Abstract
Objective:
To describe the prevalence of neurocognitive impairment (NCI) among early diagnosed and managed HIV-infected persons (HIV+) compared to HIV-negative controls.
Methods:
We performed a cross-sectional study among 200 HIV+ and 50 matched HIV-uninfected (HIV−) military beneficiaries. HIV+ patients were categorized as earlier (<6 years of HIV, no AIDS-defining conditions, and CD4 nadir >200 cells/mm3) or later stage patients (n = 100 in each group); both groups were diagnosed early and had access to care. NCI was diagnosed using a comprehensive battery of standardized neuropsychological tests.
Results:
HIV+ patients had a median age of 36 years, 91% were seroconverters (median window of 1.2 years), had a median duration of HIV of 5 years, had a CD4 nadir of 319, had current CD4 of 546 cells/mm3, and 64% were on highly active antiretroviral therapy (initiated 1.3 years after diagnosis at a median CD4 of 333 cells/mm3). NCI was diagnosed among 38 (19%, 95% confidence interval 14%–25%) HIV+ patients, with a similar prevalence of NCI among earlier and later stage patients (18% vs 20%, p = 0.72). The prevalence of NCI among HIV+ patients was similar to HIV− patients.
Conclusions:
HIV+ patients diagnosed and managed early during the course of HIV infection had a low prevalence of NCI, comparable to matched HIV-uninfected persons. Early recognition and management of HIV infection may be important in limiting neurocognitive impairment.
Despite the availability of highly active antiretroviral therapy (HAART), HIV-infected persons remain at risk for neurocognitive impairment (NCI).1 Although severe forms of neurologic disease (e.g., HIV-associated dementia) have declined, the risk of other forms of NCI remains elevated compared to the general population.1–4 The burden of NCI among HIV-infected persons remains substantial, occurring in approximately half (range 18%–73%) of patients.1,5–7
Since most studies have evaluated HIV-positive patients with unknown dates of HIV seroconversion and 35%–45% of newly diagnosed HIV-positive patients in the United States meet AIDS-defining criteria within 1 year of diagnosis,8 elevated rates of NCI may result from late diagnosis and uninhibited viral replication in the CNS causing irreversible brain injury prior to diagnosis or initiation of therapy. A history of AIDS and low nadir CD4 counts has been associated with NCI.1,2,9 However, few studies have determined the rate of NCI among HIV-positive patients managed in an optimized setting of early diagnosis, free access to care, and few concurrent comorbidities.
We determined the prevalence of NCI among US military HIV-infected persons who have routine HIV testing, mandatory follow-up evaluations after diagnosis, open access to early antiretroviral treatment, and low rates of comorbidities including active substance use. We assessed the prevalence of neurocognitive impairment evaluating HIV-positive patients both earlier (i.e., <6 years of HIV infection since diagnosis, no AIDS-defining condition, and CD4 nadir >200 cells/mm3) and later in the course of HIV infection, and compared these participants with a matched HIV-uninfected group of military beneficiaries.
METHODS
Study design and participants
We performed a cross-sectional study among 200 HIV-infected and 50 HIV-uninfected military beneficiaries (active duty members, retirees, or dependents). All active duty members, including those in this analysis, are HIV seronegative upon entry into military service and undergo repeated mandatory HIV testing. Active duty members who become HIV-positive are evaluated by an HIV specialist at least semiannually, and all military beneficiaries have open access to early antiretroviral treatment and low rates of comorbidities including active substance use.
Of the 200 HIV-infected participants, 100 were classified as earlier stage (<6 years of HIV infection since diagnosis, no prior AIDS-defining condition, and CD4 nadir >200 cells/mm3), and 100 as later stage (not meeting all 3 criteria). A control group of military beneficiaries (n = 50) were matched to the HIV-infected subjects by age (<35 vs 35–50 years), gender, race/ethnicity (Caucasian vs other), and rank (officer vs enlisted vs other including retirees and spouses). Inclusion criteria for both HIV-infected and HIV-uninfected groups included military beneficiaries who were 18–50 years of age. Exclusion criteria were current/recent suicidal ideation, inability or unwillingness to complete the neuropsychological battery, and presence of an acute medical condition that could impact the participant's ability to complete the tests (e.g., febrile illness). The control group had an HIV-negative ELISA test within 1 year of study enrollment.
Standard protocol approvals, registrations, and patient consents
All study participants provided written informed consent and the study was approved by a central military institutional review board. The trial was registered at ClinicalTrials.gov (registration #NCT00893815).
Data collection
Clinical data were abstracted from medical records including medical conditions, medications, body mass index, and fasting lipid levels. Hepatitis C virus (HCV) was defined as a positive antibody or RNA viral load. Among HIV-infected subjects, data on last HIV seronegative and first HIV seropositive dates, AIDS-defining conditions,10 CD4 cell counts (including current, nadir, and recovery [current CD4 − CD4 nadir]), HIV RNA levels, and antiretroviral therapy (type, CNS penetration effectiveness,11 duration, and percentage of time since diagnosis on medications) were collected. HIV infection in our cohort was primarily acquired by sexual routes12; data on sexual orientation were not available.
Questionnaire data included demographics, military rank and duty status, education, substance use, history of loss of consciousness or traumatic brain injury, and self-reported assessment of cognitive impairment. Illicit drug use was ascertained by a confidential questionnaire regarding drug use (past and current) and prior failure of military mandatory drug screening. Questionnaires assessed lipodystrophy, neuropathy (AIDS Clinical Trial Group Peripheral Neuropathy Screening Tool),13 current and lifetime psychiatric diagnoses (Composite International Diagnostic Interview modules A, E, F, J, K, O, and X), and current mood (Beck Depression Inventory [BDI]–II).
All participants underwent a comprehensive battery of standardized neuropsychological tests and questionnaires (administration time 3.5–4 hours) shown to be sensitive to HIV-associated neurocognitive disorders.1 The neuropsychological measures included an estimate of premorbid functioning (Wechsler Test of Adult Reading), verbal fluency (letter fluency [FAS], category fluency [animals], and action fluency [verbs]), attention/working memory (Paced Auditory Serial Addition Task, Wechsler Adult Intelligence Scale III [WAIS-III] Digit Span), visuospatial functioning (Judgment of Line Orientation Tests, form H; Hooper Visual Organization Test), speeded information processing (WAIS-III Symbol Search, WAIS-III Digit Symbol, Trail Making Test [TMT] A, Stroop Word and Color Tests), learning and recall (Hopkins Verbal Learning Test–R, Brief Visuospatial Memory Test–R), abstraction/executive functioning (Wisconsin Card Sorting Tests, 64-card version; TMT B; Stroop Word and Color Tests), motor speed and dexterity (Grooved Pegboard Test [both hands]), and effort (Hiscock Digit Memory Test). Neuropsychological tests were scored by trained psychometrists and raw scores were converted to demographically adjusted t scores corrected for effects of age, education, gender, and ethnicity. Scores were then converted to deficit scores that give differential weight to impaired rather than normal scores as previously described.14,15 The Global Deficit Score (GDS) was used to summarize neuropsychological test results by quantifying the number and degree of impaired performances. A score ≥0.5 has been shown to be a sensitive and specific indicator of global NCI,14 and a deficit score >0.5 was used within each domain. Importantly, impairment on the GDS has been found to be associated with biomarkers of HIV disease progression (e.g., CD4 count)16 as well as aspects of everyday functioning declines (e.g., medication adherence).17,18
Statistical analyses
Descriptive statistics are presented as medians with interquartile ranges (IQRs) or as counts with percents, as appropriate. The Kruskal-Wallis rank-sum test was used to compare medians, and x2 tests to compare percentages. For each analysis, there were 2 comparisons: earlier vs later stage HIV-infected participants, and HIV-infected vs HIV-uninfected participants. The relationships between self-reported cognitive problems and GDS and depression were explored with linear and logistic regression. Univariate and multivariate associations of factors with NCI were determined by logistic regression. Odds ratios (OR) for the prevalence of NCI were estimated with 95% confidence intervals (CI). Prespecified factors of interest (age, gender, race/ethnicity, years since HIV seropositivity, and cumulative years on antiretroviral therapy since diagnosis) along with factors with a p value ≤0.15 in univariate models were included in the multivariate model. All p values are 2-sided. Analyses were conducted using SAS software (version 9.1; SAS).
RESULTS
Study population
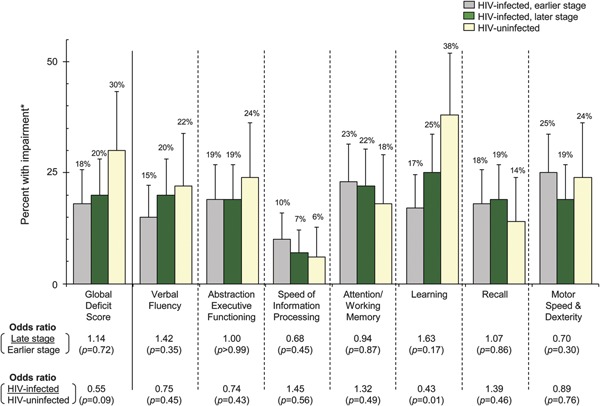
Two hundred HIV-infected persons were studied (table 1); 91% were documented HIV seroconverters with a median seroconversion window of 1.2 years. The study population consisted of a population with low prevalence of substance use—18% used tobacco, 5.5% consumed ≥6 alcoholic drinks per week, and 3.5% used illicit drugs. Sixty-four percent were receiving HAART, which was initiated a median of 1.3 years after HIV diagnosis at a median CD4 count of 333 (IQR 248–423) cells/mm3; 81% had a HIV RNA <50 copies/mL at the time of enrollment. Among those off HAART, their median CD4 count was 523 (IQR 417–685) cells/mm3. Characteristics by earlier vs later stage HIV-infected persons are shown in table 1. The HIV-negative control group (n = 50) was similar to the HIV-infected group except they were less likely to be African American and more likely to be “other” races, less likely to have a higher education degree, and less likely to have hypertension (table 1).
Table 1.
Baseline characteristics of study population
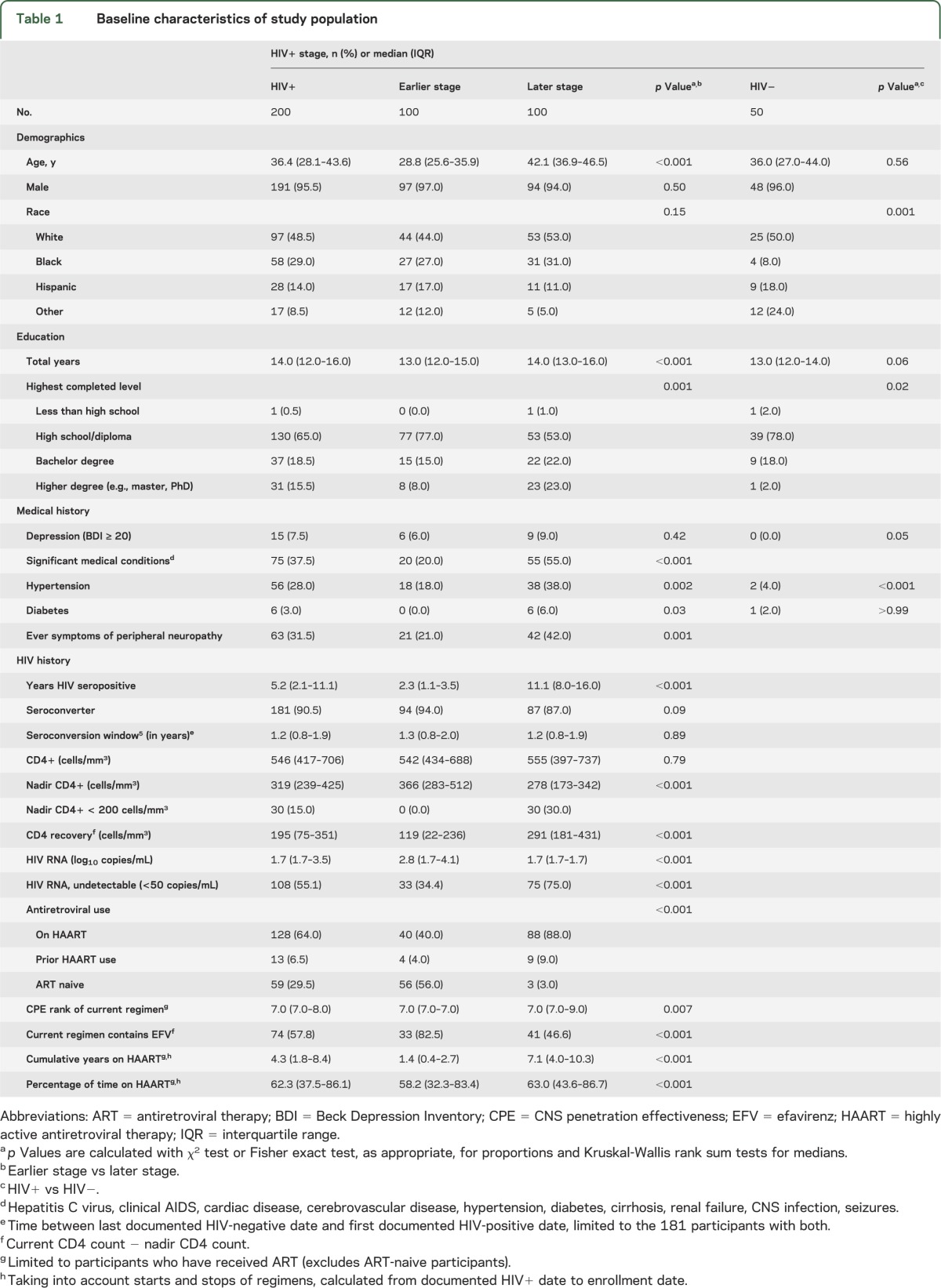
Prevalence of NCI among HIV-infected persons
NCI (GDS ≥ 0.5) was diagnosed among 38 (19%, 95% CI 14%–25%) HIV-infected participants. The prevalence of NCI was similar among earlier and later stage patients (18% vs 20%, p = 0.72). Later stage patients were more likely to have impairments in verbal fluency, learning, and recall, although none were statistically different from earlier stage patients (figure). The number of domains in which participants were impaired was also similar between earlier and later stage HIV participants (earlier 1.27 and later 1.31, p = 0.84).
Figure. Impairment by ability area.
*Domain deficit score >0.50, global deficit score ≥0.50.
We evaluated the association between self-reported cognitive problems (“Do you feel that you have a problem with memory loss or cognitive functioning?”) and battery-identified NCI and found no relationship of self-reported cognitive complaints with NCI (OR 1.3, p = 0.53) or with the mean GDS (p = 0.44). Among the 55 participants who complained of cognitive issues, 12 (22%) had NCI, whereas 26 (18%) of those who did not complain of cognitive issues had NCI (p = 0.53). Those with symptoms of depression (BDI ≥ 20) were more likely to self-report cognitive dysfunction compared to those without depression (73% vs 24%, p < 0.001); however, there was no association of depression with NCI or with GDS.
Prevalence of NCI among HIV-uninfected persons
Fifteen of the HIV-uninfected participants (30%, 95% CI 17%–43%) had NCI (figure). The percentage with NCI among HIV-infected vs HIV-uninfected persons was not statistically significantly different (p = 0.09). HIV-infected persons had elevated rates of impairment in speed of information processing, attention/working memory, and recall, but none were significantly different from HIV-uninfected persons (all p values > 0.05). HIV-uninfected participants were more likely to have impairment in learning than HIV-infected persons (p = 0.01) (figure). Additional models adjusting for years of education and ethnicity compared HIV-infected to HIV-uninfected participants for the outcomes of NCI and each domain, separately, showed similar nonsignificant findings.
Factors associated with neurocognitive impairment among HIV-infected persons
Factors associated with NCI among HIV-infected participants in the univariate models are shown in table 2. No demographic, behavioral, or medical history variable was significantly associated with NCI. A higher number of years of education was associated with NCI (OR 1.2 per year, p = 0.02). Regarding HIV-specific factors, a higher current CD4 count (OR 1.07 per 50 cells/mm3, p = 0.08) and higher CD4 recovery (OR 1.07 per 50 cells/mm3, p = 0.10) were marginally associated with NCI (table 2).
Table 2.
Factors associated with neurocognitive impairment (GDS ≥0.5) among HIV-infected persons
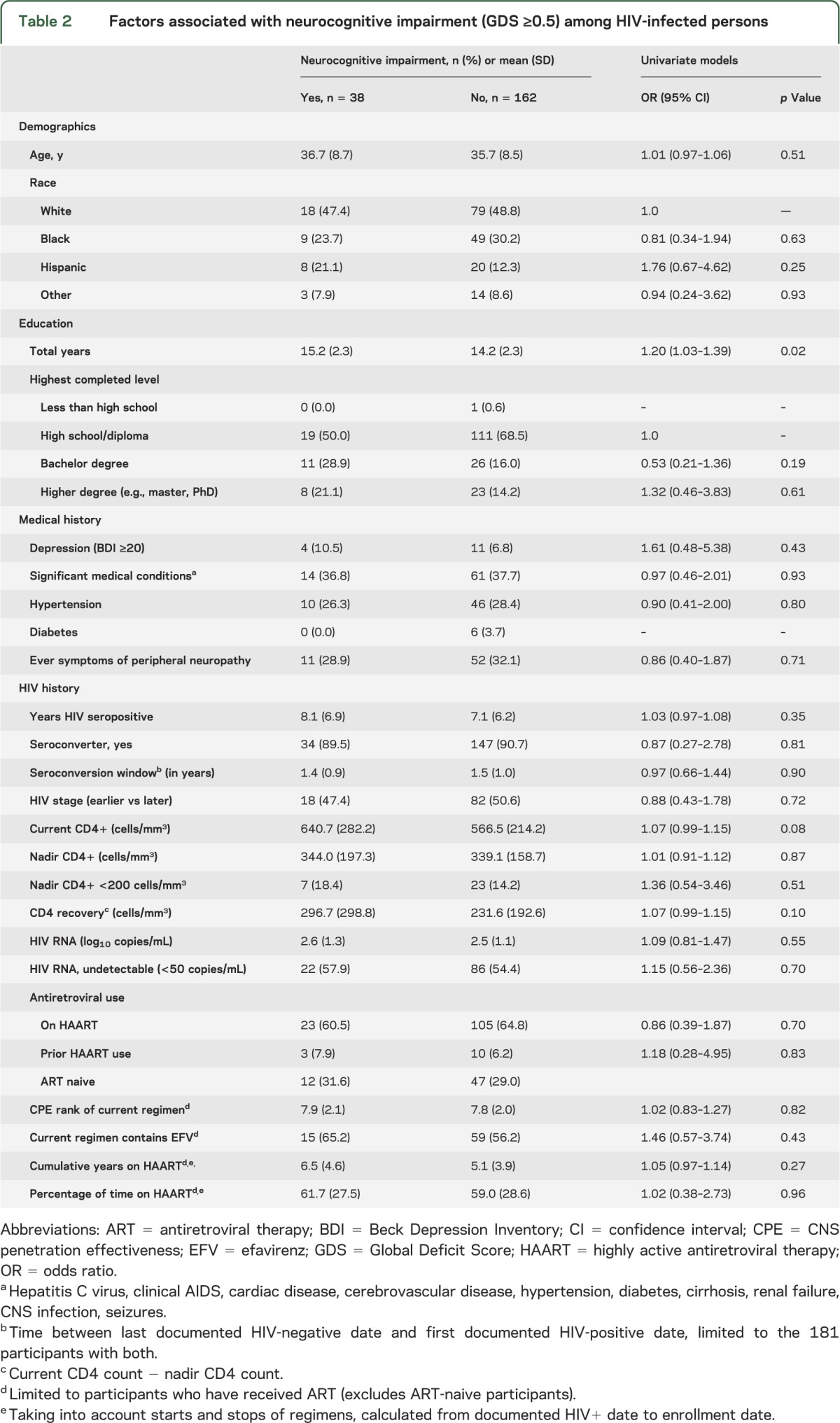
In a multivariate model including factors of interest (age, gender, race, years HIV seropositive, and cumulative years on antiretroviral therapy) and univariate factors with a p value ≤0.15, only more years of education was associated with NCI (OR 1.24 per year, p = 0.02). Current CD4 count continued to have a marginal association with NCI (OR 1.07, p = 0.07); no association was found with either CD4 nadir or CD4 recovery. Analyses were also performed stratified by earlier vs later stage and for each neurocognitive domain with similar findings (data not shown).
We repeated our analyses restricted to HIV-infected participants receiving HAART who had an HIV RNA level <50 copies/mL. Similar associations were noted with additional findings that higher CD4 cell counts (OR 1.10 per 50 cells/mm3, p = 0.04) and greater CD4 recovery (OR 1.15 per 50 cells/mm3, p = 0.01) were significantly associated with NCI. The nadir CD4 count, HIV RNA level, and HAART information were not associated with NCI (data not shown).
DISCUSSION
We found a low prevalence of NCI among HIV-infected persons diagnosed and managed early during the course of HIV infection, and nearly identical NCI rates among earlier and later HIV-infected patients in this cohort. Of note, most patients classified as later in their course of HIV infection met criteria by a longer duration of HIV infection, but the majority had preserved CD4 counts and few had prior AIDS-defining conditions. Further, the prevalence of NCI among our HIV-infected participants was not significantly greater than a matched HIV-uninfected group. These data provide important and novel information suggesting that the early recognition and management of HIV infection may be important in limiting NCI.
The importance of preventing NCI is severalfold as it impacts both the quantity and quality of life. Regarding survival, HIV-infected patients with NCI are at increased risk of death, even after controlling for other medical factors.3,19 Further, NCI can have a substantial impact on patients' daily functioning and their ability to pursue career endeavors.20 Finally, NCI may reduce antiretroviral adherence, hence adversely affecting the outcome of HIV-infected persons.17 Hence, preserving cognitive function among HIV-infected persons may be an important step in further improving quality and life expectancies of patients.
During the pre-HAART era, 16% of patients with AIDS had HIV dementia, with an annual incidence of 7%.21 After the introduction of HAART in 1996, there was a sharp decrease in HIV dementia; however, milder forms of neurocognitive disease continued to be diagnosed.4,22 Studies demonstrated that patients with symptomatic seroconverting illness as well as high HIV RNA levels and low CD4 counts early after infection were at highest risk.23,24
The prevalence of NCI in the HAART era has varied in prior studies, likely related to clinical disease stage, comorbid diseases, and other factors.6,7 A recent study found a NCI prevalence of 52% among HIV-infected patients seen at academic US HIV clinics regardless of comorbidity level.1 Our study found a much lower NCI rate (19%), but was similar to a recent study evaluating HIV-infected persons with suppressed HIV RNA levels.25 The relatively low prevalence in our study may be due to a combination of factors. Early diagnosis and active disease management, few comorbid conditions (low prevalence of concurrent medical conditions including HCV, illicit drug use, and alcohol), young age, frequent monitoring of vocational functioning, and the lack of AIDS events or low CD4 counts likely contributed to our low impairment rate. Further, despite the later group having HIV for a median of 11 years, their risk of NCI remained similar to the earlier group (median 2 years). This suggests that length of HIV duration itself may not be a risk factor if patients maintain good HIV control, avoiding AIDS-defining events and low nadir CD4 counts.6
In our study, HIV-infected persons had a similar prevalence of NCI compared to matched HIV-uninfected persons. Further, HIV-infected patients were not more likely to be impaired in any of the 7 cognitive domains. A recent Danish study found the overall risk of severe neurocognitive disorders is now similar among HIV-positive and -negative persons.3 It should be noted that the HIV-negative controls in this study are likely to be an accurate control population relative to prior studies, as the military is relatively homogeneous in regards to socioeconomic status, lifestyle, and other factors such as substance abuse.
Early events in HIV infection such as loss of vital CD4 reserves and uncontrolled HIV replication may trigger irreversible CNS damage and may cause the "residual" NCI seen in long-term survivors. Prospective studies are needed to determine if early diagnosis and initiation of antiretroviral therapy would reduce the burden of NCI among HIV-infected persons.26 A recent study showed that patients with early HIV infection had similar neurocognitive functioning compared to HIV-uninfected persons, suggesting that detrimental effects of HIV on the brain may not occur immediately, potentially providing an opportunity for early intervention.27 Clinical trials are underway, including a substudy of the Strategic Timing of Antiretroviral Treatment (START) trial, examining neurocognitive functioning among those treated immediately compared to later in their disease course.
We did not detect strong associations between immunologic or virologic control and the presence of NCI. A low CD4 nadir was not associated with NCI as seen in other studies1,2,7,9 including a recent study which suggested that these factors may lead to structural brain damage.28 Our lack of association may reflect that few of our patients experienced very low CD4 nadirs, or that CD4 nadirs are not predictive in persons who are managed early in infection and who avoid reaching very low counts (<200 cells/mm3). Regarding current HIV counts, we noted a marginal association between higher current CD4 counts and CD4 recovery with NCI. Interestingly, when restricting our analyses to participants receiving HAART with a HIV RNA <50 copies/mL, these associations became stronger. We examined the association of PI use (which may result in higher CD4 counts, but has limited CNS penetration) and found no associations between specific antiretroviral class and NCI. These data suggest a possible immunologic component, such as immune reconstitution inflammatory syndrome–like reaction, in the pathogenesis of NCI; further studies are needed. Finally, we found no associations between HAART use and NCI. Although prior studies have shown that cognition improves shortly after HAART initiation,5,29 our data suggest that NCI may persist despite ongoing HAART use, signifying that chronic neuronal inflammation and injury may continue. Since the benefit of HAART is incomplete,30,31 strategies to prevent the initial development of NCI are paramount.
The prevalence of NCI among our HIV-uninfected persons was higher than expected with the estimated rate in the general population of 16%.32 Although the reasons for this are unknown, it may have been due to self-selection bias as this group was enrolled from different settings (military bases) than the HIV-positive group (within HIV clinics). There were also differences in education and ethnicity, which may have contributed to the observed differences. The HIV-infected group had a reasonable proportion of individuals with higher degrees (e.g., Master, PhD, MD), whereas only one individual in our HIV-uninfected group had a higher degree. It may be that the more highly educated subjects in our HIV-infected group have greater levels of cognitive reserve, making declines due to HIV infection less likely. Moreover, our normative data adjust for African American and Caucasian ethnicities; the higher proportion of Hispanic and “other” ethnicities in our HIV-uninfected group may not have been appropriately corrected for among the HIV-uninfected group, leading to higher rates of NCI.
Our study had some limitations. We conducted a cross-sectional study, hence could not assess temporality or causation between factors of interest and the development of NCI. Furthermore, the low prevalence of NCI may have limited our ability to identify associated factors. We also evaluated a distinct population consisting of military members who may differ from other HIV-infected populations; however, our data provide important information about NCI in an optimized setting of early diagnosis, comprehensive medical care, stable socioeconomic factors, and few comorbidities (e.g., illicit drug use, HCV). Further, our data provide important information about the cognitive functioning of HIV-positive military personnel, and suggest that rather than disqualifying all seropositive members from performing certain occupations (e.g., aviators) due to concerns of NCI, it may be more prudent to perform neurocognitive testing in these groups.33 Additionally, our study advocates for formal neurocognitive testing as self-reports of neurocognitive complications were more strongly related to depressed mood than cognitive functioning. Since we evaluated a US military population consisting of mostly men, our study cannot be generalized to women. Finally, the main objective of the study was to determine the prevalence of NCI among HIV-infected persons, and it was not specifically powered for comparisons to the HIV-uninfected arm.
HIV-infected persons diagnosed and managed early in infection have low rates of NCI, which are comparable to those of HIV-uninfected persons. Patients with longstanding HIV infection (median >10 years) had similar NCI rates compared to those with more recent infection, suggesting that early management and avoidance of comorbid conditions may be important in preserving cognitive function.
GLOSSARY
- BDI
Beck Depression Inventory
- CI
confidence interval
- GDS
Global Deficit Score
- HAART
highly active antiretroviral therapy
- HCV
hepatitis C virus
- IQR
interquartile range
- NCI
neurocognitive impairment
- OR
odds ratio
AUTHOR CONTRIBUTIONS
Nancy F. Crum-Cianflone, MD, MPH: study concept and design, acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content, and study supervision. David J. Moore, PhD: study concept and design, critical revision of the manuscript for important intellectual content, and study supervision. Scott Letendre, MD: study concept and design, critical revision of the manuscript for important intellectual content, and study supervision. Mollie Poehlman Roediger, MS: study concept and design, analysis and interpretation, and critical revision of the manuscript for important intellectual content. Lynn Eberly, PhD: study concept and design, analysis and interpretation, and critical revision of the manuscript for important intellectual content. Amy Weintrob, MD: study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content, and study supervision. Anuradha Ganesan, MD: study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content, and study supervision. Erica Johnson, MD: study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content, and study supervision. Raechel DelRosario: acquisition of data and critical revision of the manuscript for important intellectual content. Brian K. Agan, MD: study concept and design, critical revision of the manuscript for important intellectual content, and study supervision. Braden R. Hale, MD, MPH: study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content, and study supervision.
STUDY FUNDING
Support for this work (IDCRP-016) was provided by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed through the Uniformed Services University of the Health Sciences. This project has been funded in whole, or in part, with federal funds from the National Institute of Allergy and Infectious Diseases, NIH, under Inter-Agency Agreement Y1-AI-5072. Support was also obtained by the Neurobehavioral Health Research Center from the National Institute of Mental Health (UCSD HIV Neurobehavioral Research Center, P30 MH62512). The content of this publication is the sole responsibility of the authors and does not necessarily reflect the views or policies of the NIH or the Department of Health and Human Services, the DoD, or the Departments of the Army, Navy, or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the US Government. Go to Neurology.org for full disclosures.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Heaton RK, Clifford DB, Franklin DR, Jr, et al. ; CHARTER Group HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010;75:2087–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhaskaran K, Mussini C, Antinori A, et al. ; CASCADE Collaboration Changes in the incidence and predictors of human immunodeficiency virus-associated dementia in the era of highly active antiretroviral therapy. Ann Neurol 2008;63:213–221 [DOI] [PubMed] [Google Scholar]
- 3.Lescure FX, Omland LH, Engsig FN, et al. Incidence and impact on mortality of severe neurocognitive disorders in persons with and without HIV infection: a Danish nationwide cohort study. Clin Infect Dis 2011;52:235–243 [DOI] [PubMed] [Google Scholar]
- 4.Sacktor N, McDermott MP, Marder K, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol 2002;8:136–142 [DOI] [PubMed] [Google Scholar]
- 5.Cysique LA, Vaida F, Letendre S, et al. Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology 2009;73:342–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heaton RK, Franklin DR, Ellis RJ, et al. ; CHARTER Group; HNRC Group HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011;17:3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muñoz-Moreno JA, Fumaz CR, Ferrer MJ, et al. Nadir CD4 cell count predicts neurocognitive impairment in HIV-infected patients. AIDS Res Hum Retroviruses 2008;24:1301–1307 [DOI] [PubMed] [Google Scholar]
- 8.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011;52:793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robertson KR, Smurzynski M, Parsons TD, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 2007;21:1915–1921 [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep 1992;41:1–19 [PubMed] [Google Scholar]
- 11.Letendre S, Marquie-Beck J, Capparelli E, et al. ; CHARTER Group Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 2008;65:65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brodine SK, Shaffer RA, Starkey MJ, et al. Drug resistance patterns, genetic subtypes, clinical features, and risk factors in military personnel with HIV-1 seroconversion. Ann Intern Med 1999;131:502–506 [DOI] [PubMed] [Google Scholar]
- 13.Cherry CL, Wesselingh SL, Lal L, McArther JC. Evaluation of a clinical screening tool for HIV-associated sensory neuropathies. Neurology 2005;13:1778–1781 [DOI] [PubMed] [Google Scholar]
- 14.Carey CL, Woods SP, Gonzalez R, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol 2004;26:307–319 [DOI] [PubMed] [Google Scholar]
- 15.Heaton RK, Velin RA, McCutchan JA, et al. Neuropsychological impairment in human immunodeficiency virus-infection: implications for employment: HNRC Group. Psychosom Med 1994;56:8–17 [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez R, Heaton RK, Moore DJ, et al. ; for the HNRC Group Computerizes reaction time battery versus a traditional neuropsychological battery: detecting HIV-related impairments. J Int Neuropsychol Soc 2003;9:64–71 [DOI] [PubMed] [Google Scholar]
- 17.Hinkin CH, Hardy DJ, Mason KI, et al. Medication adherence in HIV-infected adults: effect of patient care, cognitive status, and substance abuse. AIDS 2004;18:S19–S25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine AJ, Hinkin CH, Castellon SA, et al. Variations in patterns of highly active antiretroviral therapy (HAART) adherence. AIDS Behav 2004;9:355–362 [DOI] [PubMed] [Google Scholar]
- 19.Ellis RJ, Deutsch R, Heaton RK, et al. Neurocognitive impairment is an independent risk factor for death in HIV infection: San Diego HIV Neurobehavioral Research Center Group. Arch Neurol 1997;54:416–424 [DOI] [PubMed] [Google Scholar]
- 20.Heaton RK, Marcotte TD, Mindt MR, et al. ; HNRC Group The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc 2004;10:317–331 [DOI] [PubMed] [Google Scholar]
- 21.McArthur JC, Hoover DR, Bacellar H, et al. Dementia in AIDS patients: incidence and risk factors: multicenter AIDS cohort study. Neurology 1993;43:2245–2252 [DOI] [PubMed] [Google Scholar]
- 22.Sacktor N, Lyles RH, Skolasky R, et al. ; multicenter AIDS cohort study HIV-associated neurologic disease incidence changes: multicenter AIDS cohort study, 1990–1998. Neurology 2001;56:257–260 [DOI] [PubMed] [Google Scholar]
- 23.Marcotte TD, Deutsch R, McCutchan JA, et al. ; San Diego HIV Neurobehavioral Research Center (HNRC) Group Prediction of incident neurocognitive impairment by plasma HIV RNA and CD4 levels early after HIV seroconversion. Arch Neurol 2003;60:1406–1412 [DOI] [PubMed] [Google Scholar]
- 24.Wallace MR, Nelson JA, McCutchan JA, et al. Symptomatic HIV seroconverting illness is associated with more rapid neurological impairment. Sex Transm Infect 2001;77:199–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cysique LA, Brew BJ. Prevalence of non-confounded HIV-associated neurocognitive impairment in the context of plasma HIV RNA suppression. J Neurovirol 2011;17:176–183 [DOI] [PubMed] [Google Scholar]
- 26.Liner KJ, II, Ro MJ, Robertson KR. HIV, antiretroviral therapies, and the brain. Curr HIV/AIDS Rep 2010;7:85–91 [DOI] [PubMed] [Google Scholar]
- 27.Moore DJ, Letendre SL, Morris S, et al. ; CHARTER Group Neurocognitive functioning in acute or early HIV infection. J Neurovirol 2011;17:50–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jernigan TL, Archibald SL, Fennema-Notestine C, et al. ; for the CHARTER Group Clinical factors related to brain structure in HIV: the CHARTER study. J Neurovirol 2011;17:248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joska JA, Gouse H, Paul RH, Stein DJ, Flisher AJ. Does highly active antiretroviral therapy improve neurocognitive function? A systematic review. J Neurovirol 2010;16:101–114 [DOI] [PubMed] [Google Scholar]
- 30.Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci 2007;8:33–44 [DOI] [PubMed] [Google Scholar]
- 31.Tozzi V, Balestra P, Bellagamba R, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr 2007;45:174–182 [DOI] [PubMed] [Google Scholar]
- 32.Heaton RK, Miller S, Taylor M, Grant I. Revised Comprehensive Norms for an Expanded Halstead-reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults Scoring Program. Lutz: Psychological Assessment Resources; 2004 [Google Scholar]
- 33.Mapou RL, Kay GG, Rundell JR, Temoshok L. Measuring performance decrements in aviation personnel infected with the human immunodeficiency virus. Aviat Space Environ Med 1993;64:158–164 [PubMed] [Google Scholar]


