ABSTRACT
The BDADs (bis-[dichloroacetyl]-diamines) are compounds that can inhibit spermatogenesis via blocking the metabolism of vitamin A. We utilized one specific BDAD, WIN 18,446, to manipulate the endogenous production of retinoic acid (RA) in the testis to further investigate the action of this compound on mammalian sperm production. Transient treatment of adult male mice with WIN 18,446 blocked spermatogonial differentiation and induced significant changes in the cycle of the seminiferous epithelium. WIN 18,446 treatment of neonatal mice also blocked spermatogonial differentiation and, followed by injection of RA, induced synchronous spermatogenesis in adulthood. The net result was pulsatile, rather than normal continuous, release of sperm from the seminiferous epithelium. This study describes a novel technique that can enrich for specific germ cell populations within the testis, representing a valuable new tool for studying spermatogenesis.
Keywords: contraception; retinoids; spermatogenesis; testis; WIN 18,446
The treatment of mice with WIN 18,446 suppresses retinoic acid-mediated differentiation of spermatogonia and synchronizes spermatogenesis.
INTRODUCTION
One of the defining features of mammalian spermatogenesis is the constant production of sperm to ensure maximal success throughout the male reproductive lifespan. In order for this to be achieved, the mammalian testis has developed a unique way for germ cells to regularly enter their differentiation pathway, known as the cycle of the seminiferous epithelium [1, 2]. In mice, undifferentiated Aaligned spermatogonia transition to form differentiating A1 spermatogonia every 8.6 days at any given point along a testis tubule. The net result of cyclic spermatogonial differentiation is the formation of recurring sets of associated germ cells, termed stages, at distinct points in their developmental pathway [2]. While the cycle is well understood at the histological level, there is still much to be learned regarding how the cycle is propagated and maintained, although there is a growing body of evidence to suggest that the active metabolite of vitamin A, retinoic acid (RA), plays a key role in this process [3].
Vitamin A is essential for spermatogenesis. In vitamin A-deficient (VAD) rodents, the testes begin to lose all differentiating germ cells until only Sertoli cells and undifferentiated spermatogonia are left within the seminiferous epithelium (reviewed in [3]). Multiple studies have demonstrated that an injection and dietary replenishment of exogenous retinoids reinitiates spermatogenesis in VAD animals [4–6]. However, rather than sperm production being rescued in a normal manner, continual sperm production is lost and germ cell differentiation is initiated and maintained synchronously, resulting in the pulsatile release of sperm to the epididymis occurring only once every 8.6 days [4]. In addition, studies utilizing VAD rodents and chemical inhibitors of vitamin A metabolizing and signaling proteins (reviewed in [7]) have demonstrated that RA is required to stimulate undifferentiated spermatogonia to enter their differentiation pathway and commit to undergoing meiosis. However, the mechanisms governing how RA levels are regulated within the testis remain to be determined. In addition, most of the current data regarding RA activity in the testis has been generated using adult testis tissue. Only neonatal animals carrying a genetic predisposition, for example, a mutation in a retinoid storage enzyme such as LRAT, can be made VAD [8], therefore making conclusions regarding changes to testis cellular function in juvenile animals as a result of eliminating RA difficult. Therefore, very little is currently known about how RA stimulates the initial commitment of male germ cells to differentiate, known as the first wave of spermatogenesis.
Recent studies have identified WIN 18,446 as a potent inhibitor of RA activity within the testis [9, 10]. In fact, WIN 18,446 has been shown to reversibly block spermatogenesis in several species [9, 11, 12], including man, although the notion of using WIN 18,446 as a reversible human male contraceptive was discarded when it was shown to cause a disulfiram reaction when taken with alcohol. Recent in vitro studies identified WIN 18,446 as an inhibitor of the retinaldehyde dehydrogenase enzymes [9], capable of blocking RA production within cultures of whole testis explants or isolated germ cells [10]. In addition, the testes of rabbits or mice treated daily with WIN 18,446 for extended periods (16 wk in rabbits; 42 days in mice) contained only Sertoli cells and undifferentiated spermatogonia [9, 12], mimicking the morphology of a VAD testis and suggesting that WIN 18,446 generates RA-deficient testes in mammals. Based on these observations, we hypothesized that WIN 18,446 could be used to manipulate the production of RA within the testes of mice, allowing an in-depth investigation of the action of RA on male germ cell development and the in vivo effects of a contraceptive drug targeting the vitamin A-metabolizing enzymes on germ cell development. We investigated whether WIN 18,446 could be used to manipulate RA levels in vivo with only short-term treatment and whether treating neonatal animals with WIN 18,446 could allow for the analysis of RA activity on juvenile male germ cells. Our results indicate that inhibiting vitamin A metabolism in either the adult or juvenile mouse testis blocks spermatogonial differentiation and has long-lasting effects on the arrangement of germ cells within, and the timing of sperm release from, the seminiferous epithelium, two very important factors that will need to be considered during the development of a reversible male contraceptive.
MATERIALS AND METHODS
Animals and Tissues
All the animal experiments were approved by the Washington State University Animal Care and Use Committees and were conducted in accordance with the guiding principles for the care and use of research animals of the National Institutes of Health. BL/6–129 and RAREhspLacZ mouse colonies were maintained in a temperature- and humidity-controlled environment with food and water provided ad libitum. BL/6–129 and RAREhspLacZ mice, ranging from 0 to 180 days postpartum (dpp), used in these studies were collected from these colonies. The animals were euthanized by CO2 asphyxiation followed by decapitation (0–10 dpp) or cervical dissociation (10-dpp adult), and their testes dissected. Samples for RNA preparation were snap frozen immediately after collection and stored at −80°C until use. BL/6–129 samples for morphological and immunohistochemical analyses were fixed in Bouin fixative for between 2 and 8 h, depending on the sample size and then dehydrated through a graded ethanol series before being embedded in paraffin wax. Sections of 4 μm were placed on Superfrost Plus slides (Menzel-Glaser).
WIN 18,446 and RA Treatments
Adult male mice were injected subcutaneously with 40 μg/μl WIN 18,446, dissolved in 50 μl dimethyl sulfoxide (DMSO), for either 3 or 8 consecutive days. Animals receiving three consecutive daily treatments were euthanized either 24 h or 26 days posttreatment. Animals receiving eight consecutive daily treatments were either euthanized 24 h posttreatment or given an injection of 0.5 mg RA, in 100 μl 15% DMSO/85% sesame oil, and then euthanized 26 days postinjection. Control animals received injections of only 100 μl 15% DMSO/85% sesame oil.
Neonatal mice used to determine the effect of WIN 18,446 alone on spermatogonial differentiation were treated as follows: 2-dpp mice were pipette-fed 100 μg/gram body weight WIN 18,446, suspended in 1% gum tragacanth, in either a single dose or for 3 or 7 consecutive days while control animals only received 1% gum tragacanth.
Neonatal mice used to determine whether WIN 18,446 and RA could synchronize spermatogenesis were treated as follows: 2-dpp mice were pipette-fed 100 μg/gram body weight WIN 18,446, suspended in 1% gum tragacanth, for 7 consecutive days. At 9 dpp (Day 8 of treatment), these animals were given a subcutaneous injection of 200 μg of RA in 10 μl of DMSO, and then left to recover for either 1, 2, 4, or 7 days to assess the effect of this treatment on the first wave of spermatogenesis. To assess the effect of this treatment regime on adult spermatogenesis, the animals were left to recover for between 42–50, 90, and 180 days. Control animals received one of the following three different groups of vehicle combinations: 1) 1% gum tragacanth plus an injection of 10 μl DMSO; 2) 1% gum tragacanth plus an injection of 150 μg RA in 10 μl DMSO; or 3) WIN 18,446 plus an injection of 10 μl DMSO alone. All the treatment regimes are presented in table format in the supplemental data (see Supplemental Table S1 and S2; all the Supplemental Data are available online at www.biolreprod.org).
Staging Analysis of Synchronized Tissue
A minimum of 200 testis cross-sections on a minimum of two planes separated by a minimum of 50 μm from either three vehicle control group samples or three WIN 18,446/RA-treated samples were assigned as displaying a particular stage of the seminiferous epithelium based on the criteria described by Russell et al. [2]. The analysis was then graphed as a percentage of the total number of tubules displaying each stage. The Student t-test was used to determine statistical differences between tubule percentages for each cluster for the adult animals treated daily for 3 days and then left to recover for 26 days.
Beta-Galactosidase Staining and Counting
The collected RAREhspLacZ testes were fixed in 4% paraformaldehyde in PBS at 4°C for between 2 and 6 h, depending on size. Fixed tissue was washed and stained in bromo-chloro-indolyl-galactopyranoside as previously described [13]. Samples were first washed in LacZ buffer (200 mM sodium phosphate; 2 mM magnesium chloride; 0.02% Nonidet p40 substitute; 0.01% sodium deoxycholate; pH 7.4) followed by PBS three times before soaking in an equal part solution of 70% ethanol and PBS. Testes were then dehydrated in a graded series of ethanol and embedded in paraffin for sectioning.
The paraffin-embedded RAREhspLacZ neonatal testes were sectioned at 4 μm, melted onto glass slides, and then stained with Harris haematoxylin. One section every 25 μm was collected so that the sections of testes analyzed spanned a minimum of 500 μm. For beta-galactosidase activity analysis, germ cells were considered beta-galactosidase-positive if they contained two or more foci of beta-galactosidase activity (light blue staining) or definitive, diffuse cytoplasmic staining. Immunohistochemistry, described below, was used to detect STRA8 protein in sections of LacZ-stained testes. Cells were considered STRA8-positive if they contained robust brown staining. Quantification was performed on at least 500 tubule cross-sections from a minimum of three different samples. The frequency of beta-galactosidase-positive and/or STRA8-positive germ cells per tubule cross-section was determined for each treatment. The ratio of this value for each treatment compared to that of the vehicle controls was then used for all the statistical analyses. A Student t-test was performed to determine statistically significant differences between vehicle and treated samples, and the data represent mean ± SD. In all the cases, a minimum of three biological replicates and technical duplicates were utilized for the analysis.
Antibody Production, Immunohistochemistry, and TUNEL Analysis
We generated a rabbit polyclonal antibody raised against the full length STRA8 protein. Immunohistochemistry using the collected STRA8 antisera or a commercial antibody raised against ZBTB16 (sc-22839; Santa Cruz) was performed essentially as previously described [14]. Antigen retrieval was performed in 0.01 M citrate (pH 6; >90°C maintained for 5 min). STRA8 antisera was applied at a dilution of 1:2000–1:10 000 or anti-ZBTB16 was applied at 2 μg/ml for overnight incubation at room temperature in 5% normal goat serum/0.1% bovine serum albumin/PBS. Control sections were incubated with preimmune serum (STRA8) or without primary antibody (ZBTB16). The subsequent steps were performed at room temperature, with PBS washes between incubations. Binding of both primary antibodies was detected using biotinylated goat anti-rabbit antibody (Histostain kit; Invitrogen) conjugated to horseradish peroxidase using the Vectastain Elite ABC kit (Vector Laboratories) according to the manufacturer's instructions. Antibody binding was detected as a brown precipitate following development with 3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich). The sections were mounted under glass coverslips in DPX mountant (VWR International). Germ and somatic cell types were identified in Bouin fixed tissue on the basis of their nuclear morphology [2], position within the developing gonad [2], and staining for cell-specific markers (ZBTB16 and STRA8). The stages of the seminiferous epithelium were identified based on the descriptions in Russell et al. [2]. In the postnatal testis, sections from at least three BL/6–129 animals were analyzed for protein localization, and each immunohistochemistry or immunofluorescence experiment was repeated with consistent results. TUNEL (terminal deoxynucleotidyl tranferase dUTP nick end labeling) analysis was performed using the DE-ADEND kit (Promega) according to the manufacturer's instructions. TUNEL-positive cells per tubule were analyzed using a Nikon Microphot-FX microscope.
Real-Time RT-PCR
Total RNA was extracted from mouse testes using the TRIzol reagent as per the manufacturer's instructions (Invitrogen). Reverse transcription was performed to generate cDNA samples using the iScript kit (BioRad) as per the manufacturer's instructions with 1 μg of each total RNA as template. A two-step real-time RT-PCR was used to measure the expression of candidate genes as previously described [15]. The RNA samples were analyzed in triplicate with primers specific for the target genes. Stra8 primers amplified a 151-bp product (primers: 5′-GTTTCCTGCGTGTTCCACAAG-3′ and 5′-CACCCGAGGCTCAAGCTTC-3′); Kit primers amplified a 104-bp product (primers: 5′-AGCAGATCTCGGACAGCACC-3′ and 5′-CGAGTTGACCCTCACGGAAT-3′); Zbtb16 primers amplified a 150-bp product (primers: 5′-CTGCGGAAAACGGTTCCTG-3′ and 5′-GTGCCAGTATGGGTCTGTCT-3′); and control Rps2 primers amplified a 112-bp product (primers: 5′-CTGACTCCCGACCTCTGGAAA-3′ and 5′-GAGCCTGGGTCCTCTGAACA-3′). Expression of Stra8, Kit, and Zbtb16 was normalized to the Rps2 expression. The Student t-test was used to analyze all the results. Data represent mean ± SD.
RESULTS
Short-Term Treatment of Adult Male Mice with WIN 18,446 Blocks Spermatogonial Differentiation
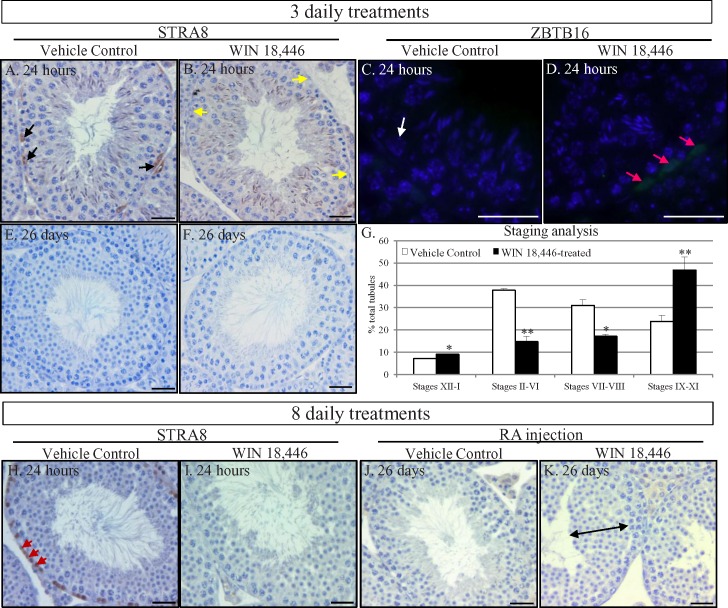
Previous studies from multiple laboratories have indicated that RA is essential for spermatogonial differentiation [8, 16–19], and we investigated whether spermatogonia were susceptible to short-term WIN 18,446 treatment regimes in the adult mouse testis. Histological analyses of testes 24 h after three daily treatments of WIN 18,446 revealed no gross morphological differences in testicular architecture compared to controls (Fig. 1, A and B). However, no STRA8 protein, the classic marker of RA activity in the testis and of differentiating A spermatogonia [19, 20], was detectable in spermatogonia in stages IX through XI of the seminiferous epithelium while these cells continued to express ZBTB16, a marker of undifferentiated spermatogonia [21, 22] (Fig. 1D). When treatment was stopped and animals were given 26 days to recover, histological analyses of their testes revealed approximately 30% (data not shown) of stage II–VI tubules were missing layers of round spermatids (Fig. 1F), and stage counts (Fig. 1G) indicated that the normal distribution of germ cell associations had been altered such that spermatogonial differentiation appeared to have been delayed. A delay in spermatogonial differentiation was also observed in animals treated with WIN 18,446 daily for 8 days or across one full cycle of the seminiferous epithelium because approximately 36% (data not shown) of stage VII and VIII tubules were missing layers of preleptotene spermatocytes 24 h posttreatment (Fig. 1I). In addition, an injection of RA appeared to induce the premature differentiation of spermatogonia in WIN 18,446-treated adult male mice as a thickened layer of round spermatids was observed in the testes of these animals 26 days postinjection (Fig. 1K).
FIG. 1.
Short-term WIN 18,446 treatment of adult mice blocks spermatogonial differentiation and alters the cycle of the seminiferous epithelium. STRA8 and ZBTB16 localization in vehicle control (A, C) and 3-day WIN 18,446-treated adult mouse testes (B, D), 24 h posttreatment. Black arrows denote STRA8-positive spermatogonia, yellow arrows denote STRA8-negative spermatogonia, white arrows denote ZBTB16-negative spermatogonia, and pink arrows denote ZBTB16-positive spermatogonia. Harris haematoxylin-stained tubule cross-sections from testes of vehicle control (E) and 3-day WIN 18,446-treated adult mice (F) left to recover for 26 days. Analysis of the different cellular associations present in vehicle control animals (white bars) and 3-day WIN 18,446-treated mice (black bars) left to recover for 26 days (G). Asterisks highlight statistical significance (*P < 0.05; **P < 0.002). STRA8 localization in vehicle control (H) and 8-day WIN 18,446-treated adult mouse testes (I), 24 h posttreatment. Red arrows denote STRA8-positive preleptotene spermatocytes. Harris haematoxylin-stained tubule cross-sections from testes of vehicle control (J) and 8-day WIN 18,446-treated adult mice (K) given an injection of RA and then left to recover for 26 days. Dual-headed black arrow denotes a thickened layer of round spermatids present in the WIN 18,446-treated animals. All bars = 20 μm.
WIN 18,446 Also Blocks Spermatogonial Differentiation in the Neonatal Testis
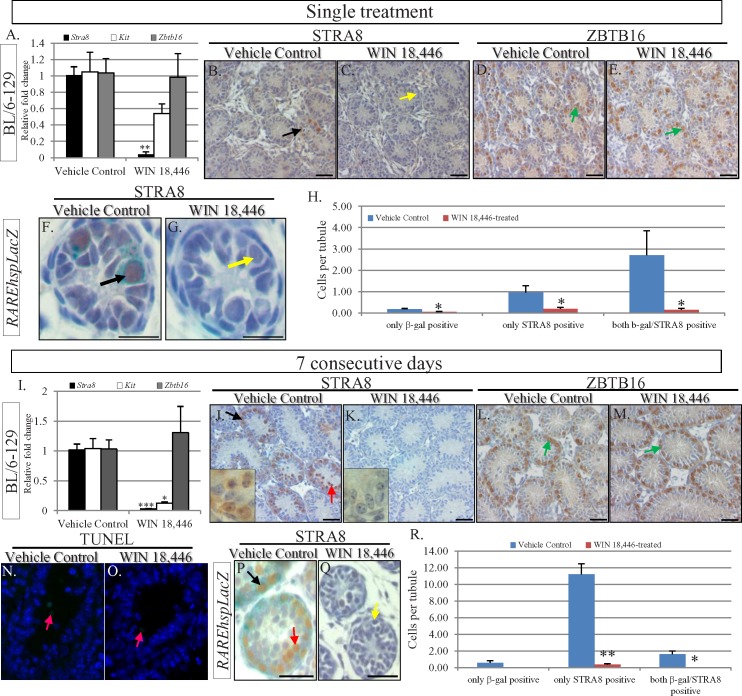
Using RAREhspLacZ mice, a transgenic line designed to express β-galactosidase under the control of an RA response element in all cells [23], RA signaling has been shown to be active in the testis beginning at 3 dpp [24], with the first differentiating spermatogonia present by 5 dpp. We used this transgenic mouse line, as well as wild-type mice, to investigate whether treatment of neonatal animals with WIN 18,446 could be used to manipulate male germ cell development before RA signaling within the testis has begun. Male mice aged 2 dpp were treated with vehicle control or either a single hit of WIN 18,446 (Fig. 2, A–H) or given consecutive daily treatments for either 3 days (see Supplemental Fig. S1) or 7 days (Fig. 2, I–R). Real-time RT-PCR and immunohistochemistry were used to assess the effect of the compound on germ cell development, and RAREhspLacZ mice was utilized to investigate the effect of WIN 18,446 on RA signaling. Stra8 transcripts and protein were very difficult to detect 24 h after all three treatment regimes (Fig. 2 and Supplemental Fig. S1) and Kit mRNA was expressed at a significantly lower level in the 7-day samples (Fig. 2I). In addition, very few LacZ-positive germ cells were present in the treated RAREhspLacZ testes when compared to controls at each time point (Fig. 2 and Supplemental Fig. S1). No significant differences in either animal or testis weights were observed for the 7 consecutive days treatment regime (Fig. 3), and there was no change in the numbers of TUNEL-positive cells observed in control and treated samples (Fig. 2, N and O), indicating that germ cell death was unlikely to be the cause of the lowered expression of markers of differentiating spermatogonia. Morphological analysis of testes subjected to the 7-day treatment regime revealed seminiferous tubules with uniform germ cell arrangements throughout the entire testis, with only Sertoli cells and spermatogonia being present (Fig. 2K). The observations that these spermatogonia were located at the basement membrane of the testis tubules and stained positive for ZBTB16 (Fig. 2M) and GFRα1 (data not shown) indicated that these cells were undifferentiated Type A spermatogonia (Fig. 2M). This is in contrast to the morphological heterogeneity and the nonuniform STRA8 and ZBTB16 staining patterns observed when comparing tubule cross-sections of control samples (Fig. 2, J and L), with undifferentiated and differentiating spermatogonia and preleptotene spermatocytes all present.
FIG. 2.
Short-term treatment of neonatal mice with WIN 18,446 holds spermatogonia in an undifferentiated state. Quantitative RT-PCR analysis of markers of undifferentiated (Zbtb16, gray bars) and differentiating spermatogonia (Stra8, black bars; Kit, white bars) after administration of a single dose (A) or seven consecutive daily treatments (I). Asterisks highlight statistical significance (*P < 0.05; **P < 0.001; ***P < 0.0001). STRA8 and ZBTB16 localization in testis cross-sections from vehicle control animals (B, D, J, L) and animals administered a single dose of WIN 18,446 (C, E) or seven consecutive daily treatments (K, M). Insets in J and K are high magnification pictures to highlight germ cell morphology in the controls and seven consecutive daily treatment samples. Black arrows denote STRA8-positive spermatogonia, yellow arrows denote STRA8-negative spermatogonia, red arrows denote STRA8-positive preleptotene spermatocytes, and green arrows denote ZBTB16-positive spermatogonia. STRA8 localization in testis cross-sections from RAREhspLacZ vehicle control animals (F, P) and mice treated with a single dose (G) or seven consecutive daily treatments (Q). Black arrows denote STRA8/β-galactosidase-positive spermatogonia, and yellow arrows denote STRA8/β-galactosidase-negative spermatogonia. The red arrows denote STRA8-positive preleptotene spermatocytes. Analysis of STRA8- and β-galactosidase-positive cells in the vehicle control (blue bars) and WIN 18,446-treated (pink bars) single dose (H) and seven consecutive daily treatment animals (R). Y axis shows the percentage of positive cells per tubule. Asterisks highlight statistical significance (*P < 0.05; **P < 0.001). TUNEL analysis of testes from vehicle control (N) and animals treated for 7 consecutive days (O). Pink arrows denote several of the very few TUNEL-positive cells that were present in either sample. All bars = 20 μm.
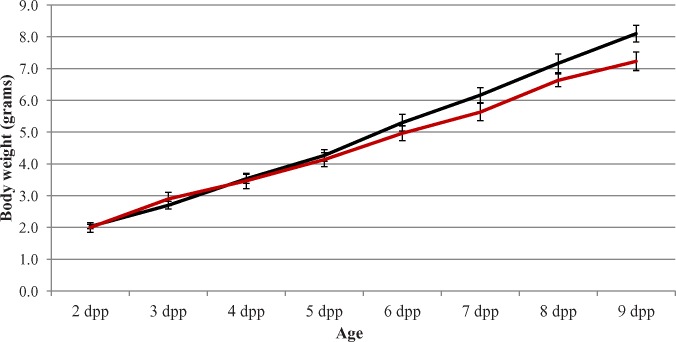
FIG. 3.
Body weight is not affected by WIN 18,446 treatment of neonatal animals. Graph depicts the daily body weight in grams of vehicle controls (black line) and animals treated daily for 7 consecutive days with WIN 18,446, beginning at 2 dpp (red line). Age is given on the X axis with body weight presented on the Y axis. There was no statistical difference between the two lines. Error bars represent ±SD.
To investigate whether WIN 18,446 could block spermatogonial differentiation in neonatal mice over a longer period of time, mice aged 2 dpp were given once daily treatments for 14 consecutive days and testis morphology was examined 24 h following the final treatment (Fig. 4). Morphological analysis of testis cross-sections revealed tubules containing only Sertoli cells and spermatogonia, with no other advanced germ cell types present. In contrast, preleptotene, leptotene, and pachytene spermatocytes, in addition to undifferentiated and differentiating spermatogonia, were present within the tubules of control animal testes (Fig. 4). Immunohistochemical staining of WIN 18,446-treated testes for STRA8 and ZBTB16 indicated that spermatogonia present within these tubules were all undifferentiated because no STRA8-positive cells were observed.
FIG. 4.
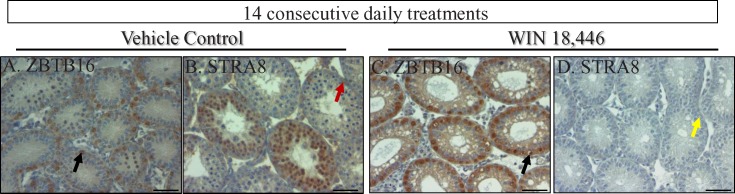
Fourteen consecutive daily treatments of neonatal mice with WIN 18,446 generates testis tubules containing only Sertoli cells and undifferentiated spermatogonia. ZBTB16 and STRA8 localization in testis cross-sections from vehicle control animals (A, B) and animals treated with WIN 18,446 for 14 consecutive days, beginning at 2 dpp (C, D). Black arrows denote ZBTB16-positive spermatogonia, red arrows denote STRA8-positive spermatogonia, and yellow arrows denote STRA8-negative spermatogonia. All bars = 50 μm.
Retinoic Acid Synchronizes the First Wave of Spermatogenesis in WIN 18,446-Treated Neonatal Mice
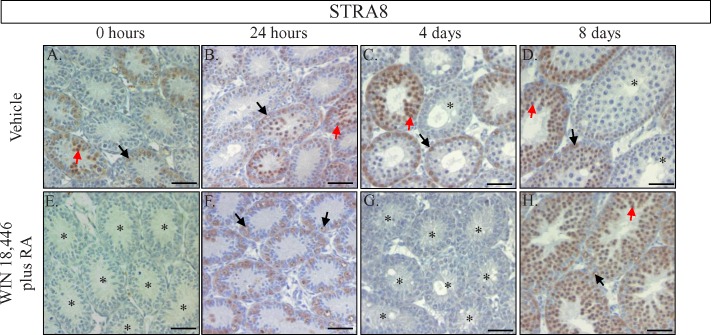
To determine whether the spermatogonia being held in an undifferentiated state during WIN 18,446 treatment could be triggered to differentiate and proceed normally through spermatogenesis, we injected WIN 18,446-treated neonatal mice with RA, allowed the animals to recover for either 24 h, 4 days, or 8 days and performed histological analyses to determine which stage of spermatogenesis had been reached by the germ cells within these testes at each time point. Interestingly, STRA8 protein appeared to be uniformly expressed by spermatogonia in every testis tubule tested 24 h postinjection (Fig. 5F). By 4-days postinjection, no STRA8-positive cells were detected yet differentiating spermatogonia could be identified based on nuclear morphology (Fig. 5G). However, ZBTB16 staining of these cross sections indicated that some undifferentiated spermatogonia remained in these testes (see Supplemental Fig. S2) and suggested that a few spermatogonia were unable to differentiate in response to the RA injection. Morphological and immunohistochemical analyses of the 8-days postinjection testes revealed the homogenous expression of STRA8 in newly differentiated Type A1 spermatogonia and preleptotene spermatocytes in every tubule cross-section across the entire testis (Fig. 5H). This staining pattern was in stark contrast to control testes that displayed a heterogeneous pattern of STRA8 signal, with some tubules containing only STRA8-positive differentiating spermatogonia, some containing STRA8-positive spermatogonia and spermatocytes, and some containing no STRA8-positive cells (Fig. 5D). In addition, there was a distinct difference in the most differentiated germ cell type present in these testes, with only preleptotene spermatocytes observed in the WIN 18,446/ RA samples and pachytene spermatocytes present in the control testes. Interestingly, the testes of animals treated with WIN 18,446 and then given an injection of the RA vehicle (DMSO) also displayed synchronous spermatogenesis throughout the first wave (data not shown), although germ cell development appeared to be delayed by approximately 24 h and the synchrony was not as precise as that observed with the RA injection (see Supplemental Fig. S3). This result suggests that an injection of RA is not required to generate synchrony but does drive it to occur faster and in a more uniform fashion.
FIG. 5.
Treatment of neonatal mice with a combination of WIN 18,446 and RA synchronizes the first wave of spermatogenesis. STRA8 localization in cross-sections of vehicle control animals (A–D) and animals treated with WIN 18,446 for 7 consecutive days followed by a single injection of RA. Testes from treated animals were collected prior to injection (0 h; E) or 24 h (F), 4 days (G), and 8 days (H) postinjection. Black arrows denote STRA8-positive spermatogonia, red arrows denote STRA8-positive preleptotene spermatocytes, and the asterisks denote tubule cross-sections containing very few or no STRA8-positive cells. All bars = 50 μm.
WIN 18,446/RA Treatment of Neonates Induces Synchronous Spermatogenesis in Adult Mice
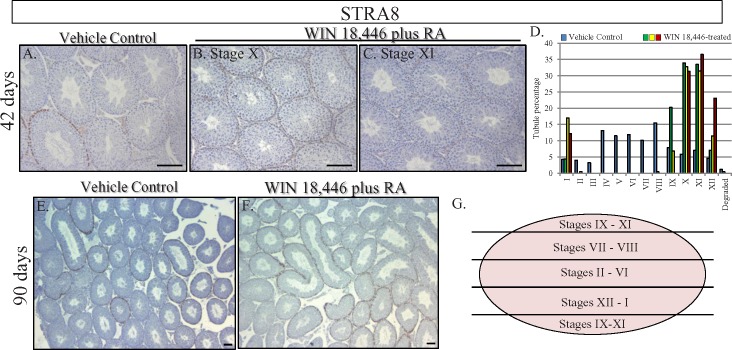
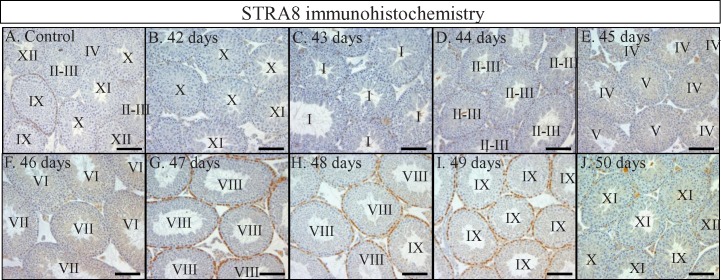
To determine whether WIN 18,446/RA-induced simultaneous spermatogonial differentiation in the neonatal testis leads to synchronous spermatogenesis in the adult testis, some WIN 18,446/RA-treated animals were left to recover for either 42, 90, or 180 days after RA injection. At 42-days postinjection, testis histology, STRA8 immunohistochemistry, and analysis of patterns of germ cell associations revealed that spermatogenesis occurred in a synchronous or pulsatile fashion (Fig. 6, B and C). In three different animals analyzed, approximately 70% of tubule cross-sections displayed only 2 of the 12 stages, stages X and XI, and a total of only four stages were present within any given sample (Fig. 6D). Interestingly, even though the treated animals were not siblings, the same two stages were overrepresented in each sample, indicating that there was very little variation when germ cell synchrony was induced in this manner. Testes were also collected daily between 42 and 50 days after WIN 18,446 treatment/RA injection (Fig. 7), therefore covering an entire single cycle of the seminiferous epithelium. Histological analysis of these testes demonstrated that this collection procedure had effectively assembled samples containing predominantly two to three stages spanning the entire cycle making it possible to collect cells from a particular stage in spermatogenesis in an accurate manner simply by calculation. Analyses at 90-days (Fig. 6) and 180-days (data not shown) postinjection revealed that testes were no longer displaying synchronous spermatogenesis, and yet WIN 18,446/RA treatment of neonatal mice did have long-lasting effects on the arrangement of spermatogenesis within the testis. At both time points, all 12 stages of the seminiferous cycle were present within the testes. However, instead of the random assortment of stages observed within cross-sections of control testes, the stages were arranged in a sequential fashion along the dorsal-ventral axis (Fig. 6F). Mating tests of WIN 18,446/RA-treated animals and of their offspring revealed these animals were fertile and produced normal litter sizes (data not shown), indicating that the spermatozoa produced after the cessation of WIN 18,446 treatment were perfectly functional.
FIG. 6.
Treatment of neonatal mice with WIN 18,446 and RA synchronizes spermatogenesis in the adult testis. STRA8 localization in vehicle control animals (A, E) and animals treated with WIN 18,446 followed by RA as neonates and left to recover for either 42 days (B, C) or 90 days (F) post-RA injection. Note the heterogeneity of stages in the vehicle control samples compared to the uniformity of stages present in the WIN 18,446/RA-treated samples 42 days postinjection. Analysis of the cellular associations present in an average vehicle control 42 days postinjection testis (blue bars) and in three different WIN 18,446/RA-treated 42 days postinjection samples (green, yellow, and red bars) (D). Data are presented as the percentage of total tubules analyzed displaying each individual stage in each sample, with tubule percentage given on the Y axis. G) Schematic drawing of the sequential arrangement of stages present in the WIN 18,446/RA-treated samples at 90 days postinjection. This sequential arrangement is maintained through until at least 180 days postinjection. All bars = 100 μm
FIG. 7.
WIN 18,446/RA treatment of neonatal mice can generate testes containing only very specific stages of the seminiferous epithelium. STRA8 localization in a representative vehicle control animal (A) and animals collected at 42 (B), 43 (C), 44 (D), 45 (E), 46 (F), 47 (G), 48 (H), 49 (I), and 50 (J) days postinjection. Roman numerals in each tubule designate the stage represented by the cellular association present. All bars = 100 μm.
DISCUSSION
The intricate arrangement of germ cells within the mammalian testis makes it an incredibly complex organ to study. For spermatozoa to be continuously released from the seminiferous epithelium at a high enough concentration for fertility, large numbers of germ cells must proceed through numerous different, highly specialized differentiation steps at the same time within the testis. As a result, investigating each individual step in germ cell differentiation is a difficult task. This study describes a novel technique that, via manipulating RA levels within the testis, can alter the normal, asynchronous production of sperm and produce testes enriched for single populations of germ cells or containing only two to three stages of the seminiferous epithelium. Therefore, this technique represents a novel and powerful tool for studying spermatogenesis.
Our data indicate that eliminating RA production in either the adult or neonatal testis has long-lasting effects on the progression of spermatogenesis. Treatment of either adult or neonatal animals with WIN 18,446 lowered RA levels within the testis so that spermatogonia were held in an undifferentiated state. When treatment was either ceased or followed by an injection of RA, the normal cycle of the seminiferous epithelium was perturbed upon recovery. These observations imply that when RA levels are reduced within the testis, undifferentiated spermatogonia continue to proliferate but fail to differentiate. Therefore, when RA either returns naturally or is introduced exogenously, there appears to be more undifferentiated spermatogonia ready to transition to type A1 differentiating spermatogonia, and as a result, more germ cells simultaneously undergo the same steps as they develop into spermatozoa. This is evident in the adult animals that received treatments for 3 days were left to recover for 26 days: there was an overrepresentation of stages IX–XI in these samples (Fig. 1G).
The effect of treating neonatal male mice with WIN 18,446/RA was particularly dramatic in terms of changing the progression of the first wave of spermatogenesis. A single injection of RA after WIN 18,446 treatment drove the majority of undifferentiated spermatogonia to differentiate simultaneously and near perfectly synchronized the entire first wave of spermatogenesis. In the normal neonatal testis, the first wave of spermatogenesis proceeds in an asynchronous fashion [24], with spermatogonia constantly being triggered to differentiate and generate a juvenile testis full of germ cells in all the different stages of differentiation. Retinoic acid injection following WIN 18,446 treatment of neonatal mice did induce the undifferentiated spermatogonia to enter their differentiation pathway. However, rather than being triggered in a normal, asynchronous fashion, this differentiation occurred in a coordinated wave across the entire testis. Other studies have reported no significant differences in serum levels of testosterone after WIN 18,446 treatment [9, 11], indicating that the negative effects on spermatogenesis resulting from WIN 18,446 are nonhormonal in nature. Collectively, these observations suggest that there are very precise control mechanisms governing RA levels within the seminiferous epithelium, and perturbing these levels has significant repercussions regarding when germ cells commit to spermatogenesis.
The observation that WIN 18,446/RA treatment of neonates results in the altered arrangement of sperm production within the adult testis for at least 6 mo posttreatment indicates that the asynchronous production of sperm in mammals is likely triggered by the precise regulation of RA levels within the newly formed seminiferous tubules at the very beginning of spermatogenesis. This hypothesis is also supported by the observation that there appear to be patches of RA activity along the length of a neonatal testis tubule [24] and that injecting neonatal mice with RA can induce synchronous spermatogenesis in the adult [25]. Understanding how these patches of RA activity are established within the newborn testis and determining the mechanism that drives the reinforcement of this periodic activity in the adult testis, and therefore asynchronous spermatogenesis, will be the focus of future research.
This study also supports the hypothesis that RA is required during very specific stages of the adult seminiferous epithelium. Short-term WIN 18,446 treatment appeared to only delay the development of spermatogonia at stages VII and VIII of the cycle. Progress has been made in terms of quantitating RA concentrations in the serum [26], however, accurate measurements of RA levels in the neonatal testis and across the cycle of the seminiferous epithelium have still yet to be performed. While expression of all types of vitamin A-metabolizing enzymes can be detected throughout the cycle, there are RA synthesis enzymes that display a peak in expression during stages VII and VIII, when the A to A1 transition is taking place [27, 28] and RA degradation enzymes that display a peak during stages VIII–XII [28]. These expression studies, combined with our observations, imply that there may be an increase in endogenous RA levels at stages VII–VIII, leading to spermatogonial differentiation, followed by a decrease in local RA levels, which could ultimately lead to RA maintaining the cycle of the seminiferous epithelium in the adult testis.
In addition, this study has important implications for the development of a male contraceptive that interferes with the production of RA within the testis and/or with spermatogonial differentiation. Our data suggest that blocking the metabolism of vitamin A within the human testis may lead to alterations in the arrangement of the developing germ cells within the seminiferous epithelium or even result in the pulsatile, rather than continuous, release of sperm. It is possible that any reversible contraceptive that holds spermatogonia in an undifferentiated state may produce this result once treatment has ceased. Therefore, the long-term effects of treatment on the arrangement of germ cells within the testis is an important issue that will needed to be carefully examined for any potential male contraceptive and is especially critical if the target is one of the proteins involved in vitamin A metabolism.
Utilizing WIN 18,446 to investigate how drugs targeting vitamin A metabolism affect the testis morphologically, we have developed a unique and novel tool for studying all aspects of spermatogenesis. Male germ cell development is difficult to study at the molecular level because once spermatogonia differentiate, the testis contains germ cells at every stage of development. Therefore, trying to extract molecular information from one or a few specific testicular germ cell types from this complex population is extremely difficult because the presence of many different germ cell types dilutes specific signals. Our WIN 18,446/RA treatment regime generates testes full of germ cells progressing through the first wave of spermatogenesis normally but simultaneously and hence is an extremely powerful technique for investigating a specific subset of male germ cells or a particular spermatogenic process. This includes the spermatogonial stem cell population because maintaining neonatal mice on WIN 18,446 treatment longer than 7 days produces a testis full of only undifferentiated spermatogonia and Sertoli cells. Synchronization of spermatogenesis using the VAD model is possible and has been used to investigate stage-specific gene expression [29]. However, synchronization induced via dietary deficiency takes a long time to achieve (approximately 28 wk), the animal's health is compromised, and the effects of RA deficiency in the neonatal testis can only be studied in animals carrying genetic mutations that predispose them to easily become VAD [8]. The WIN 18,446/RA technique described here offers a faster, safer and more reproducible alternative to dietary VAD, allows for significant headway to be made into our understanding of how vitamin A regulates spermatogenesis, and represents a powerful new tool for the reproductive biology scientific community.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Patricia Hunt for her critical reading of the manuscript and the Laboratory for Biotechnology and Bioanalysis at Washington State University for the use of their equipment.
Footnotes
Supported by a Contraceptive Center Grant U54 42454 and by HD 10808 from the NIH to M.D.G. and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, a division of the NIH through cooperative agreements #U01 HD060408 to J.K.A. Presented in part at the 2011 Mammalian Gametogenesis and Embryogenesis Gordon Conference, 21–26 August, 2011, Waterville Valley, New Hampshire.
REFERENCES
- Oakberg EF. A description of spermiogenesis in the mouse and its use in analysis of the cycle of the seminiferous epithelium and germ cell renewal. Am J Anat 1956; 99: 391 413 [DOI] [PubMed] [Google Scholar]
- Russell LD, Ettlin RA, Sinha Hikim AP, Clegg EP. Histological and Histopathological Evaluation of the Testis. St. Louis, MO: Cache River Press; 1990. [Google Scholar]
- Hogarth CA, Griswold MD. The key role of vitamin A in spermatogenesis. J Clin Investig 2010; 120: 956 962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales CG, Griswold MD. Retinol-induced stage synchronization in seminiferous tubules of the rat. Endocrinology 1987; 121 (1): 432 434 [DOI] [PubMed] [Google Scholar]
- Snyder EM, Davis JC, Zhou Q, Evanoff R, Griswold MD. Exposure to retinoic acid in the neonatal but not adult mouse results in synchronous spermatogenesis. Biol Reprod 2011; 84: 886 893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beek ME, Meistrich ML. A method for quantifying synchrony in testes of rats treated with vitamin A deprivation and readministration. Biol Reprod 1990; 42: 424 431 [DOI] [PubMed] [Google Scholar]
- Griswold MD, Hogarth CA, Bowles J, Koopman P. Initiating meiosis: the case for retinoic acid. Biol Reprod 2012; 86: 35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Palczewski K, Baehr W, Clagett-Dame M. Vitamin A deficiency results in meiotic failure and accumulation of undifferentiated spermatogonia in prepubertal mouse testis. Biol Reprod 2011; 84: 336 341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amory JK, Muller CH, Shimshoni JA, Isoherranen N, Paik J, Moreb JS, Amory DW, Sr,, Evanoff R, Goldstein AS, Griswold MD. Suppression of spermatogenesis by bisdichloroacetyldiamines is mediated by inhibition of testicular retinoic acid biosynthesis. J Androl 2011; 32: 111 119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth CA, Evanoff R, Snyder E, Kent T, Mitchell D, Small C, Amory JK, Griswold MD. Suppression of Stra8 expression in the mouse gonad by WIN 18,446. Biol Reprod 2011; 84: 957 965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller CG, Moore DJ, Paulsen CA. Suppression of spermatogenesis and chronic toxicity in men by a new series of bis(dichloroacetyl) diamines. Toxicol Appl Pharmacol 1961; 3: 1 11 [DOI] [PubMed] [Google Scholar]
- Brooks NL, van der Horst G. Short-term effects of N′N-bis(dichloroacetyl)-1,8-octamethylenediamine (WIN 18446) on the testes, selected sperm parameters and fertility of male CBA mice. Lab Anim 2003; 37: 363 373 [DOI] [PubMed] [Google Scholar]
- McLean DJ, Russell LD, Griswold MD. Biological activity and enrichment of spermatogonial stem cells in vitamin A-deficient and hyperthermia-exposed testes from mice based on colonization following germ cell transplantation. Biol Reprod 2002; 66: 1374 1379 [DOI] [PubMed] [Google Scholar]
- Loveland KL, Hogarth C, Szczepny A, Prabhu SM, Jans DA. Expression of nuclear transport importins beta 1 and beta 3 is regulated during rodent spermatogenesis. Biol Reprod 2006; 74: 67 74 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Shima JE, Nie R, Friel PJ, Griswold MD. Androgen-regulated transcripts in the neonatal mouse testis as determined through microarray analysis. Biol Reprod 2005; 72: 1010 1019 [DOI] [PubMed] [Google Scholar]
- van Pelt AM, de Rooij DG. Synchronization of the seminiferous epithelium after vitamin A replacement in vitamin A-deficient mice. Biol Reprod 1990; 43: 363 367 [DOI] [PubMed] [Google Scholar]
- Griswold MD, Bishop PD, Kim KH, Ping R, Siiteri JE, Morales C. Function of vitamin A in normal and synchronized seminiferous tubules. Ann N Y Acad Sci 1989; 564: 154 172 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Li Y, Nie R, Friel P, Mitchell D, Evanoff RM, Pouchnik D, Banasik B, McCarrey JR, Small C, Griswold MD. Expression of stimulated by retinoic acid gene 8 (Stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol Reprod 2008; 78: 537 545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini M, Filipponi D, Gori M, Barrios F, Lolicato F, Grimaldi P, Rossi P, Jannini EA, Geremia R, Dolci S. ATRA. and KL promote differentiation toward the meiotic program of male germ cells. Cell Cycle 2008; 7: 3878 3888 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Nie R, Li Y, Friel P, Mitchell D, Hess RA, Small C, Griswold MD. Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: an in vivo study in vitamin A-sufficient postnatal murine testes. Biol Reprod 2008; 79: 35 42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet 2004; 36: 647 652 [DOI] [PubMed] [Google Scholar]
- Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, Orwig KE, Wolgemuth DJ, Pandolfi PP. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet 2004; 36: 653 659 [DOI] [PubMed] [Google Scholar]
- Rossant J, Zirngibl R, Cado D, Shago M, Giguere V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev 1991; 5: 1333 1344 [DOI] [PubMed] [Google Scholar]
- Snyder EM, Small C, Griswold MD. Retinoic acid availability drives the asynchronous initiation of spermatogonial differentiation in the mouse. Biol Reprod 2010; 83: 783 790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Davis JC, Zhou Q, Evanoff R, Griswold MD. Exposure to retinoic acid in the neonatal but not adult mouse results in synchronous spermatogenesis. Biol Reprod 2011; 84: 886 893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SL, Amory JK, Walsh TJ, Isoherranen N. A sensitive and specific method for measurement of multiple retinoids in human serum with UHPLC-MS/MS. J Lipid Res 2012; 53: 587 598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet N, Dennefeld C, Rochette-Egly C, Oulad-Abdelghani M, Chambon P, Ghyselinck NB, Mark M. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology 2006; 147: 96 110 [DOI] [PubMed] [Google Scholar]
- Sugimoto R, Nabeshima Y, Yoshida S. Retinoic acid metabolism links the periodical differentiation of germ cells with the cycle of Sertoli cells in mouse seminiferous epithelium. Mech Dev 2012; 128: 610 624 [DOI] [PubMed] [Google Scholar]
- Luk JM, Mok BW, Shum CK, Yeung WS, Tam PC, Tse JY, Chow JF, Woo J, Kam K, Lee KF. Identification of novel genes expressed during spermatogenesis in stage-synchronized rat testes by differential display. Biochem Biophys Res Comm 2003; 307: 782 790 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.