Abstract
Purpose
To compare the efficacy and safety of 1.25 mg versus 2.5 mg intravitreal bevacizumab (IVB) for treatment of choroidal neovascularization (CNV) associated with agerelated macular degeneration (AMD).
Methods
In this randomized clinical trial, consecutive patients with active CNV associated with AMD received 1.25 mg or 2.5 mg IVB. Best corrected visual acuity (BCVA), foveal thickness and side effects of therapy were evaluated one and three months after intervention.
Results
Overall 86 subjects were enrolled and completed the scheduled follow-up. Forty seven and 39 patients received 1.25 and 2.5 mg IVB respectively. The study groups were balanced in terms of baseline characteristics such as age, BCVA and foveal thickness. Mean improvement in BCVA was 0.06±0.3 logMAR in the 1.25 mg group and 0.07±0.34 logMAR in the 2.5 mg group (P=0.9). Mean decrease in foveal thickness was 49±36 μm in the 1.25 mg group and 65±31μm in the 2.5 mg group (P=0.6). Three cases of vitreous reaction and one case of massive subretinal hemorrhage were observed in the 2.5 mg group.
Conclusion
Double dose (2.5 mg) IVB does not seem to be more effective than regular dose (1.25 mg) injections for treatment of CNV due to AMD and may lead to more complications.
INTRODUCTION
Age-related macular degeneration (AMD) is the leading cause of irreversible visual loss in the elderly; severe visual loss in such cases is most often due to choroidal neovascularization (CNV).1–4 Increased expression of vascular endothelial growth factor (VEGF) has been shown in human eyes with neovascular AMD.5,6 In recent years there has been an increasing trend toward intravitreal injection of VEGF inhibitors for treatment of exudative maculopathy and the outcomes have been relatively favorable as compared to other treatment modalities.7–15
Currently, two closely related anti-VEGF agents, bevacizumab and ranibizumab (both from Genentech Inc., South San Francisco, CA, USA), are used for treatment of neovascular AMD. Bevacizumab (Avastin), the full-length humanized monoclonal anti-VEGF antibody, was approved for treatment of colorectal cancer in 2004.12,13 Bevacizumab has been designed for intravenous injection; however when injected into the vitreous cavity, it has been shown to be effective for preventing visual loss and improving visual acuity in patients with neovascular AMD.7–9,16 Ranibizumab, an antibody fragment of bevacizumab, received FDA approval for intravitreal injection for AMD-associated CNV in 2006. Although bevacizumab has not yet been approved for this route of administration, many ophthalmologists continue to use it instead of ranibizumab due to much lower price.17 Despite its widespread use, the “safe and effecttive” dose for intravitreal bevacizumab is not well established.
Intravitreal bevacizumab (IVB) was first used for treatment of CNV associated with AMD by Rosenfeld et al18 who injected a single dose of 1.0 mg bevacizumab. Based on phase III trial reports on monthly intravitreal injections of 0.3 to 0.5 mg ranibizumab and considering the fact that the molecular weight of bevacizumab is approximately three times that of ranibizumab, the dose of bevacizumab containing the same number of molecules would be 0.9 to 1.5 mg. Considering other factors such as the presence of two antigen binding sites on the bevacizumab molecule as compared to a single site on ranibizumab and that ranibizumab has been genetically engineered for increased affinity to VEGF, the authors eventually estimated that 1.0 to 1.25 mg of bevacizumab seems to be suitable for conducting a dose-response study. Thereafter, most authors have used 1.25 mg IVB for treatment of neovascular AMD.7–9 Some investigators however opted to use higher doses hoping to achieve higher efficacy with varying results.19–22
With the paucity of reports comparing different doses of IVB, the optimal dose for highest efficacy and minimal toxicity is not clear. This randomized clinical trial was conducted to compare the outcomes of 1.25 mg vs 2.5 mg intravitreal bevacizumab in eyes with CNV associated with AMD.
METHODS
This prospective, randomized clinical trial was performed on consecutive patients aged 50 or more with AMD and active subfoveal CNV of any angiographic subtype with best-corrected visual acuity (BCVA) of 20/40 to 20/2000 (equivalent to counting fingers at 2 feet). CNV activity was judged according to history of recent visual loss, presence of subretinal fluid with or without subretinal hemorrhage and/or exudates, and obvious leakage on fluorescein angiography (FA) 1 week prior to enrollment.
Exclusion criteria included CNV due to disorders other than AMD, vision limiting conditions other than AMD such as diabetic retinopathy, recent or old retinal vein occlusion involving the fovea, and any sign of ocular inflammation. Patients were also excluded if they had previous intraocular surgery other than uncomplicated cataract surgery more than 3 months before, previous photodynamic therapy, history of external beam radiation to the skull region and inability to comply with the study protocol.
Ophthalmic examinations included BCVA measurement with the Early Treatment of Diabetic Retinopathy Study (ETDRS) chart, intraocular pressure measurement, slitlamp biomicroscopy and dilated funduscopy using an indirect ophthalmoscope and at the slitlamp using a +90 diopters lens. Paraclinical evaluations included FA and optical coherence tomography (Stratus III OCT, Carl Zeiss, Dublin, California, USA).
Patients were randomly assigned to receive 1.25 or 2.5 mg IVB (equivalent to 0.05 and 0.1 ml of commercially available Avastin, respectively). The injections were performed according to a previously described protocol.7 A cotton-tipped applicator was placed adjacent to the needle and rolled over the injection site immediately upon needle withdrawal while applying mild pressure for 30 seconds. Indirect ophthalmoscopy was then performed to ascertain patency of the central retinal artery. The eye was then patched following instillation of one drop of 5% povidone-iodine. The patients were instructed to remain in supine position for 8 hours and to call the emergency room in case of pain or blurred vision.
All patients were visited 48 hours after IVB injection and one week, one month and 3 months thereafter. One month after each injecttion, treatment was repeated in case of persistent subretinal fluid with or without hemorrhage, or if leakage was observed on FA. Maximum numbers of injections for each patient was three. OCT was obtained to determinefoveal thickness at baseline and repeated 1 and 3 months after the first injection. Outcome measures included changes in BCVA and foveal thickness at 3 months.
Changes in BCVA were compared within each group using paired t-test and between the 2 groups using Chi-square and t-tests with significance level set at 0.05. The Institutional Review Board and Ethics Committee of the eye research center approved the study and informed consent was obtained from all patients.
RESULTS
Initially 95 patients had been enrolled in the study; however, one patient in the 1.25 mg group and 8 subjects in the 2.5 mg group were lost to follow-up and therefore excluded. Eventually, 86 eyes of 86 patients including 47 and 39 patients in the 1.25 and 2.5 mg IVB groups who completed the scheduled followup were analyzed. The 1.25 mg group included 32 (66.1%) male and 15 (31.9%) female subjects with mean age of 73.8±8.5 (range 48–85) years and the 2.5 mg group included 26 (66.7%) male and 13 (33.3%) female subjects with mean age of 71.1±9.2 (range 49–87) years. Baseline patient characteristics and outcomes of the study are shown in table 1. There was no difference between the 2 groups regarding age, sex, baseline BCVA and pretreatment foveal thickness.
Table 1.
Patient characteristics
| Parameters | Groups |
P Value | |
|---|---|---|---|
| 1.25 mg | 2.5 mg | ||
| Male (n) Female (n) |
32 15 |
26 13 |
0.8* |
|
| |||
| Age (years): M±SD | 73.8±8.5 | 71.1±9.2 | 0.18** |
|
| |||
| Baseline BCVA (logMAR): M±SD | 1.13±0.5 | 1.26±0.46 | 0.2** |
|
| |||
| Baseline foveal thickness (μm): M±SD | 325±54 | 339±62 | 0.8** |
|
| |||
| Third month BCVA (logMAR): M±SD | 1.0±0.49 | 1.1±0.49 | 0.2** |
|
| |||
| Third month foveal thickness (μm): M±SD | 276±55 | 274±50 | 0.8** |
M, mean; SD, standard deviation; BCVA, best-corrected visual acuity.
Chi-square test.
t-test
Mean increase in BCVA was 0.06±0.3 logMAR in the 1.25 mg group and 0.07±0.34 logMAR in the 2.5 mg group (P=0.9). Mean decrease in foveal thickness was 49±36 μm in the 1.25 mg group and 65±31 μm in the 2.5 mg group (P=0.6). Twelve (25.5%) patients in the 1.25 mg group and 9 (23.1%) patients in the 2.5 mg group experienced at least 3 lines (15 letters) of improvement in BCVA (P=0.7). In contrast 6 (12.8%) patients in the 1.25 mg group and 4 (10.3%) subjects in the 2.5 mg group lost more than 3 lines (15 letters) of BCVA (P=0.7).
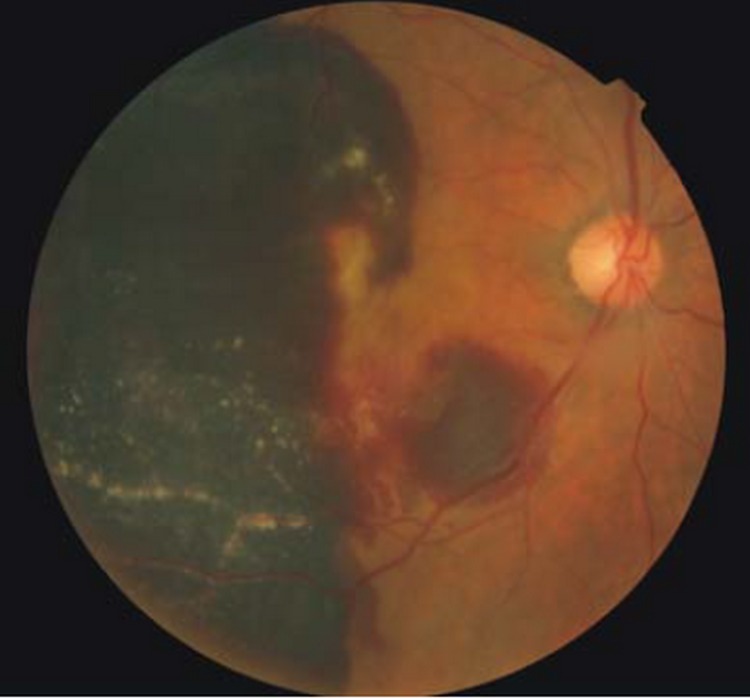
Mean number of injections was 1.5±0.5 (range 1–3) in the 1.25 mg group versus 1.8±0.8 (range 1–3) in the 2.5 mg group (P=0.07). No adverse events were observed in the 1.25 mg group; however, 3 cases (7.7%) of intravitreal inflammation were observed in the 2.5 mg group within 48 hours (Fig. 1). Vitritis was associated with decreased visual acuity and trace anterior chamber reaction in all 3 cases; the grade of vitritis was +3 in one patient and +2 in two others. The more severe case was treated with oral prednisolone 50 mg daily for 1 week and topical betamethasone for 3 weeks. Intraocular inflammation gradually subsided with visual improvement over 1 month in all 3 cases. Two (5.1%) patients in the 2.5 mg group developed acute posterior vitreous detachment (PVD) associated with pinpoint peripheral retinal hemorrhages in one case. One patient with predominantly classic CNV developed sudden visual loss 6 days after 2.5 mg IVB injection due to massive subretinal hemorrhage on funduscopy (Fig. 2). The patient refused intravitreal tissue plasminogen activator and gas injection.
Figure 1.
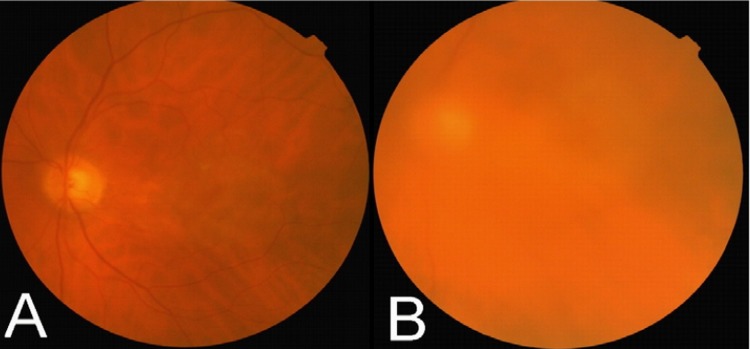
(A) Fundus photograph of a patient with choroidal neovascularization associated with age-related macular degeneration before injection of intravitreal bevacizumab (IVB). (B) Marked vitreous reaction developed 2 days after 2.5 mg IVB.
Figure 2.
Fundus photograph of a patient with choroidal neovascularization associated with age-related macular degeneration 6 days after injection of 2.5 mg bevacizumab. Note the huge subretinal hemorrhage.
DISCUSSION
The optimal dosage of intravitreal bevacizumab has not been clearly established. We found that 1.25 and 2.5 mg IVB are comparable in terms of visual improvement and reduction in macular thickness in patients with CNV due to AMD. A dose escalating study by Costa et al19 has shown progressively higher efficacy for both 1.5 and 2 mg IVB as compared to 1 mg IVB in eyes with CNV secondary to AMD. However, Wu et al22 reported similar efficacy for 1.25 and 1.5 mg intravitreal bevacizumab in 45 patients with branch retinal vein occlusion. These studies were retrospective or data were gathered from several centers located in different countries. To the best of our knowledge, this is the only prospective study comparing different doses of IVB in the same treatment setting.
Uveitis has been reported after intravitreal injections of both bevacizumab and ranibizumab. 23–25 Bakri et al25 reported 4 cases of intraocular inflammation including 2 cases of iritis and 2 cases of vitritis following IVB and stressed the importance of avoiding unnecessary treatment for endophthalmitis. Although the authors did not mention the dosage, they apparently used the usual 1.25 mg dose.
Adverse effects of IVB have been studied in animal experiments. Manzano et al26 studied intravitreal injection of different doses of bevacizumab up to 5 mg in the rabbit and reported vitreous inflammation only with the 5 mg dosage but observed no histological or electroretinographic evidence of retinal toxicity. Similarly, Feiner et al27 did not find any abnormalities on photopic or scotopic electroretinograms (ERG) and no histological evidence of toxicity in rabbit eyes following intravitreal injection of 1.25 and 2.5 mg bevacizumab. Shahar and coworkers28 observed full-thickness retinal penetration of the agent following 2.5 mg IVB in rabbit eyes but reported no sign of toxicity on ERG or visual evoked potential. In the current study, we encountered 3 cases (7.7%) of clinically significant inflammation using the same dosage. This difference may be in part due to differences in the immunologic response in human and rabbit eyes. In clinical experiments, no inflammation was observed with 2.5 mg dosage in some studies,19,20,22 while apparent in another study using the 1.25 mg dosage.25
Studies in which intraocular inflammation have not been reported are generally retrospective; giving rise to the possibility that occasional cases of mild reaction remained unnoticed or unreported. Another possible explanation is changes in formulation of bevacizumab by the manufacturer. However, in an inquiry from Genentech technical representatives during the American Academy of Ophthalmology meeting in November 2007 by one of the authors (MM), they denied any change in the formulation of bevacizumab up to the time of our study.
In our series, PVD was observed in 2 patients in the 2.5 mg group accompanied by peripheral pinpoint bleedings in one case. Although PVD was uneventful in both patients, it may predispose to retinal detachment. Retinal detachment has actually been reported following IVB injections.29 We also encountered a single dose of large subretinal hemorrhage. It is hard to directly attribute the hemorrhage to bevacizumab since CNV itself may lead to subretinal hemorrhage at any time. On the other hand, it is conceivable that rapid shrinkage of CNV following IVB may increase the chance for subretinal hemorrhage. This complication has previously been reported by Chieh and Fekrat30 after 1.25 mg IVB.
Despite limited follow-up, our study has the advantages of being prospective and being performed at a single center. We found 2.5 mg IVB to be equally effective as 1.25 mg but the higher dose seems to be associated with more adverse effects.
REFERENCES
- 1.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137:486–495. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 2.Age-Related Eye Disease Study Research Group Potential public health impact of Age-Related Eye Disease Study results: AREDS report no. 11. Arch Ophthalmol. 2003;121:1621–1624. doi: 10.1001/archopht.121.11.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eye Diseases Prevalence Research Group Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 4.Ferris FL III, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102:1640–1642. doi: 10.1001/archopht.1984.01040031330019. [DOI] [PubMed] [Google Scholar]
- 5.Amin R, Puklin JE, Frank RN. Growth factor localization in choroidal neovascular membrane of age-related macular degeneration. Invest Ophthalmol Vis Sci. 1994;35:3178–3188. [PubMed] [Google Scholar]
- 6.Kliffen M, Sharma HS, Mooy CM, Kerkvliet S, de Jong PT. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol. 1997;81:154–162. doi: 10.1136/bjo.81.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–372. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Spaide RF, Laud K, Fine HF, Klancnik JM, Meyerle CB, Yannuzzi LA. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006;26:383–390. doi: 10.1097/01.iae.0000238561.99283.0e. [DOI] [PubMed] [Google Scholar]
- 9.Bashshur ZF, Bazarbachi A, Schakal A, Haddad ZA, El Haibi CP, Noureddin BN. Intravitreal bevacizumab for the management of choroidal neovascularization in age-related macular degeneration. Am J Ophthalmol. 2006;142:1–9. doi: 10.1016/j.ajo.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 10.Bayer DS, Autoszyk AN, Awh CC, Bhisitkul RB, Shapiro H, Acharya NR, MARINA Study Group Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114:246–252. doi: 10.1016/j.ophtha.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 11.Heier JS, Antoszyk AN, Pavan PR, Leff SR, Rosenfeld PJ, Ciulla TA, et al. Ranibizumab for treatment of neovascular age-related macular degeneration. A phase I/II multicenter, controlled, multidose study. Ophthalmology. 2006;113:633–642. doi: 10.1016/j.ophtha.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nature. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 13.Presta LG, Chen H, O’Connor SJ, Chisholm V, Meng YG, Krummen L, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–459. [PubMed] [Google Scholar]
- 14.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 15.Brow DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporphin for neovascular age-related macular degeneration. N Eng J Med. 2006;335:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 16.Yoganathan P, Deramo VA, Lai JC, Tibrewala RK, Fastenberg DM. Visual improvement following intravitreal bevacizumab (Avastin) in exudative age-related macular degeneration. Retina. 2006;26:994–998. doi: 10.1097/01.iae.0000244380.34082.67. [DOI] [PubMed] [Google Scholar]
- 17.Freeman WR, Falkenstein I. Avastin and new treatments for AMD: where are we? Retina. 2006;26:853–858. doi: 10.1097/01.iae.0000244722.35073.7c. [DOI] [PubMed] [Google Scholar]
- 18.Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–335. [PubMed] [Google Scholar]
- 19.Costa RA, Jorge R, Calucci D, Cardillo JA, Melo LA Jr, Scott IU. Intravitreal bevacizumab for choroidal neovascularization caused by AMD (IBeNA Study): results of a phase 1 dose-escalation study. Invest Ophthalmol Vis Sci. 2006;47:4569–4578. doi: 10.1167/iovs.06-0433. [DOI] [PubMed] [Google Scholar]
- 20.Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, Sanchez JG, Wu L, Maia M, et al. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology. 2007;114:743–750. doi: 10.1016/j.ophtha.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 21.Hernández-Rojas ML, Quiroz-Mercado H, Dalma-Weiszhausz J. Short-term effects of intravitreal bevacizumab for subfoveal choroidal neovascularization in pathologic myopia. Retina. 2007;27:707–712. doi: 10.1097/GIM.0b013e3180a03276. [DOI] [PubMed] [Google Scholar]
- 22.Wu L, Arevalo JF, Roca JA, Maia M, Berrocal MH, Rodriguez FJ, et al. Comparison of two doses intravitreal bevacizumab (Avastin) for treatment of macular edema secondary to branch retinal vein occlusion: Results from the Pan-American Collaborative Retina Study Group at 6 months of follow-up. Retina. 2008;28:212–219. doi: 10.1097/IAE.0b013e3181619bee. [DOI] [PubMed] [Google Scholar]
- 23.Rosenfeld PJ, Heier JS, Hantsbarger G, Shams N. Tolerability and efficacy of multiple escalating doses of ranibizumab (Lucentis) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:623–632. doi: 10.1016/j.ophtha.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 24.Rosenfeld PJ, Schwartz SD, Blumenkranz MS, Miller JW, Haller JA, Reimann JD, et al. Maximum tolerated dose of a humanized anti-vascular endothelial growth factor antibody fragment for treating neovascular age-related macular degeneration. Ophthalmology. 2005;112:1048–1053. doi: 10.1016/j.ophtha.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 25.Bakri SJ, Larson TA, Edwards AO. Intraocular inflammation following intravitreal injection of bevacizumab. Graefes Arch Clin Exp Ophthalmol. 2008;17 doi: 10.1007/s00417-007-0754-7. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 26.Manzano RP, Peyman GA, Khan P, Kivilcim M. Testing intravitreal toxicity of bevacizumab (Avastin) Retina. 2006;26:257–261. doi: 10.1097/00006982-200603000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Feiner L, Barr EE, Shui YB, Holekamp NM, Brantley MA Jr. Safety of intravitreal injection of bevacizumab in rabbit eyes. Retina. 2006;26:882–888. doi: 10.1097/01.iae.0000230717.85319.f5. [DOI] [PubMed] [Google Scholar]
- 28.Shahar J, Avery RL, Heilweil G, Barak A, Zemel E, Lewis GP, et al. Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (Avastin) Retina. 2006;26:262–269. doi: 10.1097/00006982-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Fung AE, Rosenfeld PJ, Reichel E. The International Intravitreal Bevacizumab Safety Survey: using the internet to assess drug safety worldwide. Br J Ophthalmol. 2006;90:1344–1349. doi: 10.1136/bjo.2006.099598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chieh JJ, Fekrat S. Large subretinal hemorrhage after intravitreal bevacizumab (Avastin) for agerelated macular degeneration. Ann Ophthalmol (Skokie) 2007;39:51–52. doi: 10.1007/BF02697326. [DOI] [PubMed] [Google Scholar]