Abstract
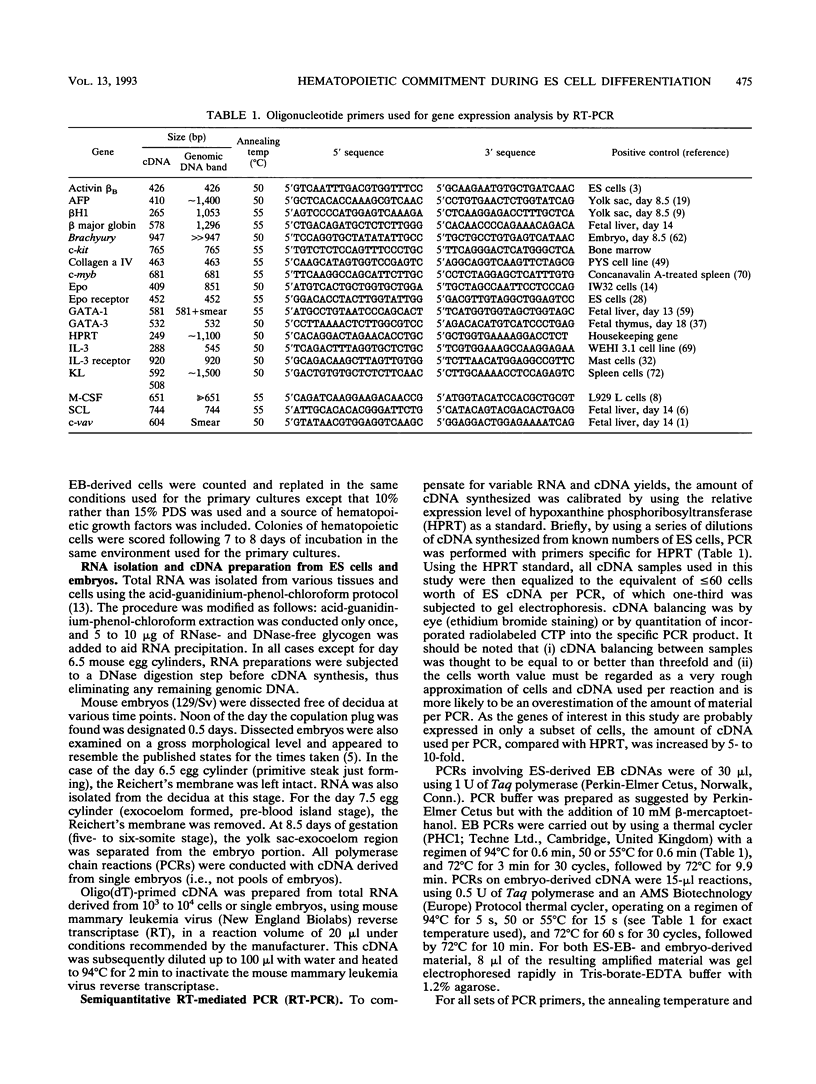
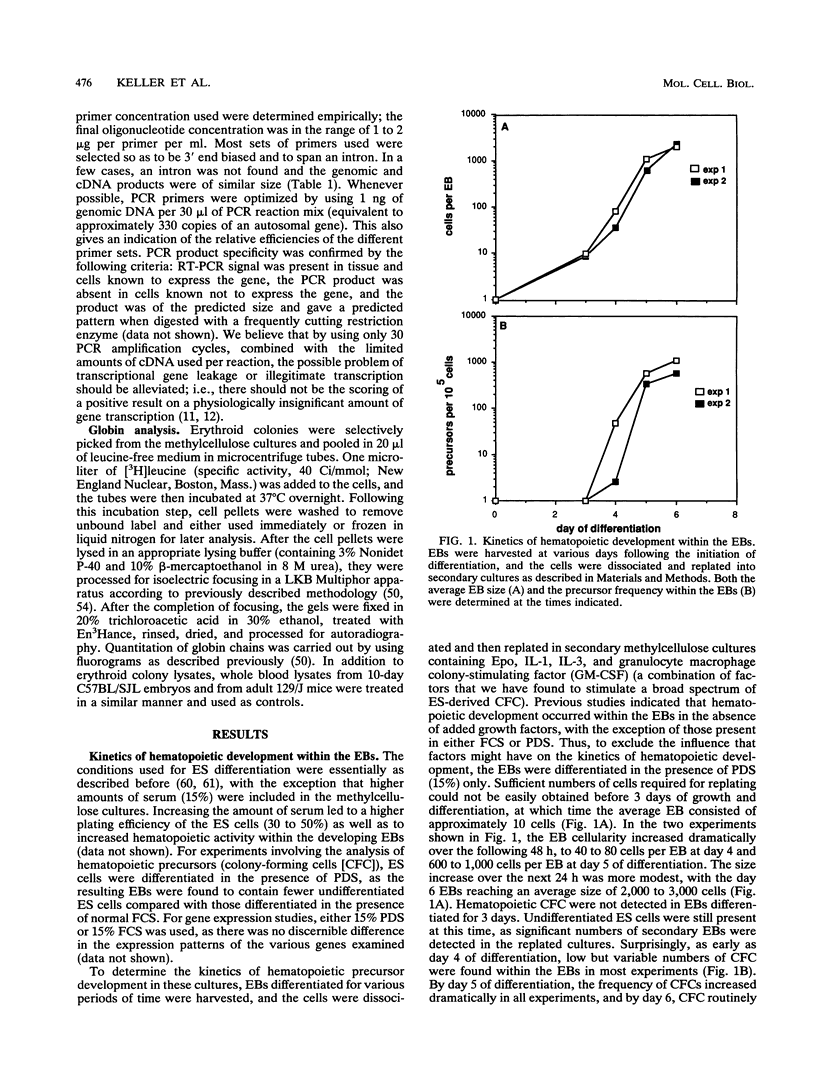
We report that embryonic stem cells efficiently undergo differentiation in vitro to mesoderm and hematopoietic cells and that this in vitro system recapitulates days 6.5 to 7.5 of mouse hematopoietic development. Embryonic stem cells differentiated as embryoid bodies (EBs) develop erythroid precursors by day 4 of differentiation, and by day 6, more than 85% of EBs contain such cells. A comparative reverse transcriptase-mediated polymerase chain reaction profile of marker genes for primitive endoderm (collagen alpha IV) and mesoderm (Brachyury) indicates that both cell types are present in the developing EBs as well in normal embryos prior to the onset of hematopoiesis. GATA-1, GATA-3, and vav are expressed in both the EBs and embryos just prior to and/or during the early onset of hematopoiesis, indicating that they could play a role in the early stages of hematopoietic development both in vivo and in vitro. The initial stages of hematopoietic development within the EBs occur in the absence of added growth factors and are not significantly influenced by the addition of a broad spectrum of factors, including interleukin-3 (IL-3), IL-1, IL-6, IL-11, erythropoietin, and Kit ligand. At days 10 and 14 of differentiation, EB hematopoiesis is significantly enhanced by the addition of both Kit ligand and IL-11 to the cultures. Kinetic analysis indicates that hematopoietic precursors develop within the EBs in an ordered pattern. Precursors of the primitive erythroid lineage appear first, approximately 24 h before precursors of the macrophage and definitive erythroid lineages. Bipotential neutrophil/macrophage and multilineage precursors appear next, and precursors of the mast cell lineage develop last. The kinetics of precursor development, as well as the growth factor responsiveness of these early cells, is similar to that found in the yolk sac and early fetal liver, indicating that the onset of hematopoiesis within the EBs parallels that found in the embryo.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Houston H., Allen J., Lints T., Harvey R. The hematopoietically expressed vav proto-oncogene shares homology with the dbl GDP-GTP exchange factor, the bcr gene and a yeast gene (CDC24) involved in cytoskeletal organization. Oncogene. 1992 Apr;7(4):611–618. [PubMed] [Google Scholar]
- Adamson E. D., Ayers S. E. The localization and synthesis of some collagen types in developing mouse embryos. Cell. 1979 Apr;16(4):953–965. doi: 10.1016/0092-8674(79)90110-7. [DOI] [PubMed] [Google Scholar]
- Azoulay M., Webb C. G., Sachs L. Control of hematopoietic cell growth regulators during mouse fetal development. Mol Cell Biol. 1987 Sep;7(9):3361–3364. doi: 10.1128/mcb.7.9.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley C. G., Aplan P. D., Denning S. M., Haynes B. F., Waldmann T. A., Kirsch I. R. The gene SCL is expressed during early hematopoiesis and encodes a differentiation-related DNA-binding motif. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10128–10132. doi: 10.1073/pnas.86.24.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley C. G., Visvader J., Green A. R., Aplan P. D., Metcalf D., Kirsch I. R., Gough N. M. Molecular cloning and chromosomal localization of the murine homolog of the human helix-loop-helix gene SCL. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):869–873. doi: 10.1073/pnas.88.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltz-Nitulescu G., Wiltschke C., Holzinger C., Fellinger A., Scheiner O., Gessl A., Förster O. Differentiation of rat bone marrow cells into macrophages under the influence of mouse L929 cell supernatant. J Leukoc Biol. 1987 Jan;41(1):83–91. doi: 10.1002/jlb.41.1.83. [DOI] [PubMed] [Google Scholar]
- Brotherton T. W., Chui D. H., Gauldie J., Patterson M. Hemoglobin ontogeny during normal mouse fetal development. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2853–2857. doi: 10.1073/pnas.76.6.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkert U., von Rüden T., Wagner E. F. Early fetal hematopoietic development from in vitro differentiated embryonic stem cells. New Biol. 1991 Jul;3(7):698–708. [PubMed] [Google Scholar]
- Chelly J., Concordet J. P., Kaplan J. C., Kahn A. Illegitimate transcription: transcription of any gene in any cell type. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2617–2621. doi: 10.1073/pnas.86.8.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelly J., Kaplan J. C., Maire P., Gautron S., Kahn A. Transcription of the dystrophin gene in human muscle and non-muscle tissue. Nature. 1988 Jun 30;333(6176):858–860. doi: 10.1038/333858a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Choppin J., Casadevall N., Lacombe C., Wendling F., Goldwasser E., Berger R., Tambourin P., Varet B. Production of erythropoietin by cloned malignant murine erythroid cells. Exp Hematol. 1985 Aug;13(7):610–615. [PubMed] [Google Scholar]
- Cline M. J., Moore M. A. Embryonic origin of the mouse macrophage. Blood. 1972 Jun;39(6):842–849. [PubMed] [Google Scholar]
- Coppola J., Bryant S., Koda T., Conway D., Barbacid M. Mechanism of activation of the vav protooncogene. Cell Growth Differ. 1991 Feb;2(2):95–105. [PubMed] [Google Scholar]
- Doetschman T. C., Eistetter H., Katz M., Schmidt W., Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985 Jun;87:27–45. [PubMed] [Google Scholar]
- Dyson P. J., Poirier F., Watson R. J. Expression of c-myb in embryonal carcinoma cells and embryonal stem cells. Differentiation. 1989 Oct;42(1):24–27. doi: 10.1111/j.1432-0436.1989.tb00603.x. [DOI] [PubMed] [Google Scholar]
- Dziadek M. A., Andrews G. K. Tissue specificity of alpha-fetoprotein messenger RNA expression during mouse embryogenesis. EMBO J. 1983;2(4):549–554. doi: 10.1002/j.1460-2075.1983.tb01461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. G., Chan D. C., Leder P. Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell. 1991 Mar 8;64(5):1025–1035. doi: 10.1016/0092-8674(91)90326-t. [DOI] [PubMed] [Google Scholar]
- Froesch E. R., Schmid C., Schwander J., Zapf J. Actions of insulin-like growth factors. Annu Rev Physiol. 1985;47:443–467. doi: 10.1146/annurev.ph.47.030185.002303. [DOI] [PubMed] [Google Scholar]
- Gabbianelli M., Sargiacomo M., Pelosi E., Testa U., Isacchi G., Peschle C. "Pure" human hematopoietic progenitors: permissive action of basic fibroblast growth factor. Science. 1990 Sep 28;249(4976):1561–1564. doi: 10.1126/science.2218497. [DOI] [PubMed] [Google Scholar]
- Gorman D. M., Itoh N., Kitamura T., Schreurs J., Yonehara S., Yahara I., Arai K., Miyajima A. Cloning and expression of a gene encoding an interleukin 3 receptor-like protein: identification of another member of the cytokine receptor gene family. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5459–5463. doi: 10.1073/pnas.87.14.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haar J. L., Ackerman G. A. Ultrastructural changes in mouse yolk sac associated with the initiation of vitelline circulation. Anat Rec. 1971 Aug;170(4):437–455. doi: 10.1002/ar.1091700406. [DOI] [PubMed] [Google Scholar]
- Hara T., Miyajima A. Two distinct functional high affinity receptors for mouse interleukin-3 (IL-3). EMBO J. 1992 May;11(5):1875–1884. doi: 10.1002/j.1460-2075.1992.tb05239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein C., Fischer K. D., Stoffel M., Nowock J., Ford A., Tessmer U., Stocking C. The gene for erythropoietin receptor is expressed in multipotential hematopoietic and embryonal stem cells: evidence for differentiation stage-specific regulation. Mol Cell Biol. 1992 Apr;12(4):1815–1826. doi: 10.1128/mcb.12.4.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann B. G., Labeit S., Poustka A., King T. R., Lehrach H. Cloning of the T gene required in mesoderm formation in the mouse. Nature. 1990 Feb 15;343(6259):617–622. doi: 10.1038/343617a0. [DOI] [PubMed] [Google Scholar]
- Ho I. C., Vorhees P., Marin N., Oakley B. K., Tsai S. F., Orkin S. H., Leiden J. M. Human GATA-3: a lineage-restricted transcription factor that regulates the expression of the T cell receptor alpha gene. EMBO J. 1991 May;10(5):1187–1192. doi: 10.1002/j.1460-2075.1991.tb08059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B. L., Taylor A., Adamson E. Cell interactions modulate embryonal carcinoma cell differentiation into parietal or visceral endoderm. Nature. 1981 May 21;291(5812):235–237. doi: 10.1038/291235a0. [DOI] [PubMed] [Google Scholar]
- Ikuta K., Weissman I. L. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1502–1506. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Schreurs J., Gorman D. M., Maruyama K., Ishii A., Yahara I., Arai K., Miyajima A. Cloning of an interleukin-3 receptor gene: a member of a distinct receptor gene family. Science. 1990 Jan 19;247(4940):324–327. doi: 10.1126/science.2404337. [DOI] [PubMed] [Google Scholar]
- Johnson G. R., Barker D. C. Erythroid progenitor cells and stimulating factors during murine embryonic and fetal development. Exp Hematol. 1985 Mar;13(3):200–208. [PubMed] [Google Scholar]
- Johnson G. R., Metcalf D. Sources and nature of granulocyte-macrophage colony stimulating factor in fetal mice. Exp Hematol. 1978 Mar;6(3):327–335. [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Katzav S., Martin-Zanca D., Barbacid M. vav, a novel human oncogene derived from a locus ubiquitously expressed in hematopoietic cells. EMBO J. 1989 Aug;8(8):2283–2290. doi: 10.1002/j.1460-2075.1989.tb08354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labastie M. C., Thiery J. P., Le Douarin N. M. Mouse yolk sac and intraembryonic tissues produce factors able to elicit differentiation of erythroid burst-forming units and colony-forming units, respectively. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1453–1456. doi: 10.1073/pnas.81.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbaum M. H., Grosveld F. An in vitro globin gene switching model based on differentiated embryonic stem cells. Genes Dev. 1990 Dec;4(12A):2075–2085. doi: 10.1101/gad.4.12a.2075. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Eisenman R. N. New light on Myc and Myb. Part II. Myb. Genes Dev. 1990 Dec;4(12B):2235–2241. doi: 10.1101/gad.4.12b.2235. [DOI] [PubMed] [Google Scholar]
- Matsui Y., Zsebo K. M., Hogan B. L. Embryonic expression of a haematopoietic growth factor encoded by the Sl locus and the ligand for c-kit. Nature. 1990 Oct 18;347(6294):667–669. doi: 10.1038/347667a0. [DOI] [PubMed] [Google Scholar]
- Miura Y., Wilt F. H. Tissue interaction and the formation of the first erythroblasts of the chick embryo. Dev Biol. 1969 Feb;19(2):201–211. doi: 10.1016/0012-1606(69)90055-4. [DOI] [PubMed] [Google Scholar]
- Moore M. A., Metcalf D. Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol. 1970 Mar;18(3):279–296. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- Mortensen R. M., Conner D. A., Chao S., Geisterfer-Lowrance A. A., Seidman J. G. Production of homozygous mutant ES cells with a single targeting construct. Mol Cell Biol. 1992 May;12(5):2391–2395. doi: 10.1128/mcb.12.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucenski M. L., McLain K., Kier A. B., Swerdlow S. H., Schreiner C. M., Miller T. A., Pietryga D. W., Scott W. J., Jr, Potter S. S. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991 May 17;65(4):677–689. doi: 10.1016/0092-8674(91)90099-k. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Vaessin H., Caudy M., Jan L. Y., Jan Y. N., Cabrera C. V., Buskin J. N., Hauschka S. D., Lassar A. B. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989 Aug 11;58(3):537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Nicola N. A. Hemopoietic cell growth factors and their receptors. Annu Rev Biochem. 1989;58:45–77. doi: 10.1146/annurev.bi.58.070189.000401. [DOI] [PubMed] [Google Scholar]
- Nocka K., Majumder S., Chabot B., Ray P., Cervone M., Bernstein A., Besmer P. Expression of c-kit gene products in known cellular targets of W mutations in normal and W mutant mice--evidence for an impaired c-kit kinase in mutant mice. Genes Dev. 1989 Jun;3(6):816–826. doi: 10.1101/gad.3.6.816. [DOI] [PubMed] [Google Scholar]
- Oohira A., Wight T. N., McPherson J., Bornstein P. Biochemical and ultrastructural studies of proteoheparan sulfates synthesized by PYS-2, a basement membrane-producing cell line. J Cell Biol. 1982 Feb;92(2):357–367. doi: 10.1083/jcb.92.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Kurachi S., Brice M., Nakamoto B., Stamatoyannopoulos G. Asynchronous synthesis of HbF and HbA during erythroblast maturation. II. Studies of G gamma, A gamma, and beta chain synthesis in individual erythroid clones from neonatal and adult BFU-E cultures. Blood. 1981 Mar;57(3):531–536. [PubMed] [Google Scholar]
- Paul S. R., Bennett F., Calvetti J. A., Kelleher K., Wood C. R., O'Hara R. M., Jr, Leary A. C., Sibley B., Clark S. C., Williams D. A. Molecular cloning of a cDNA encoding interleukin 11, a stromal cell-derived lymphopoietic and hematopoietic cytokine. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7512–7516. doi: 10.1073/pnas.87.19.7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevny L., Simon M. C., Robertson E., Klein W. H., Tsai S. F., D'Agati V., Orkin S. H., Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991 Jan 17;349(6306):257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- Quesniaux V. F., Clark S. C., Turner K., Fagg B. Interleukin-11 stimulates multiple phases of erythropoiesis in vitro. Blood. 1992 Sep 1;80(5):1218–1223. [PubMed] [Google Scholar]
- Righetti P. G., Gianazza E., Gianni A. M., Comi P., Giglioni B., Ottolenghi S., Secchi C., Rossi-Bernardi L. Human globin chain separation by isoelectric focusing. J Biochem Biophys Methods. 1979;1(1):45–57. doi: 10.1016/0165-022x(79)90045-9. [DOI] [PubMed] [Google Scholar]
- Robertson E., Bradley A., Kuehn M., Evans M. Germ-line transmission of genes introduced into cultured pluripotential cells by retroviral vector. Nature. 1986 Oct 2;323(6087):445–448. doi: 10.1038/323445a0. [DOI] [PubMed] [Google Scholar]
- Schmitt R. M., Bruyns E., Snodgrass H. R. Hematopoietic development of embryonic stem cells in vitro: cytokine and receptor gene expression. Genes Dev. 1991 May;5(5):728–740. doi: 10.1101/gad.5.5.728. [DOI] [PubMed] [Google Scholar]
- Simon M. C., Pevny L., Wiles M. V., Keller G., Costantini F., Orkin S. H. Rescue of erythroid development in gene targeted GATA-1- mouse embryonic stem cells. Nat Genet. 1992 May;1(2):92–98. doi: 10.1038/ng0592-92. [DOI] [PubMed] [Google Scholar]
- Smith A. G., Heath J. K., Donaldson D. D., Wong G. G., Moreau J., Stahl M., Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988 Dec 15;336(6200):688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- WILT F. H. ERYTHROPOIESIS IN THE CHICK EMBRYO: THE ROLE OF ENDODERM. Science. 1965 Mar 26;147(3665):1588–1590. doi: 10.1126/science.147.3665.1588. [DOI] [PubMed] [Google Scholar]
- Whitelaw E., Tsai S. F., Hogben P., Orkin S. H. Regulated expression of globin chains and the erythroid transcription factor GATA-1 during erythropoiesis in the developing mouse. Mol Cell Biol. 1990 Dec;10(12):6596–6606. doi: 10.1128/mcb.10.12.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiles M. V., Keller G. Multiple hematopoietic lineages develop from embryonic stem (ES) cells in culture. Development. 1991 Feb;111(2):259–267. doi: 10.1242/dev.111.2.259. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., Herrmann B. G. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature. 1990 Feb 15;343(6259):657–659. doi: 10.1038/343657a0. [DOI] [PubMed] [Google Scholar]
- Williams R. L., Hilton D. J., Pease S., Willson T. A., Stewart C. L., Gearing D. P., Wagner E. F., Metcalf D., Nicola N. A., Gough N. M. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988 Dec 15;336(6200):684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- Witte O. N. Steel locus defines new multipotent growth factor. Cell. 1990 Oct 5;63(1):5–6. doi: 10.1016/0092-8674(90)90280-r. [DOI] [PubMed] [Google Scholar]
- Wong P. M., Chung S. W., Chui D. H., Eaves C. J. Properties of the earliest clonogenic hemopoietic precursors to appear in the developing murine yolk sac. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3851–3854. doi: 10.1073/pnas.83.11.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. M., Chung S. W., Eaves C. J., Chui D. H. Ontogeny of the mouse hemopoietic system. Prog Clin Biol Res. 1985;193:17–28. [PubMed] [Google Scholar]
- Wong P. M., Chung S. W., Reicheld S. M., Chui D. H. Hemoglobin switching during murine embryonic development: evidence for two populations of embryonic erythropoietic progenitor cells. Blood. 1986 Mar;67(3):716–721. [PubMed] [Google Scholar]
- Ymer S., Tucker W. Q., Sanderson C. J., Hapel A. J., Campbell H. D., Young I. G. Constitutive synthesis of interleukin-3 by leukaemia cell line WEHI-3B is due to retroviral insertion near the gene. Nature. 1985 Sep 19;317(6034):255–258. doi: 10.1038/317255a0. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Wang Z., Lee A., Huang D. P., Chiu J. F. Differential expression of cellular oncogenes during rat liver development. Cancer Lett. 1988 Aug 15;41(2):147–155. doi: 10.1016/0304-3835(88)90111-5. [DOI] [PubMed] [Google Scholar]
- Zon L. I., Mather C., Burgess S., Bolce M. E., Harland R. M., Orkin S. H. Expression of GATA-binding proteins during embryonic development in Xenopus laevis. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10642–10646. doi: 10.1073/pnas.88.23.10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsebo K. M., Williams D. A., Geissler E. N., Broudy V. C., Martin F. H., Atkins H. L., Hsu R. Y., Birkett N. C., Okino K. H., Murdock D. C. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990 Oct 5;63(1):213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]