Abstract
Background
Uncertainty exists about the appropriate use of screening mammography among older women because comorbid illnesses may diminish the benefit of screening. We examined the risk of adverse tumor characteristics and false positive rates according to screening interval, age, and comorbidity.
Methods
From January 1999 to December 2006, data were collected prospectively on 2993 older women with breast cancer and 137 949 older women without breast cancer who underwent mammography at facilities that participated in a data linkage between the Breast Cancer Surveillance Consortium and Medicare claims. Women were aged 66 to 89 years at study entry to allow for measurement of 1 year of preexisting illnesses. We used logistic regression analyses to calculate the odds of advanced (IIb, III, IV) stage, large (>20 millimeters) tumors, and 10-year cumulative probability of false-positive mammography by screening frequency (1 vs 2 years), age, and comorbidity score. The comorbidity score was derived using the Klabunde approximation of the Charlson score. All statistical tests were two-sided.
Results
Adverse tumor characteristics did not differ statistically significantly by comorbidity, age, or interval. Cumulative probability of a false-positive mammography result was higher among annual screeners than biennial screeners irrespective of comorbidity: 48.0% (95% confidence interval [CI] = 46.1% to 49.9%) of annual screeners aged 66 to 74 years had a false-positive result compared with 29.0% (95% CI = 28.1% to 29.9%) of biennial screeners.
Conclusion
Women aged 66 to 89 years who undergo biennial screening mammography have similar risk of advanced-stage disease and lower cumulative risk of a false-positive recommendation than annual screeners, regardless of comorbidity.
The rapid aging of the population has profound implications for an increasing multimorbidity burden that includes cancer (1). In the United States, the majority of women aged 65 years and older (hereafter referred to as “older” women) are regularly offered mammography screening (2–4), and there is little evidence that the presence of comorbidity influences choices about screening frequency (5,6). In fact, studies have found that women with comorbidities have high mammography utilization rates, probably because of their frequent exposure to medical providers (6,7). The benefits of screening mammography among older women remain ambiguous, especially because few screening trials have included women aged more than 74 years (8). In addition, life expectancy among older women is variable and greatly influenced by comorbidity (9).
Current guidelines reflect the uncertainty surrounding screening mammography among older women: the US Preventive Services Task Force breast cancer screening guidelines recommend universal screening for women aged 50 to 74 years (10), whereas the American Cancer Society proposes annual screening for all women aged older than 40 years with no upper age limit (11). Although some observational studies suggest that mammography screening may benefit healthy older women (1,12,13), the benefit may not apply to individuals with severe comorbidity (13). Women who may not benefit should be spared the potential harms associated with screening, including anxiety associated with detection of nonbiologically life-threatening lesions (8).
Few studies have reported on whether comorbidity combined with screening mammography interval influences outcomes. For instance, Mandelblatt et al. (14) examined hypothetical cohorts and showed that benefits of biennial screening in terms of life years saved were important for older women with mild hypertension but were substantially lower for those with heart disease. A recent decision analysis model showed that biennial screening provides a better balance of benefits and harms than annual intervals, with overdiagnosis increasing substantially after age 69 years (15). Our study extends this literature by reporting whether the benefits (detection of early-stage disease) and harms (false-positive mammography or biopsy recommendation) differ by screening interval and comorbidity among older women undergoing screening mammography in community practice.
Methods
Study Setting and Data Sources
Data were obtained from the four Breast Cancer Surveillance Consortium (BCSC) mammography registries (http://breastscreening.cancer.gov) (16) that participated in linkage of BCSC records and Medicare claims data—Carolina Mammography Registry, New Hampshire Mammography Network, San Francisco Mammography Registry, and Vermont Breast Cancer Surveillance System—and from a fifth registry, Group Health Cooperative in western Washington, an integrated health-care system from which claims data were also available for the assessment of comorbidity.
Registries collected data, including patient characteristics and clinical information at each mammogram, from community radiology facilities. Radiologists’ assessments and recommendations were based on the American College of Radiology’s Breast Imaging Reporting and Data System (BI-RADS) (17). Breast cancer diagnoses and tumor characteristics were obtained by linking BCSC data to hospital-based pathology services, regional Surveillance, Epidemiology, and End Results (SEER) programs, and/or state tumor registries. Data were pooled at a central statistical coordinating center. Registries and the coordinating center received institutional review board approval for active or passive consenting processes or a waiver of consent to enroll participants, link data, and perform analysis. All procedures were Health Insurance Portability and Accountability Act compliant, and registries and the coordinating center received a Federal Certificate of Confidentiality and other protections for the identities of women, physicians, and facilities.
Women receiving mammograms between 1999 and 2006 at one of the four registries participating in the Medicare linkage were linked to the Center for Medicare and Medicaid Services’ Medicare Program Master Enrollment file by unique Medicare identifiers such as name, date of birth, and social security number (86.8% match rate). Medicare enrollment information for this period and all Medicare claims data were included in the database. Claims data from 1999 to 2006, including diagnostic and procedure codes for all health services obtained from Group Health Cooperative, were also available for women receiving a mammogram within the Group Health registry.
Participants
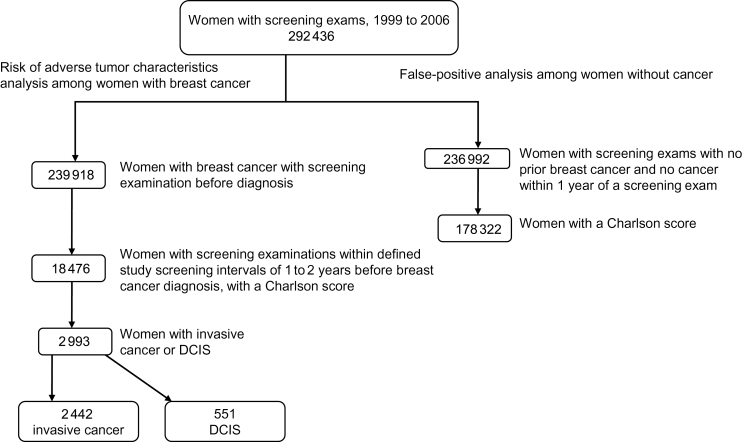
Figure 1 shows a flow chart of the two study populations: women diagnosed with breast cancer and those without breast cancer. Women who received a screening mammogram captured by one of the participating BCSC registries were included if they were aged 66 to 89 years at the time of the mammogram and were continuously enrolled in Medicare parts A and B and not enrolled in Medicare Advantage or were continuously enrolled in Group Health Cooperative for 1 year before the mammogram. We made this restriction to ensure complete capture of administrative claims data to assess comorbidity status. To further ensure complete capture of claims, we excluded mammograms in the BCSC database for which a corresponding Medicare claim or Group Health record for a mammogram could not be found within 7 days before or after the exam date recorded in the BCSC database. To be included in the analysis, women needed to have sufficient data to calculate a Charlson score.
Figure 1.
Flowchart of study population of 292 436 women who had mammograms at five sites from 1999 to 2006 when aged 66 to 89 years. DCIS = ductal carcinoma in situ.
Participants in the Analyses of Tumor Characteristics.
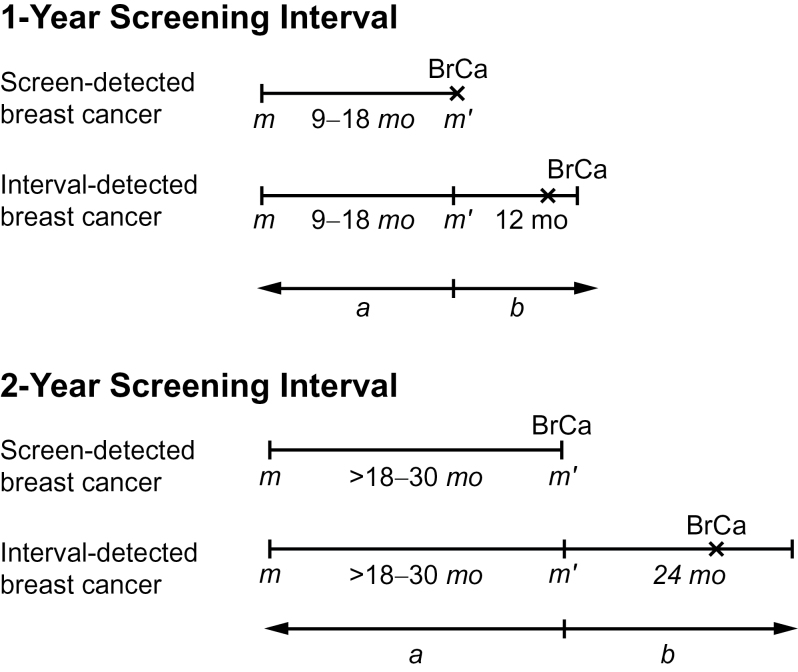
Analyses of breast tumor characteristics included women meeting the above eligibility criteria who were diagnosed with an incident invasive breast cancer or ductal carcinoma in situ, either screen-detected or interval cancer, between 1999 and 2006 and who had at least two screening mammograms before diagnosis. Women were classified based on the time between the two screening examinations as annual (9–18 months) or biennial (>18–30 months) screeners (Figure 2). We restricted analyses to breast cancers diagnosed within a specified follow-up period after a woman’s index examination (prior screening mammography closest to breast cancer diagnosis): within 1 year for annual screeners and 2 years for biennial screeners. To allow adequate follow-up, we included only index examinations that occurred at least 1 year before the end of complete cancer data collection by a woman’s BCSC registry for annual screeners and at least 2 years for biennial screeners (Figure 2).
Figure 2.
Overview of study design. a = screening interval; b = follow-up period for cancer ascertainment; BrCA = breast cancer; m = screening mammogram, m’ = index mammogram.
Participants in the Cumulative Analyses of False-Positive Probabilities.
To assess cumulative false-positive probabilities, we included the first and subsequent screening mammography examinations from 1999 to 2006 on women without a personal history of breast cancer and without a breast cancer diagnosis within 1 year of screening mammography. To ensure an accurate count of mammography examinations, we censored women at their prior screening examination if their self-reported time since last examination differed from that in the BCSC database by more than 6 months.
Measures and Definitions
Comorbidity.
Of comorbidity measures developed to date, the Charlson index has been most extensively studied (18,19). Each condition included in the original Charlson index conferred an independent relative risk of death of 1.2. The comorbid conditions in the original Charlson index were weighted so that those leading to relative risks between 1.2 and less than 1.5 were scored as 1; those leading to relative risks between 1.5 and less than 2.5 were scored as 2; those leading to relative risks between 2.5 and less than 3.5 were scored as 3; and two conditions with a relative risk of 6 or more were scored as 6. The total scores calculated by tallying these weighted scores range from 1 to 6 (0 if comorbidity is absent) and are then collapsible into four summary categories: 0, 1 to 2, 3 to 4, and 5 points. Scores based on the Charlson index were estimated using claims data for the 1-year period before each screening mammogram (18). We used Medicare claims data from the inpatient, outpatient, and physician files and Group Health records for all medical encounters. These different data sources have been shown to be complementary for identifying comorbid conditions (20), and both claims-based and medical record–based comorbidity measures have demonstrated their utility in survival models (21). We used International Classification of Disease, Ninth Revision, Clinical Modification and Healthcare Common Procedure Coding System codes to estimate Charlson scores, using the modification of Klabunde and colleagues (22) to account for possible misclassification using codes from physician claims. Comorbidity scores were calculated using an SAS macro provided by the National Cancer Institute (http://healthservices.cancer.gov/seermedicare/program/comorbidity.html). (Supplementary Table 1, available online, shows the Charlson conditions and their weights).
Demographic Variables.
At each mammogram, patient health surveys included questions on age and race/ethnicity. We used self-reported race/ethnicity to categorize women as non-Hispanic white, non-Hispanic black, Hispanic, Asian/Native Hawaiian/Pacific Islander, Native American/Native Alaskan or other/mixed race. If self-reported race/ethnicity was missing, we used information from cancer registries for the analysis of tumor characteristics.
Anthropometry.
Self-reported data on weight and height were used to calculate body mass index (kg/m2). Body mass index was calculated according to categories of the World Health Organization: underweight (<18.5kg/m2), normal (18.5–24.9kg/m2), overweight (25–29.9kg/m2), obese I (30–34.9kg/m2), obese II/III (≥35).
Breast Cancer Classification.
Breast cancers were classified according to the American Joint Committee on Cancer (AJCC) staging system, sixth edition (23). We defined advanced stage disease as AJCC stages IIB, III, or IV and large tumors as greater than or equal to 20mm. For 4.8% of invasive cancers, stage was classified as early or advanced based on summary stage and other tumor characteristics because of missing AJCC stage.
Mammography Examinations.
Examinations were considered screening based on the indication reported by radiologists. To minimize the risk of misclassifying diagnostic mammography as screening, we excluded mammography examinations that were unilateral or were preceded by a breast-imaging examination within the prior 9 months. “First mammography” refers to examinations in women with no prior mammograms in the BCSC database, no indication of comparison films, and no self-report of a prior mammogram. “Subsequent mammography” refers to examinations that occur after a first screening mammogram.
A false positive recall or biopsy recommendation was defined as a positive screening examination with no invasive carcinoma or ductal carcinoma in situ diagnosis within 1 year or before the next screening examination, whichever occurred first. A screening examination was considered positive for recall if the initial BI-RADS assessment was: 0, needs additional imaging evaluation; 4, suspicious abnormality; 5, highly suggestive of malignancy; or 3, probably benign finding with a recommendation for immediate follow-up. A screening examination was considered positive for biopsy recommendation if the final BI-RADS assessment after all imaging workup and within 90 days after the screening exam (final assessment) was 4 or 5—or was 0 or 3 with a recommendation for biopsy, fine needle aspiration, or surgical consult. Exams were excluded from the biopsy recommendation analysis if the final assessment 90 days after the screening mammography was BI-RADS 0 with a recommendation for additional imaging, nonspecified workup, or missing a recommendation.
Statistical Analysis
We estimated the distribution of risk factors among women with and without breast cancer stratified by annual or biennial screening intervals. Among breast cancer cases, we estimated the proportion with invasive cancer vs ductal carcinoma in situ. Among women with invasive cancer, we estimated distributions of tumor stage, size, and node positivity at diagnosis by interval, comorbidity, and age group (66–74 vs 75–89 years). We used logistic regression to estimate odds ratios and 95% confidence intervals (CIs) of adverse (vs more favorable) tumor stage, tumor size, and nodes associated with biennial vs annual screening by comorbidity and age group. Odds ratios in logistic regression models were adjusted for age in years, race/ethnicity, and BCSC registry.
We estimated the cumulative probability of false-positive results using previously developed methods (24). Women were able to contribute multiple screening mammograms to this analysis, and screening interval was treated as a time-varying covariable calculated at each mammogram based on the elapsed time since the most recent prior screening mammogram. Briefly, we fit logistic regression models for false-positive results at the first mammogram and at each subsequent screening round conditional on screening round number, total number of screening rounds before censoring, screening interval, comorbidity, and BCSC registry. All estimates were stratified by age group (66–74 vs 75–89 years of age) and by the Charlson comorbidity score (≥1 vs 0). We combined estimates of the false-positive risk at each subsequent screening round to obtain woman-level cumulative false-positive probabilities after 10 years of repeat screening. We report fitted values from this model by comorbidity, screening interval, and age and standardized to the BCSC registry distribution using indirect (marginal) standardization.
Analyses of tumor characteristics were performed in SAS software, version 9.2 (SAS Institute, Cary, NC). Analyses of cumulative false-positive probabilities were performed using R 2.10.1 (R Foundation for Statistical Computing, Vienna, Austria). All statistical tests were two-sided.
Results
Characteristics of the Study Population for Analysis of Tumor Characteristics
We included 2993 women with invasive breast cancer or ductal carcinoma in situ who had screening examinations within 1- or 2-year intervals and a Charlson score (Figure 1). The majority were aged 66 to 74 years, white, overweight, and with minimal comorbidity, as reflected by the Charlson score of 0. Specifically, just less than 27% of the breast cancer cases had a Charlson score greater than or equal to 1 (Table 1).
Table 1.
Characteristics of older women (aged 66–89 years) diagnosed with incident breast cancer who underwent screening mammography between 1999 and 2006 in the Breast Cancer Surveillance Consortium
| Characteristic | 1-year interval | 2 year-interval |
|---|---|---|
| breast cancer* | breast cancer† | |
| Total number of women | 1946 | 1047 |
| Median screening interval, months | 13 | 24 |
| Age, years, % | ||
| 66–74 | 57.6 | 53.4 |
| 75–89 | 42.4 | 46.6 |
| Charlson score, % | ||
| 0 | 73.4 | 73.2 |
| 1 | 19.7 | 19.6 |
| 2 | 4.8 | 5.2 |
| ≥3 | 2.1 | 2 |
| Race/ethnicity, %‡ | ||
| White | 83.3 | 84.6 |
| Black | 5.2 | 4.9 |
| Hispanic | 1.4 | 1.7 |
| Asian/Pacific Islander | 2.2 | 3.4 |
| Unknown/other | 7.9 | 5.4 |
| Body mass index, %‡, kg/m2 | ||
| Underweight (<18.5) | 0.6 | 0.9 |
| Normal (18.5–24.9) | 15.8 | 19.8 |
| Overweight (25–29.9) | 14.2 | 18.6 |
| Obese I (30–34.9) | 6.8 | 11.1 |
| Obese II/III (≥35) | 3.9 | 5.3 |
| Missing§ | 58.7 | 44.3 |
| Type of detection, %|| | ||
| Screen-detected | 76.6 | 66.7 |
| Interval cancer | ||
| 0–12 months | 23.4 | 13.8 |
| 13–24 months | 19.4 | |
* Cancer diagnosed within 12 months of screening examination.
† Cancer diagnosed within 24 months of screening examination.
‡ Body mass index is calculated when weight is between 50 and 500 pounds and height is between 48 and 87 inches. Only resulting body mass index values between 15 and 90 are considered valid, and other values outside this range are set to missing.
§ Proportions were computed from among nonmissing values.
|| Screen-detected breast cancer is defined as diagnosis after a positive screening mammography result and before the next screening examination.
Risk of Adverse Tumor Characteristics by Screening Interval
In general, the proportion of invasive tumors associated with less favorable prognostic characteristics (stage IIb or higher, size >20mm, and positive lymph nodes) was higher in the group aged 66 to 74 years than in the group aged 75 to 89 years (Table 2). Even though the proportion with adverse tumor characteristics was generally higher for women aged 66 to 74 years with a Charlson score greater than or equal to 1 within screening intervals (Table 2), there was no statistically significant association between comorbidity and tumor characteristics (Table 3). The proportion with adverse tumor characteristics was similar among annual and biennial screeners (Table 2). We did not observe more adverse tumor characteristics at diagnosis associated with biennial vs annual screening in adjusted models (Table 3).
Table 2.
Unadjusted distribution of tumor characteristics by age, comorbidity, and screening interval*
| Charlson score = 0 | Charlson score ≥ 1 | ||||
|---|---|---|---|---|---|
| Screening interval | Screening interval | ||||
| Age, years | Tumor characteristic | 1 y | 2 y | 1 y | 2 y |
| 66–74 | All cancers (n = 1680), No. | 824 | 403 | 297 | 156 |
| DCIS, % | 18.4 | 19.9 | 22.2 | 23.1 | |
| Invasive cancer, % | 81.6 | 80.1 | 77.8 | 76.9 | |
| Invasive only (n = 1346), No. | 672 | 323 | 231 | 120 | |
| Advanced stage (IIB–IV), % | 12.6 | 10.9 | 15.8 | 11.1 | |
| Large size (>20mm), % | 18.5 | 18.1 | 26.1 | 19.7 | |
| Positive lymph nodes, % | 21.2 | 20.6 | 24.1 | 17.8 | |
| 75–89 | All cancers (n = 1313), No. | 604 | 363 | 221 | 125 |
| DCIS, % | 18.9 | 15.2 | 14.0 | 13.6 | |
| Invasive cancer, % | 81.1 | 84.8 | 86.0 | 86.4 | |
| Invasive only (n = 1096), No. | 490 | 308 | 190 | 108 | |
| Advanced stage (IIB–IV), % | 9.4 | 10.7 | 11.7 | 5.7 | |
| Large size (>20mm), % | 15.7 | 19.0 | 16.9 | 17.6 | |
| Positive lymph nodes, % | 16.3 | 15.7 | 16.6 | 12.4 | |
* DCIS = ductal carcinoma in situ.
Table 3.
Adjusted odds ratios of adverse invasive breast cancer characteristics associated with screening interval by comorbidity and age*
| Charlson score = 0 | Charlson score ≥ 1 | |
|---|---|---|
| 2 vs 1 year | 2 vs 1 year | |
| Age group and tumor characteristics | OR (95% CI) | OR (95% CI) |
| 66–74 years | n = 1227 | n = 453 |
| Invasive (vs. DCIS) | 0.83 (0.59 to 1.17) | 0.92 (0.54 to 1.56) |
| Advanced stage (IIB-IV) | 0.75 (0.46 to 1.22) | 0.99 (0.48 to 2.04) |
| Large size (>20mm) | 0.83 (0.55 to 1.24) | 0.91 (0.50 to 1.65) |
| Positive lymph nodes | 0.84 (0.57 to 1.23) | 0.76 (0.41 to 1.43) |
| 75–89 years | n = 967 | n = 346 |
| Invasive (vs DCIS) | 1.07 (0.71 to 1.60) | 1.02 (0.51 to 2.03) |
| Advanced stage (IIB-IV) | 1.27 (0.72 to 2.25) | 0.37 (0.13 to 1.04) |
| Large size (>20mm) | 1.30 (0.83 to 2.05) | 1.38 (0.70 to 2.73) |
| Positive lymph nodes | 0.83 (0.51 to 1.33) | 0.62 (0.29 to 1.34) |
* Adjusted for the Breast Cancer Surveillance Consortium registry, age and race/ethnicity. Logistic regression analyses were used to estimate odds ratios and 95% confidence intervals of adverse (vs more favorable) tumor stage, tumor size, and nodes associated with biennial vs annual screening by comorbidity and age group. All statistical tests were two-sided. CI = confidence interval; DCIS = ductal carcinoma in situ; OR = odds ratio.
Cumulative Probability of False-Positive Mammography and Biopsy
We included 137 949 women with screening exams without breast cancer (Table 4). Twenty-eight percent of the women in the analysis of the cumulative probability of false-positive mammography and biopsy had one mammogram during the study period (1999–2006), approximately 21% had two mammograms, 17% had three, 13% had four, and approximately 21% had five or more mammograms (Table 4). Cumulative probability of a false positive recall was higher among annual than biennial screeners irrespective of comorbidity (Table 5). When screening women aged 66 to 74 years with comorbidity, the cumulative probability of a woman receiving at least one false-positive recall after 10 years was 48.0% (95% CI = 46.1% to 49.9%) with annual and 29.0% (95% CI = 28.1% to 29.9%) with biennial screening (Table 5). Estimates were similar for women aged 66 to 74 years with no comorbidity. Among women aged 75 to 89 years with comorbidity, estimates were 48.4% (95% CI = 46.1% to 50.8%) with annual and 27.4% (95% CI = 26.5% to 28.4%) with biennial screening. Slightly lower estimates were obtained for women in this age group with no comorbidity.
Table 4.
Population characteristics for older women without breast cancer (aged 66 to 89 years) who underwent screening mammography between 1999 and 2006 in the Breast Cancer Surveillance Consortium (N = 137 949)*
| Characteristics† | % |
|---|---|
| Age, years | |
| 66–74 | 67.9 |
| 75–89 | 32.1 |
| Charlson score | |
| 0 | 75.8 |
| 1 | 18.4 |
| 2 | 4.1 |
| ≥3 | 1.7 |
| Race/ethnicity | |
| White | 79.2 |
| Black | 6.6 |
| Hispanic | 1.7 |
| Asian/Pacific Islander | 3.5 |
| Unknown/Other | 9.0 |
| Body mass index‡, kg/m2 | |
| Underweight (<18.5) | 0.7 |
| Normal (18.5–24.9) | 16.2 |
| Overweight (25–29.9) | 13.7 |
| Obese I (30–34.9) | 6.6 |
| Obese II/III (≥35) | 3.1 |
| Missing | 59.7 |
| Number of mammograms | |
| 1 | 28.0 |
| 2 | 21.3 |
| 3 | 17.1 |
| 4 | 13.0 |
| ≥5 | 20.6 |
* Includes women with annual and biennial screening intervals.
† These characteristics are based on the first exam that a woman contributed to the analysis.
‡ Body mass index is calculated when weight is between 50 and 500 pounds and height is between 48 and 87 inches. Only resulting body mass index values between 15 and 90 are considered valid and other values outside this range are set to missing.
Table 5.
Percentage of false-positive results at first mammography and percentage with at least one false-positive result after 10 years of subsequent mammography by comorbidity and interval among women without breast cancer who underwent screening mammography between 1999 and 2006 (N = 137 949)*
| Result | Age | Screening interval | Charlson score = 0 | Charlson score ≥ 1 |
|---|---|---|---|---|
| % (95% CI) | % (95% CI) | |||
| False-positive recall | 66–74 | |||
| First mammography | 8.6 (8.3 to 8.8) | 8.9 (8.5 to 9.3) | ||
| 1 year | 49.7 (47.8 to 51.5) | 48.0 (46.1 to 49.9) | ||
| 2 year | 30.2 (29.4 to 31.1) | 29.0 (28.1 to 29.9) | ||
| False-positive recall | 75–89 | |||
| First mammography | 8.0 (7.6 to 8.4) | 8.8 (8.2 to 9.4) | ||
| 1 year | 47.2 (44.9 to 49.5) | 48.4 (46.1 to 50.8) | ||
| 2 year | 26.6 (25.7 to 27.5) | 27.4 (26.5 to 28.4) | ||
| False-positive biopsy recommendation | ||||
| 66–74 | ||||
| First mammography | 1.2 (1.1 to 1.3) | 1.7 (1.5 to 1.9) | ||
| 1 year | 9.8 (8.4 to 11.3) | 11.8 (10.1 to 13.8) | ||
| 2 year | 4.6 (4.2 to 5.1) | 5.6 (5.1 to 6.2) | ||
| False-positive biopsy recommendation | 75–89 | |||
| First mammography | 1.2 (1.1 to 1.4) | 1.7 (1.4 to 2.0) | ||
| 1 year | 9.2 (7.5 to 11.2) | 11.3 (9.3 to 13.6) | ||
| 2 year | 4.1 (3.7 to 4.6) | 5.1 (4.5 to 5.7) |
* Adjusted for the Breast Cancer Surveillance Consortium (BCSC) registry. First mammography refers to examinations in women with no prior mammograms in the BCSC database, no indication of comparison films, and no self-report of a prior mammogram. Subsequent mammography refers to examinations that occur after a first screening mammogram. Logistic regression models for false-positive results at the first mammogram and at each subsequent screening round were fit conditional on screening round number, total number of screening rounds before censoring, screening interval, comorbidity, and BCSC registry. All estimates were stratified by age group (66–74 vs 75–89 years of age) and by the Charlson comorbidity score (≥1 vs 0). Estimates of the false-positive risk at each subsequent screening round were combined to obtain woman-level cumulative false-positive probabilities after 10 years of repeat screening. Fitted values are reported from this model by comorbidity, screening interval, and age and standardized to the BCSC registry distribution using indirect (marginal) standardization. All statistical tests were two-sided. CI = confidence interval.
Estimates of the cumulative probability of a woman receiving at least one false-positive biopsy recommendation after 10 years had a similar pattern to that of false-positive recall. In summary, cumulative risk decreased as screening interval increased, and cumulative risk was higher among women with comorbidity (Table 5).
Discussion
We found that biennial screening mammography for women aged 66 to 89 years, irrespective of comorbidity, results in a similar risk of presenting with advanced-stage disease as annual screening mammography. This suggests that annual screening for women without comorbidity, as reflected by a Charlson score of 0, would not lead to a better balance of benefits vs harms. As is the case in younger women, most older women who undergo annual mammography are at high risk of false-positive mammography results and biopsy recommendations without added benefit from more frequent screening.
Our findings are generally consistent with earlier work showing that biennial screening compared with annual screening at age 50 to 74 years retains most benefits but results in fewer harms (15,25,26). Previous work in the BCSC did not find an increase in adverse breast tumor characteristics among women aged 50 to 89 years with breast cancer with a 2-year vs a 1-year screening mammography interval (26). A meta-analysis of the association between screening interval and mortality compared eight randomized controlled trials in relation to the length of the screening interval among the trials (rather than within the trials) (25); mortality reductions for screening every 18 to 33 months were similar to reductions for annual screening for women aged 40 to 49 and 50 to 74 years. However, the trials included in the meta-analysis did not enroll women aged more than 74 years.
Our analyses indicated no association between comorbidity, screening interval, and tumor stage at diagnosis. These results are in contrast with an earlier population-based analysis of SEER–Medicare data, which indicated that stable comorbidities—a different measure of comorbidity to the one used in this study—and ambulatory visits were independently and inversely associated with a risk of advanced-stage disease whereas unstable comorbidities were associated with an increased risk of advanced stage, after adjusting for mammography use (6). Fleming et al. (27) found that different comorbidities were differentially associated with localized vs advanced stage of breast cancer at diagnosis. For example, women with diabetes were 19% more likely to be diagnosed at advanced stage, whereas women with cardiovascular disease had 13% lower odds of being diagnosed at advanced stage (27). Moreover, women with two or more limiting conditions, such as arthritis, were found to have 50% lower odds of advanced disease (28). One of the key differences between our study and these earlier reports pertains to the measurement of comorbidity. We used the Charlson index, which has been widely applied in cancer research, and did not examine the effect of individual comorbidities. Efforts to investigate this question further will require a better understanding of the extent to which comorbidity serves as a marker for health-care contact, health or lifestyle behaviors, or care-seeking style (29) and the extent to which specific comorbid conditions increase risk of more (or less) aggressive disease via biological pathways. If conditions such as diabetes increase the risk of more aggressive tumors, they may also increase risk of death from non–breast cancer causes, so screening at all or screening frequency may not affect breast cancer–specific mortality.
Our estimates of the probability of receiving at least one false-positive recall or biopsy recommendation after 10 years of annual screening are similar to those recently reported for women aged 40 to 59 years (24) and higher than in some earlier reports (30,31). Unlike these previous studies, our analysis focused specifically on older women and, for the first time, examined the extent to which false-positive rates vary by comorbidity. The lack of screening interval–related difference in adverse tumor characteristics in our study should be considered in the context of the large increase in false-positive examinations and biopsy recommendations, particularly among older women with comorbidities screened annually. We estimate that there are 4.9 million US women aged 66 to 89 years in the population with comorbidities and 14.3 million women without comorbidities (32). If these women undergo annual instead of biennial mammography, this could result in approximately one million additional false-positive examinations and 0.29 million additional false-positive biopsy recommendations among women with comorbidity plus 2.86 million additional false-positive examinations and 0.86 million additional false-positive biopsy recommendations among women without comorbidity. Thus, if older women undergo annual screening without consideration of the presence of comorbidity, it could result in substantial morbidity from screening mammography (32).
The prevalence of multiple comorbidities among older women reported here was comparable with results from earlier studies (6,33) but somewhat lower than results previously reported by Fleming et al. (27). This difference is probably a result of our use of Medicare claims data from the inpatient, outpatient, and physician files and Group Health medical records associated with International Classification of Disease, Ninth Revision, Clinical Modification diagnosis codes. We used these codes to estimate Charlson comorbidity scores with the modification of the algorithm developed by Klabunde et al. (22) to account for possible misclassification using codes from physician claims, which captured only clinician-assigned codes related to comorbid conditions.
One of the limitations of this study concerns potential underreporting of chronic conditions, which is a well-recognized limitation of administrative data. Despite the large sample, our data are also limited by a relatively small number of breast cancer cases. Our definition of screening interval is based on the time since the woman’s previous screening mammogram and does not necessarily represent long-term patterns for individuals. Still, our study design, in which the follow-up time for identifying cases corresponds with the screening interval, reduces length bias (34). Our analysis of tumor characteristics includes only women with multiple screening mammograms. Because we did not examine women who screened less frequently, who had screened only once, or who had never screened, we cannot address outcome variations related to infrequent or lack of screening. Although reduction in breast cancer deaths would be preferred to assessing tumor characteristics at diagnosis as a measure of benefit, advanced-stage disease strongly predicts breast cancer mortality (35). Comorbidity is clearly related to life expectancy and probability of death from non–breast cancer causes (14,36) and could affect the decision of whether to screen older women. Because there is heterogeneity in life expectancy by both chronological and physiological age (36), it will be important to consider these factors to inform future recommendations about upper age limits for screening cessation.
The strengths of our study include its representative sample of women receiving screening mammography in community practice, large sample size, geographic and racial/ethnic diversity, and use of the validated claims-based methods to estimate comorbidity scores. These data represent the largest available screening mammography dataset in the United States linked to both cancer diagnosis from tumor registries and to administrative data from Medicare claims to identify comorbid diagnoses. Finally, our study focuses on an important gap in screening research because accountable care organizations and care quality indicators do not address outcomes of screening mammography in women of advanced age or with significant comorbidities.
In conclusion, this large, population-based study of women aged 66 to 89 years indicates that undergoing biennial screening mammography is associated with similar rates of advanced-stage disease and lower cumulative rates of a false-positive recall and biopsy recommendation as annual screening, regardless of comorbidity. Because a randomized controlled trial of mammography in older women is unlikely, more high-quality observational research examining additional measures of comorbidity and breast cancer mortality may facilitate improved understanding of the benefits and harms of different screening mammography frequencies among older women and, ultimately, inform clinical and policy decisions about the appropriate use of screening in this growing population.
Funding
This work was supported by grants from the National Cancer Institute–funded Breast Cancer Surveillance Consortium (U01CA63740, U01CA86076, U01CA86082, U01CA63736, U01CA70013, U01CA69976, U01CA63731, U01CA70040, HHSN261201100031C) and the National Cancer Institute (R03CA150007 and RC2CA148577). DB was in part funded by the Mentored Research Scholar Award (121891-MRSG-12-007-01-CPHPS) from the American Cancer Society. KJW was in part funded by Agency for Healthcare Quality and Research Comparative Effectiveness Career Development Award (K12 HS019482). JSM was funded by grants from the National Cancer Institute (U01CA088283, U01CA152958, and KO5CA96940). The collection of cancer data used in this study was supported in part by several state public health departments and cancer registries throughout the United States. For a full description of these sources, please see: http://breastscreening.cancer.gov/work/acknowledgement.html. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Supplementary Material
We thank the participating women, mammography facilities, and radiologists for the data they have provided for this study. We are grateful to Robert A. Hiatt for helpful comments on the original draft. A list of BCSC investigators and procedures for requesting BCSC data for research purposes are at http://breast screening.cancer.gov/.
References
- 1. Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001; 285(7):885–892 [DOI] [PubMed] [Google Scholar]
- 2. Schonberg MA, Silliman RA, Marcantonio ER. Weighing the benefits and burdens of mammography screening among women age 80 years or older. J Clin Oncol. 2009; 27(11):1774–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bellizzi KM, Breslau ES, Burness A, Waldron W. Prevalence of cancer screening in older, racially diverse adults: still screening after all these years. Arch Intern Med. 2011; 171(22):2031–2037 [DOI] [PubMed] [Google Scholar]
- 4. Schonberg MA, McCarthy EP, Davis RB, Phillips RS, Hamel MB. Breast cancer screening in women aged 80 and older: results from a national survey. J Am Geriatr Soc. 2004; 52(10):1688–1695 [DOI] [PubMed] [Google Scholar]
- 5. Heflin MT, Oddone EZ, Pieper CF, Burchett BM, Cohen HJ. The effect of comorbid illness on receipt of cancer screening by older people. J Am Geriatr Soc. 2002; 50(10):1651–1658 [DOI] [PubMed] [Google Scholar]
- 6. Yasmeen S, Xing G, Morris C, Chlebowski RT, Romano PS. Comorbidities and mammography use interact to explain racial/ethnic disparities in breast cancer stage at diagnosis. Cancer. 2011; 117(14):3252–3261 [DOI] [PubMed] [Google Scholar]
- 7. Smith-Bindman R, Quale C, Chu PW, Rosenberg R, Kerlikowske K. Can Medicare billing claims data be used to assess mammography utilization among women ages 65 and older?. Med Care. 2006; 44(5):463–470 [DOI] [PubMed] [Google Scholar]
- 8. Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001; 285(21):2750–2756 [DOI] [PubMed] [Google Scholar]
- 9. Hillner BE, Mandelblatt J. Caring for older women with breast cancer: can observational research fill the clinical trial gap?. J Natl Cancer Inst. 2006; 98(10):660–661 [DOI] [PubMed] [Google Scholar]
- 10. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009; 151(10):716–726 [DOI] [PubMed] [Google Scholar]
- 11. Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2010; 60(2):99–119 [DOI] [PubMed] [Google Scholar]
- 12. Badgwell BD, Giordano SH, Duan ZZ, et al. Mammography before diagnosis among women age 80 years and older with breast cancer. . J Clin Oncol. 2008; 26(15):2482–2488 [DOI] [PubMed] [Google Scholar]
- 13. McPherson CP, Swenson KK, Lee MW. The effects of mammographic detection and comorbidity on the survival of older women with breast cancer. J Am Geriatr Soc. 2002; 50(6):1061–1068 [DOI] [PubMed] [Google Scholar]
- 14. Mandelblatt JS, Wheat ME, Monane M, Moshief RD, Hollenberg JP, Tang J. Breast cancer screening for elderly women with and without comorbid conditions. A decision analysis model. Ann Intern Med. 1992; 116(9):722–730 [DOI] [PubMed] [Google Scholar]
- 15. Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009; 151(10):738–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ballard-Barbash R, Taplin SH, Yankaskas BC, et al. Breast Cancer Surveillance Consortium: a national mammography screening and outcomes database. AJR Am J Roentgenol. 1997; 169(4):1001–1008 [DOI] [PubMed] [Google Scholar]
- 17. Land LH, Dalton SO, Jensen MB, Ewertz M. Impact of comorbidity on mortality: a cohort study of 62,591 Danish women diagnosed with early breast cancer. 1990–2008 Breast Cancer Res Treat. 2012; 131(3): 1013–1020 [DOI] [PubMed] [Google Scholar]
- 18. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. . J Chronic Dis. 1987; 40(5):373–383 [DOI] [PubMed] [Google Scholar]
- 19. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994; 47(11):1245–1251 [DOI] [PubMed] [Google Scholar]
- 20. Klabunde CN, Harlan LC, Warren JL. Data sources for measuring comorbidity: a comparison of hospital records and medicare claims for cancer patients. Med Care. 2006; 44(10):921–928 [DOI] [PubMed] [Google Scholar]
- 21. Newschaffer CJ, Bush TL, Penberthy LT. Comorbidity measurement in elderly female breast cancer patients with administrative and medical records data. J Clin Epidemiol. 1997; 50(6):725–733 [DOI] [PubMed] [Google Scholar]
- 22. Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000; 53(12):1258–1267 [DOI] [PubMed] [Google Scholar]
- 23. American Joint Committee on Cancer Manual for Staging of Cancer. 6th ed Philadelphia: JB Lippincott; 2002. [Google Scholar]
- 24. Hubbard RA, Miglioretti DL, Smith RA. Modelling the cumulative risk of a false-positive screening test. Stat Methods Med Res. 2010; 19(5):429–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kerlikowske K, Grady D, Rubin SM, Sandrock C, Ernster VL. Efficacy of screening mammography. A meta-analysis. JAMA. 1995; 273(2):149–154 [PubMed] [Google Scholar]
- 26. White E, Miglioretti DL, Yankaskas BC, et al. Biennial versus annual mammography and the risk of late-stage breast cancer. J Natl Cancer Inst. 2004; 96(24):1832–1839 [DOI] [PubMed] [Google Scholar]
- 27. Fleming ST, Pursley HG, Newman B, Pavlov D, Chen K. Comorbidity as a predictor of stage of illness for patients with breast cancer. Med Care. 2005; 43(2):132–140 [DOI] [PubMed] [Google Scholar]
- 28. Vaeth PA, Satariano WA, Ragland DR. Limiting comorbid conditions and breast cancer stage at diagnosis. J Gerontol A Biol Sci Med Sci. 2000; 55(10):M593–600 [DOI] [PubMed] [Google Scholar]
- 29. Mandelblatt JS, Gold K, O’Malley AS, et al. Breast and cervix cancer screening among multiethnic women: role of age, health, and source of care. Prev Med. 1999; 28(4):418–425 [DOI] [PubMed] [Google Scholar]
- 30. Elmore JG, Barton MB, Moceri VM, Polk S, Arena PJ, Fletcher SW. Ten-year risk of false positive screening mammograms and clinical breast examinations. N Engl J Med. 1998; 338(16):1089–1096 [DOI] [PubMed] [Google Scholar]
- 31. Xu JL, Fagerstrom RM, Prorok PC, Kramer BS. Estimating the cumulative risk of a false-positive test in a repeated screening program. Biometrics. 2004; 60(3):651–660 [DOI] [PubMed] [Google Scholar]
- 32. US Census Bureau Current Population Reports, P25-1095. Table US-EST90INT-04 - Intercensal Estimates of the United States Resident Population by Age Groups and Sex. , 1990–2000: Selected Months, September 2002. http://www.census .gov/popest/archives/EST90INTERCENSAL/US-EST90INT-04.html; and 2010 Census Redistricting Data (P.L. 94-171) Summary File, http://www.census.gov/rdo/data/2010_census_redistricting_data_pl_94-171_summary_files.html [Google Scholar]
- 33. Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007; 17(8):584–590 [DOI] [PubMed] [Google Scholar]
- 34. Zelen M, Feinleib M. On the theory of screening for chronic diseases. Biometrika. 1969; 56(3):601–614 [Google Scholar]
- 35. Autier P, Hery C, Haukka J, Boniol M, Byrnes G. Advanced breast cancer and breast cancer mortality in randomized controlled trials on mammography screening. J Clin Oncol. 2009; 27(35):5919–23 [DOI] [PubMed] [Google Scholar]
- 36. Mandelblatt JS, Schechter CB, Yabroff KR, et al. Toward optimal screening strategies for older women. Costs, benefits, and harms of breast cancer screening by age, biology, and health status. J Gen Intern Med. 2005; 20(6):487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.