Abstract
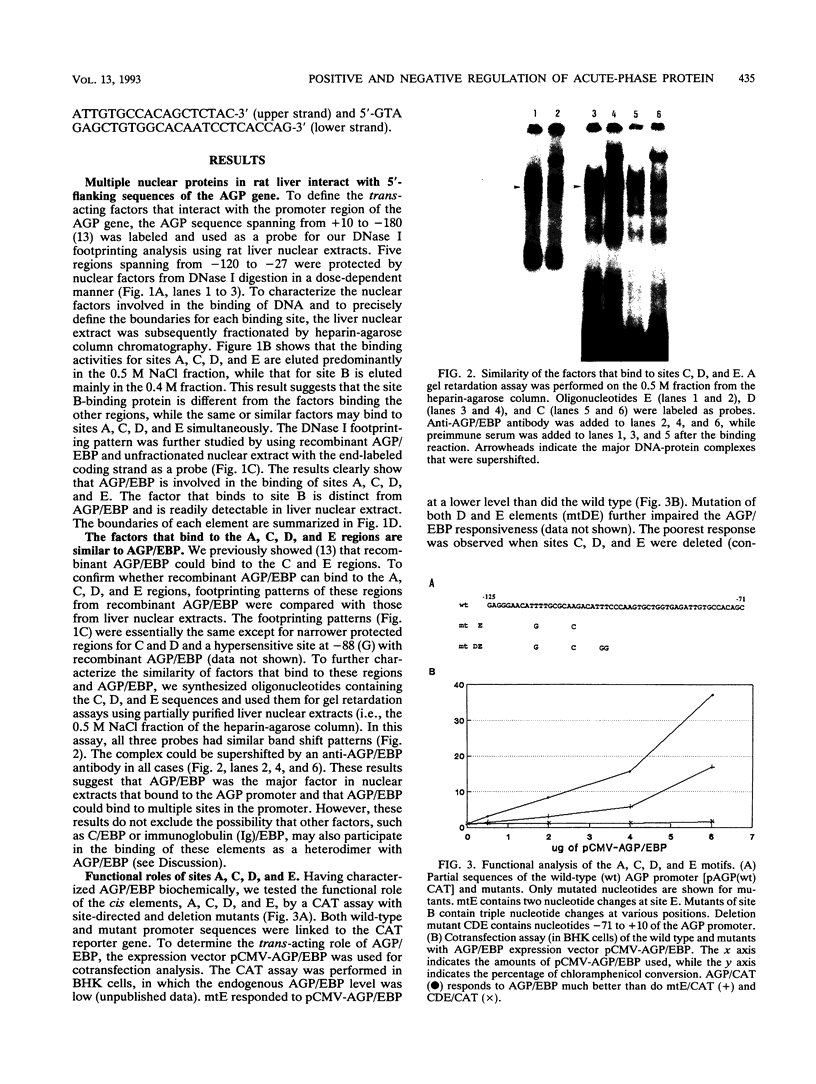
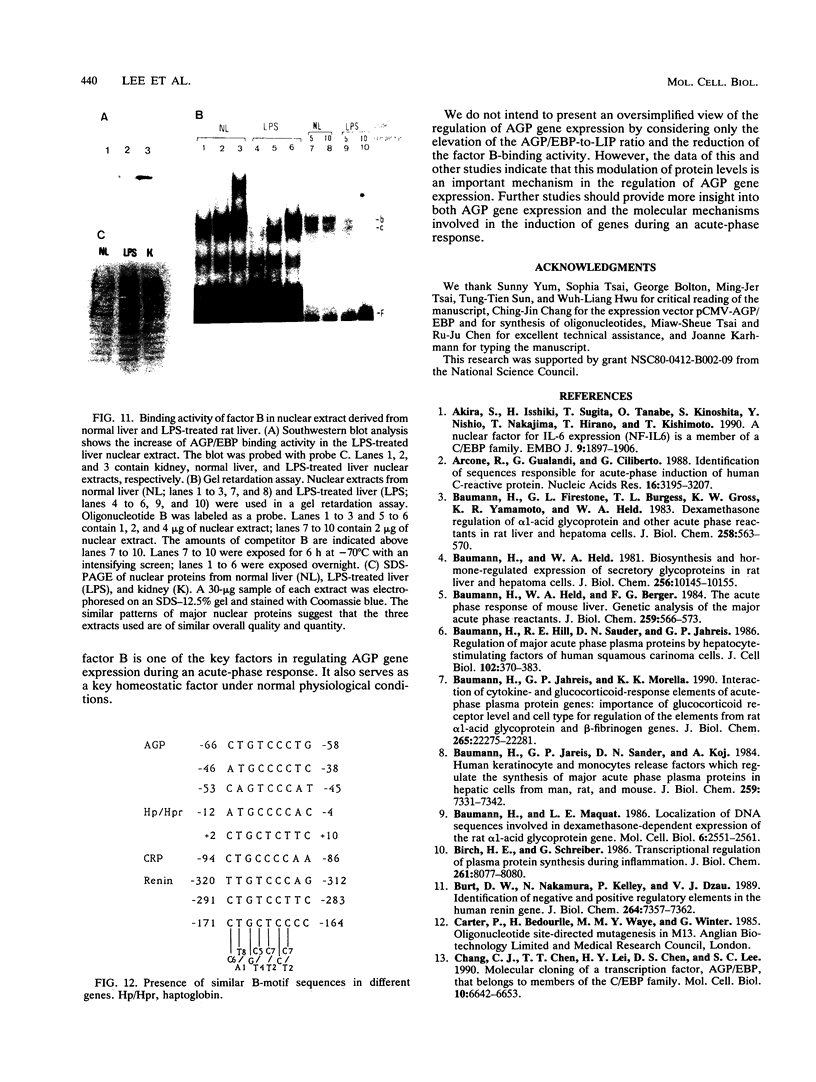
Expression of the alpha-1 acid glycoprotein (AGP) gene is liver specific and acute phase responsive. Within the 180-bp region of the AGP promoter, at least five cis elements have been found to interact with trans-acting factors. Four of these elements (A, C, D, and E) interacted with AGP/EBP, a liver-enriched transcription factor, as shown by footprinting analysis and by an anti-AGP/EBP antibody-induced supershift in a gel retardation assay. Modification of these sites by site-directed mutagenesis coupled with transfection analysis indicated that AGP/EBP binding to all of these sites resulted in positive regulation of the promoter. Dose-response data suggest that AGP/EBP binding to these sites results in the cooperative activation of the promoter. In contrast, functional assays showed that element B is a negative regulatory element; this element is recognized by heat-stable DNA-binding factors which are found in many cells and tissues. The regulation of these binding proteins was studied in rat liver treated with lipopolysaccharide (LPS), which induced an acute-phase reaction. We found that LPS treatment resulted in a two- to threefold increase in AGP/EBP activity and a severalfold decrease in the activity of factors that bind to element B in the liver. These results indicate that expression of the AGP gene can be regulated by both positive and negative factors and that the modulation of these factors can account for the LPS induction of the AGP gene.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Isshiki H., Sugita T., Tanabe O., Kinoshita S., Nishio Y., Nakajima T., Hirano T., Kishimoto T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990 Jun;9(6):1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcone R., Gualandi G., Ciliberto G. Identification of sequences responsible for acute-phase induction of human C-reactive protein. Nucleic Acids Res. 1988 Apr 25;16(8):3195–3207. doi: 10.1093/nar/16.8.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H., Firestone G. L., Burgess T. L., Gross K. W., Yamamoto K. R., Held W. A. Dexamethasone regulation of alpha 1-acid glycoprotein and other acute phase reactants in rat liver and hepatoma cells. J Biol Chem. 1983 Jan 10;258(1):563–570. [PubMed] [Google Scholar]
- Baumann H., Held W. A., Berger F. G. The acute phase response of mouse liver. Genetic analysis of the major acute phase reactants. J Biol Chem. 1984 Jan 10;259(1):566–573. [PubMed] [Google Scholar]
- Baumann H., Held W. A. Biosynthesis and hormone-regulated expression of secretory glycoproteins in rat liver and hepatoma cells. Effect of glucocorticoids and inflammation. J Biol Chem. 1981 Oct 10;256(19):10145–10155. [PubMed] [Google Scholar]
- Baumann H., Hill R. E., Sauder D. N., Jahreis G. P. Regulation of major acute-phase plasma proteins by hepatocyte-stimulating factors of human squamous carcinoma cells. J Cell Biol. 1986 Feb;102(2):370–383. doi: 10.1083/jcb.102.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H., Jahreis G. P., Morella K. K. Interaction of cytokine- and glucocorticoid-response elements of acute-phase plasma protein genes. Importance of glucocorticoid receptor level and cell type for regulation of the elements from rat alpha 1-acid glycoprotein and beta-fibrinogen genes. J Biol Chem. 1990 Dec 25;265(36):22275–22281. [PubMed] [Google Scholar]
- Baumann H., Jahreis G. P., Sauder D. N., Koj A. Human keratinocytes and monocytes release factors which regulate the synthesis of major acute phase plasma proteins in hepatic cells from man, rat, and mouse. J Biol Chem. 1984 Jun 10;259(11):7331–7342. [PubMed] [Google Scholar]
- Baumann H., Maquat L. E. Localization of DNA sequences involved in dexamethasone-dependent expression of the rat alpha 1-acid glycoprotein gene. Mol Cell Biol. 1986 Jul;6(7):2551–2561. doi: 10.1128/mcb.6.7.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch H. E., Schreiber G. Transcriptional regulation of plasma protein synthesis during inflammation. J Biol Chem. 1986 Jun 25;261(18):8077–8080. [PubMed] [Google Scholar]
- Burt D. W., Nakamura N., Kelley P., Dzau V. J. Identification of negative and positive regulatory elements in the human renin gene. J Biol Chem. 1989 May 5;264(13):7357–7362. [PubMed] [Google Scholar]
- Burt D. W., Nakamura N., Kelley P., Dzau V. J. Identification of negative and positive regulatory elements in the human renin gene. J Biol Chem. 1989 May 5;264(13):7357–7362. [PubMed] [Google Scholar]
- Chang C. J., Chen T. T., Lei H. Y., Chen D. S., Lee S. C. Molecular cloning of a transcription factor, AGP/EBP, that belongs to members of the C/EBP family. Mol Cell Biol. 1990 Dec;10(12):6642–6653. doi: 10.1128/mcb.10.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. J., Lai M. Y., Chen D. S., Lee S. C. Structure and expression of mouse alpha 1-acid glycoprotein gene-3 (AGP-3). DNA Cell Biol. 1992 May;11(4):315–320. doi: 10.1089/dna.1992.11.315. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Grayson D. R., Xanthopoulos K. G., Darnell J. E., Jr A liver-specific DNA-binding protein recognizes multiple nucleotide sites in regulatory regions of transthyretin, alpha 1-antitrypsin, albumin, and simian virus 40 genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3840–3844. doi: 10.1073/pnas.85.11.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois G., Morgan J. G., Campbell L. A., Fourel G., Crabtree G. R. Interaction of a liver-specific nuclear factor with the fibrinogen and alpha 1-antitrypsin promoters. Science. 1987 Oct 30;238(4827):688–692. doi: 10.1126/science.3499668. [DOI] [PubMed] [Google Scholar]
- Darlington G. J., Wilson D. R., Lachman L. B. Monocyte-conditioned medium, interleukin-1, and tumor necrosis factor stimulate the acute phase response in human hepatoma cells in vitro. J Cell Biol. 1986 Sep;103(3):787–793. doi: 10.1083/jcb.103.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Descombes P., Chojkier M., Lichtsteiner S., Falvey E., Schibler U. LAP, a novel member of the C/EBP gene family, encodes a liver-enriched transcriptional activator protein. Genes Dev. 1990 Sep;4(9):1541–1551. doi: 10.1101/gad.4.9.1541. [DOI] [PubMed] [Google Scholar]
- Descombes P., Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991 Nov 1;67(3):569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo D., Williams P., Ringold G. Identification of two distinct nuclear factors with DNA binding activity within the glucocorticoid regulatory region of the rat alpha-1-acid glycoprotein promoter. Biochem Biophys Res Commun. 1991 May 15;176(3):1326–1332. doi: 10.1016/0006-291x(91)90431-6. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Döbbeling U., Ross K., Klein-Hitpass L., Morley C., Wagner U., Ryffel G. U. A cell-specific activator in the Xenopus A2 vitellogenin gene: promoter elements functioning with rat liver nuclear extracts. EMBO J. 1988 Aug;7(8):2495–2501. doi: 10.1002/j.1460-2075.1988.tb03096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Flanagan J. R., Becker K. G., Ennist D. L., Gleason S. L., Driggers P. H., Levi B. Z., Appella E., Ozato K. Cloning of a negative transcription factor that binds to the upstream conserved region of Moloney murine leukemia virus. Mol Cell Biol. 1992 Jan;12(1):38–44. doi: 10.1128/mcb.12.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbourn S., Burstein H., Maniatis T. The human beta-interferon gene enhancer is under negative control. Cell. 1986 May 23;45(4):601–610. doi: 10.1016/0092-8674(86)90292-8. [DOI] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Grayson D. R., Costa R. H., Xanthopoulos K. G., Darnell J. E. One factor recognizes the liver-specific enhancers in alpha 1-antitrypsin and transthyretin genes. Science. 1988 Feb 12;239(4841 Pt 1):786–788. doi: 10.1126/science.3257586. [DOI] [PubMed] [Google Scholar]
- Hata A., Ohno S., Akita Y., Suzuki K. Tandemly reiterated negative enhancer-like elements regulate transcription of a human gene for the large subunit of calcium-dependent protease. J Biol Chem. 1989 Apr 15;264(11):6404–6411. [PubMed] [Google Scholar]
- Hsu S. I., Cohen D., Kirschner L. S., Lothstein L., Hartstein M., Horwitz S. B. Structural analysis of the mouse mdr1a (P-glycoprotein) promoter reveals the basis for differential transcript heterogeneity in multidrug-resistant J774.2 cells. Mol Cell Biol. 1990 Jul;10(7):3596–3606. doi: 10.1128/mcb.10.7.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isshiki H., Akira S., Sugita T., Nishio Y., Hashimoto S., Pawlowski T., Suematsu S., Kishimoto T. Reciprocal expression of NF-IL6 and C/EBP in hepatocytes: possible involvement of NF-IL6 in acute phase protein gene expression. New Biol. 1991 Jan;3(1):63–70. [PubMed] [Google Scholar]
- Johnson P. F. Transcriptional activators in hepatocytes. Cell Growth Differ. 1990 Jan;1(1):47–52. [PubMed] [Google Scholar]
- Kageyama R., Pastan I. Molecular cloning and characterization of a human DNA binding factor that represses transcription. Cell. 1989 Dec 1;59(5):815–825. doi: 10.1016/0092-8674(89)90605-3. [DOI] [PubMed] [Google Scholar]
- Klein E. S., DiLorenzo D., Posseckert G., Beato M., Ringold G. M. Sequences downstream of the glucocorticoid regulatory element mediate cycloheximide inhibition of steroid induced expression from the rat alpha 1-acid glycoprotein promoter: evidence for a labile transcription factor. Mol Endocrinol. 1988 Dec;2(12):1343–1351. doi: 10.1210/mend-2-12-1343. [DOI] [PubMed] [Google Scholar]
- Klein E. S., Reinke R., Feigelson P., Ringold G. M. Glucocorticoid-regulated expression from the 5'-flanking region of the rat alpha 1-acid glycoprotein gene. Requirement for ongoing protein synthesis. J Biol Chem. 1987 Jan 15;262(2):520–523. [PubMed] [Google Scholar]
- Kulkarni A. B., Reinke R., Feigelson P. Acute phase mediators and glucocorticoids elevate alpha 1-acid glycoprotein gene transcription. J Biol Chem. 1985 Dec 15;260(29):15386–15389. [PubMed] [Google Scholar]
- Lee S. C., Chang C. J., Lee Y. M., Lei H. Y., Lai M. Y., Chen D. S. Molecular cloning of cDNAs corresponding to two genes of alpha 1-acid glycoprotein and characterization of two alleles of AGP-1 in the mouse. DNA. 1989 May;8(4):245–251. doi: 10.1089/dna.1.1989.8.245. [DOI] [PubMed] [Google Scholar]
- Lichtsteiner S., Wuarin J., Schibler U. The interplay of DNA-binding proteins on the promoter of the mouse albumin gene. Cell. 1987 Dec 24;51(6):963–973. doi: 10.1016/0092-8674(87)90583-6. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Mortensen R. F., Shapiro J., Lin B. F., Douches S., Neta R. Interaction of recombinant IL-1 and recombinant tumor necrosis factor in the induction of mouse acute phase proteins. J Immunol. 1988 Apr 1;140(7):2260–2266. [PubMed] [Google Scholar]
- Mueller C. R., Maire P., Schibler U. DBP, a liver-enriched transcriptional activator, is expressed late in ontogeny and its tissue specificity is determined posttranscriptionally. Cell. 1990 Apr 20;61(2):279–291. doi: 10.1016/0092-8674(90)90808-r. [DOI] [PubMed] [Google Scholar]
- Muglia L., Rothman-Denes L. B. Cell type-specific negative regulatory element in the control region of the rat alpha-fetoprotein gene. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7653–7657. doi: 10.1073/pnas.83.20.7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkubo H., Nakayama K., Tanaka T., Nakanishi S. Tissue distribution of rat angiotensinogen mRNA and structural analysis of its heterogeneity. J Biol Chem. 1986 Jan 5;261(1):319–323. [PubMed] [Google Scholar]
- Oliviero S., Morrone G., Cortese R. The human haptoglobin gene: transcriptional regulation during development and acute phase induction. EMBO J. 1987 Jul;6(7):1905–1912. doi: 10.1002/j.1460-2075.1987.tb02450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K., Atchison M. L. Isolation of a candidate repressor/activator, NF-E1 (YY-1, delta), that binds to the immunoglobulin kappa 3' enhancer and the immunoglobulin heavy-chain mu E1 site. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9804–9808. doi: 10.1073/pnas.88.21.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prowse K. R., Baumann H. Hepatocyte-stimulating factor, beta 2 interferon, and interleukin-1 enhance expression of the rat alpha 1-acid glycoprotein gene via a distal upstream regulatory region. Mol Cell Biol. 1988 Jan;8(1):42–51. doi: 10.1128/mcb.8.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers E. F., Yang J. Q., Marcu K. B. A negative transcriptional control element located upstream of the murine c-myc gene. EMBO J. 1986 May;5(5):899–904. doi: 10.1002/j.1460-2075.1986.tb04301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricca G. A., Hamilton R. W., McLean J. W., Conn A., Kalinyak J. E., Taylor J. M. Rat alpha 1-acid glycoprotein mRNA. Cloning of double-stranded cDNA and kinetics of induction of mRNA levels following acute inflammation. J Biol Chem. 1981 Oct 25;256(20):10362–10368. [PubMed] [Google Scholar]
- Sassone-Corsi P., Verma I. M. Modulation of c-fos gene transcription by negative and positive cellular factors. Nature. 1987 Apr 2;326(6112):507–510. doi: 10.1038/326507a0. [DOI] [PubMed] [Google Scholar]
- Schmid K., Kaufmann H., Isemura S., Bauer F., Emura J., Motoyama T., Ishiguro M., Nanno S. Structure of 1 -acid glycoprotein. The complete amino acid sequence, multiple amino acid substitutions, and homology with the immunoglobulins. Biochemistry. 1973 Jul 3;12(14):2711–2724. doi: 10.1021/bi00738a026. [DOI] [PubMed] [Google Scholar]
- Shi Y., Seto E., Chang L. S., Shenk T. Transcriptional repression by YY1, a human GLI-Krüppel-related protein, and relief of repression by adenovirus E1A protein. Cell. 1991 Oct 18;67(2):377–388. doi: 10.1016/0092-8674(91)90189-6. [DOI] [PubMed] [Google Scholar]
- Sibler A. P., Dirheimer G., Martin R. P. Yeast mitochondrial tRNAIle and tRNAMetm: nucleotide sequence and codon recognition patterns. Nucleic Acids Res. 1985 Feb 25;13(4):1341–1345. doi: 10.1093/nar/13.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner C., Muller M., Baniahmad A., Renkawitz R. Lysozyme gene activity in chicken macrophages is controlled by positive and negative regulatory elements. Nucleic Acids Res. 1987 May 26;15(10):4163–4178. doi: 10.1093/nar/15.10.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Vannice J. L., Taylor J. M., Ringold G. M. Glucocorticoid-mediated induction of alpha 1-acid glycoprotein: evidence for hormone-regulated RNA processing. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4241–4245. doi: 10.1073/pnas.81.14.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P., Ratajczak T., Lee S. C., Ringold G. M. AGP/EBP(LAP) expressed in rat hepatoma cells interacts with multiple promoter sites and is necessary for maximal glucocorticoid induction of the rat alpha-1 acid glycoprotein gene. Mol Cell Biol. 1991 Oct;11(10):4959–4965. doi: 10.1128/mcb.11.10.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong N. C., Perez-Castillo A. M., Sanders M. M., Schwartz H. L., Oppenheimer J. H. Thyroid hormone and circadian regulation of the binding activity of a liver-specific protein associated with the 5'-flanking region of the S14 gene. J Biol Chem. 1989 Mar 15;264(8):4466–4470. [PubMed] [Google Scholar]
- Yee J. K. A liver-specific enhancer in the core promoter region of human hepatitis B virus. Science. 1989 Nov 3;246(4930):658–661. doi: 10.1126/science.2554495. [DOI] [PubMed] [Google Scholar]














