% placebo, NNH 5).24 This is similar to the observed results for the SPAN-100 status in the present study (OR 3.50, 95 CI 1.45–8.46 for sICH and OR 4.93, 95% CI 2.64–9.16 for any ICH). Further, both the iScore ≥200 and SPAN-100 positive were associated with a similarly lower favorable composite outcome at 3 months after adjustment for tPA (iScore ≥200: OR 0.16, 95% CI 0.09–0.27; SPAN-100: OR 0.05, 95% CI 0.02–0.16).
The HAT score is a 5-point scale based on NIHSS score, extent of hypodensity on CT scan, serum glucose at baseline, and history of diabetes to predict the risk of hemorrhage after thrombolysis. The rate of any ICH in the NINDS tPA arm was strongly related to the HAT score (c statistic 0.70).19
The DRAGON is a 10-point score that includes early infarct signs or hyperdense cerebral artery, prestroke mRS, age, onset to treatment time, and stroke severity. It showed good predictive ability (AUC 0.84) for a favorable outcome (mRS 0–2) after thrombolysis in both the derivation (n = 1,319) and validation (n = 333) cohorts.15 The ASTRAL score, which includes age, severity of stroke, stroke onset to admission time, range of visual fields, glucose on admission, and level of consciousness, also showed a good predictive value (AUC 0.85) for unfavorable outcome (mRS > 2) in 1,645 patients with an ischemic stroke.20 There was no specific cutoff reported for the ASTRAL or DRAGON scores for the group of patients who may not benefit from tPA.15,20
Although potentially useful for prognostication, these scores require online access or the use of handheld devices, as well as the expertise and time to perform the necessary calculations, thus representing a barrier for busy clinicians assisting patients in an emergency setting. The SPAN-100 index is a simple and easy to calculate score that provides useful information on both clinical response to tPA (favorable and poor outcomes) at different points in time (24 hours, 3 months, and 1 year) as well as risk of ICH after thrombolysis. It does not require the interpretation of imaging, stroke subtype, or other precise measures to provide outcome estimations.
Our study has some limitations that deserve comment. First, the use of only 2 variables to create a score may be too simplistic. As showed by our group and others, other factors influence clinical outcomes and the risk of bleeding.13,14,19,21 Some clinicians may argue that elderly patients (age >80) with a low NIHSS may still have a good outcome. However, the lowest NIHSS among SPAN-100 patients was 15, suggesting the SPAN-100 index captures high-risk patients and provides relevant clinical information.
Second, our results should be interpreted with caution considering this relatively small cohort (n = 624). The small number of patients classified as SPAN-100 may affect the accuracy of estimation of outcomes as reflected by the low PAR for ICH and the wide CIs for fatal ICH or catastrophic outcomes at 12 months. Consequently, these results need confirmation in larger and more contemporary datasets from stroke registries or clinical trials. Third, the 100 cutoff for the SPAN index has been artificially created and not derived from analysis of receiver operating characteristic (ROC) curves. Although lower cutoff points had marginally better ROC values, we would argue this would not justify the complexity. The intention of the SPAN-100 was to create a simple and easy to remember index for clinicians that would not depend on the availability and access to Internet. Finally, ICH post-tPA is not always associated with poor outcomes.
How might results from this study help clinicians? Previous investigators demonstrated the inaccuracy of clinicians' perception on the risk and benefit of thrombolysis. In their survey, only 11% (95% CI 0%–22%) of emergency physicians and neurologists were able to correctly identify the benefit of tPA treatment and less than 40% were able to estimate the risk of ICH.25 What our study shows is that SPAN-100 patients had over 40% risk of ICH, 50% death rate, and only a 5% chance of a composite favorable outcome at 3 months after tPA. Further, poor long-term outcomes were 3-fold more common among SPAN-100–positive patients compared to their SPAN-100–negative counterparts (catastrophic outcomes at 12 months: 85.7% vs 30.0%). This represents one catastrophic outcome every 18 patients due to SPAN-100. For SPAN-100–positive patients, the NNH for ICH was 4 when comparing tPA vs placebo. Further, the NNH for ICH was 3 when comparing SPAN-100–positive with SPAN-100–negative patients receiving tPA.
Some families may still agree to proceed with IV tPA in SPAN-100–positive patients in the absence of contraindications despite knowing the high risk of bleeding and the limited clinical benefit (compared to patients not receiving thrombolysis). Clinicians may proceed with caution when facing older patients with high NIHSS (e.g., fulfilling SPAN-100 criteria).
The SPAN-100 index is a simple method, applicable in virtually all clinical settings, and easy to calculate and remember, especially for emergency physicians, internists, and non-stroke neurologists, which can be used when counseling patients and families. Although tPA robustly improves the likelihood of favorable outcome, the SPAN-100 status can differentially identify patients who more likely have poor outcomes. Confirmation of these results in larger, more contemporary datasets of tPA-treated patients is warranted.
Supplementary Material
GLOSSARY
- AR
attributable risk
- AUC
area under the curve
- CI
confidence interval
- ECASS
European Cooperative Acute Stroke Study
- HAT
Hemorrhage after Thrombolysis
- ICH
intracerebral hemorrhage
- mRS
modified Rankin Scale
- NIHSS
NIH Stroke Scale
- NINDS
National Institute of Neurological Disorders and Stroke
- NNH
number needed to harm
- NNT
number needed to treat
- OR
odds ratio
- PAR
population attributable risk
- ROC
receiver operating characteristic
- sICH
symptomatic ICH
- SPAN
Stroke Prognostication using Age and NIH Stroke Scale
- tPA
tissue plasminogen activator
- UCSD
University of California, San Diego
Footnotes
Editorial, page 15
AUTHOR CONTRIBUTIONS
G. Saposnik: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, contribution of vital reagents/tools/patients, acquisition of data, statistical analysis, study supervision, obtaining funding. A. Guzik: drafting/revising the manuscript. B. Ovbiagele: drafting/revising the manuscript, study concept or design, analysis or interpretation of data. M. Reeves: drafting/revising the manuscript, analysis or interpretation of data, statistical analysis. S.C. Johnston: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, study supervision.
STUDY FUNDING
Dr. Saposnik is supported by the Distinguished Clinician-Scientist Award from the Heart and Stroke Foundation of Canada (HSFC).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Weimar C, Konig IR, Kraywinkel K, Ziegler A, Diener HC. Age and National Institutes of Health Stroke Scale score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: development and external validation of prognostic models. Stroke 2004;35:158–162 [DOI] [PubMed] [Google Scholar]
- 2.Konig IR, Ziegler A, Bluhmki E, et al. Predicting long-term outcome after acute ischemic stroke: a simple index works in patients from controlled clinical trials. Stroke 2008;39:1821–1826 [DOI] [PubMed] [Google Scholar]
- 3.Predicting outcome after acute ischemic stroke: an external validation of prognostic models. Neurology 2004;62:581–585 [DOI] [PubMed] [Google Scholar]
- 4.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke: The European Cooperative Acute Stroke Study (ECASS). JAMA 1995;274:1017–1025 [PubMed] [Google Scholar]
- 5.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II): Second European-Australasian Acute Stroke Study Investigators. Lancet 1998;352:1245–1251 [DOI] [PubMed] [Google Scholar]
- 6.Clark WM, Albers GW, Madden KP, Hamilton S. The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (a0276g): results of a double-blind, placebo-controlled, multicenter study: Thrombolytic Therapy in Acute Ischemic Stroke Study Investigators. Stroke 2000;31:811–816 [DOI] [PubMed] [Google Scholar]
- 7.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–1329 [DOI] [PubMed] [Google Scholar]
- 8.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587 [DOI] [PubMed] [Google Scholar]
- 9.Mishra NK, Ahmed N, Andersen G, et al. Thrombolysis in very elderly people: controlled comparison of SITS international stroke thrombolysis registry and virtual international stroke trials archive. BMJ 2010;341:c6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sylaja PN, Cote R, Buchan AM, Hill MD. Thrombolysis in patients older than 80 years with acute ischaemic stroke: Canadian Alteplase for Stroke Effectiveness Study. J Neurol Neurosurg Psychiatry 2006;77:826–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garg AX, Adhikari NK, McDonald H, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA 2005;293:1223–1238 [DOI] [PubMed] [Google Scholar]
- 12.Saposnik G, Cote R, Phillips S, et al. Stroke outcome in those over 80: a multicenter cohort study across Canada. Stroke 2008;39:2310–2317 [DOI] [PubMed] [Google Scholar]
- 13.Kent DM, Selker HP, Ruthazer R, Bluhmki E, Hacke W. The stroke-thrombolytic predictive instrument: a predictive instrument for intravenous thrombolysis in acute ischemic stroke. Stroke 2006;37:2957–2962 [DOI] [PubMed] [Google Scholar]
- 14.Saposnik G, Kapral MK, Liu Y, et al. iScore: a risk score to predict death early after hospitalization for an acute ischemic stroke. Circulation 2011;123:739–749 [DOI] [PubMed] [Google Scholar]
- 15.Strbian D, Meretoja A, Ahlhelm FJ, et al. Predicting outcome of IV thrombolysis-treated ischemic stroke patients: the DRAGON score. Neurology 2012;78:427–432 [DOI] [PubMed] [Google Scholar]
- 16.The NINDS t-PA Stroke Study Group Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke 1997;28:2109–2118 [DOI] [PubMed] [Google Scholar]
- 17.Saposnik G, Young B, Silver B, et al. Lack of improvement in patients with acute stroke after treatment with thrombolytic therapy: predictors and association with outcome. JAMA 2004;292:1839–1844 [DOI] [PubMed] [Google Scholar]
- 18.Altman DG. Confidence intervals for the number needed to treat. BMJ 1998;317:1309–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lou M, Safdar A, Mehdiratta M, et al. The HAT score: a simple grading scale for predicting hemorrhage after thrombolysis. Neurology 2008;71:1417–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ntaios G, Faouzi M, Ferrari J, Lang W, Vemmos K, Michel P. An integer-based score to predict functional outcome in acute ischemic stroke: the ASTRAL score. Neurology 2012;78:1916–1922 [DOI] [PubMed] [Google Scholar]
- 21.Saposnik G, Fang J, Kapral MK, et al. The iScore predicts effectiveness of thrombolytic therapy for acute ischemic stroke. Stroke 2012;43:1315–1322 [DOI] [PubMed] [Google Scholar]
- 22.Guzik AK, Raman R, Ernstron K, et al. Clinical outcomes of the ‘Stroke 100 club.’ Stroke 2012;43:A3572 [Google Scholar]
- 23.Saposnik G, Raptis S, Kapral MK, et al. The iScore predicts poor functional outcomes early after hospitalization for an acute ischemic stroke. Stroke 2011;42:3421–3428 [DOI] [PubMed] [Google Scholar]
- 24.Saposnik G, Demchuk A, Tu JV, Johnston SC, Stroke Outcomes Research Canada (SORCan) Working Group. The iScore predicts efficacy and risk of bleeding in the National Institute of Neurological Disorders and Stroke tissue plasminogen activator stroke trial. J Stroke Cerebrovasc Dis Epub 2012 Oct 24 [DOI] [PubMed] [Google Scholar]
- 25.Merino JG, Silver B, Wong E, Demaerschalk B, Tamayo A, Hachinski V. Physician knowledge of the benefits, risks, and contraindications of tissue plasminogen activator for acute ischemic stroke. Stroke 2001;32:2208–2209 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.