Abstract
Sensory neurons in the dorsal root ganglia (DRG) express a subset of voltage dependent sodium channels (NaV) including NaV1.1, 1.6, 1.7, 1.8 and 1.9. Previous work supported preferential localization of NaV1.8 channels to small-medium diameter, nociceptive afferent neurons. However, we recently published evidence that NaV1.8 was the dominant NaV channel expressed in the somas of small, medium and large diameter muscle afferent neurons, which is consistent with other reports. Here, we extend those results to show that NaV1.8 expression is not correlated with afferent neuron diameter. Using immunocytochemistry, we found NaV1.8 expression in ~50% of sensory afferent neurons with diameters ranging from 20 to 70 µm. In addition, electrophysiological analysis shows that the kinetic and inactivation properties of NaV1.8 current are invariant with neuron size. These data add further support to the idea that NaV1.8 contributes to the electrical excitability of both nociceptive and non-nociceptive sensory neurons.
Keywords: cutaneous afferents, muscle afferents, dorsal root ganglia neurons, Tetrodotoxin-resistant (TTX-R)
Introduction
NaV1.8 channels are tetrodotoxin-resistant (TTX-R) channels that play a role in action potential generation in the soma of small diameter sensory neurons1-3 and these channels have been shown to be involved in nociception and chronic pain.4-9 Thus, the role of NaV1.8 channels in small unmyelinated and thinly myelinated sensory neurons has been well established3,10
However, there is evidence that NaV1.8 channels are also expressed in non-nociceptor sensory afferent neurons. Using both electrophysiology and immunocytochemistry, we recently showed dominant expression of NaV1.8 channels in small, medium and large diameter (> 40 µm) rat muscle afferent neurons.11 While this was inconsistent with some studies showing minimal expression of NaV1.8 in large diameter cutaneous afferents,10 it was consistent with other studies using mouse,12,13 rat14 and human15 sensory neurons. Here, we extend our previous results to examine the expression of NaV1.8 channels in sensory neurons, and show that the kinetic properties of these channels do not vary with cell size.
Results
We used immunocytochemistry to assess NaV1.8 expression in sensory afferent neurons isolated from lumbar dorsal root ganglia. The fluorescent intensity and neuronal diameter were measured using ImageJ64 (as previously described).11 Out of a total of 277 sensory neurons imaged, 140 were stained positive for NaV1.8 (51%) (Fig. 1A). The neuronal diameter for NaV1.8 positive neurons ranged from 14–75 µm, while the range for unlabeled neurons was 17–72 µm. The size distribution of NaV1.8 positive neurons showed a peak between 25–35 µm with a reduction of labeled neurons at larger diameters (Fig. 1B). However, expressing this histogram as a percentage of NaV1.8 positive neurons showed roughly similar percentages of labeled neurons with diameters ranging from 20 to 70 µm (Fig. 1C). The percentage of labeled neuron varied between 33–74% (bins with six or more neurons), but there was no clear trend with cell diameter. Thus, there was no preferential labeling of small to medium diameter afferent neurons in this study.
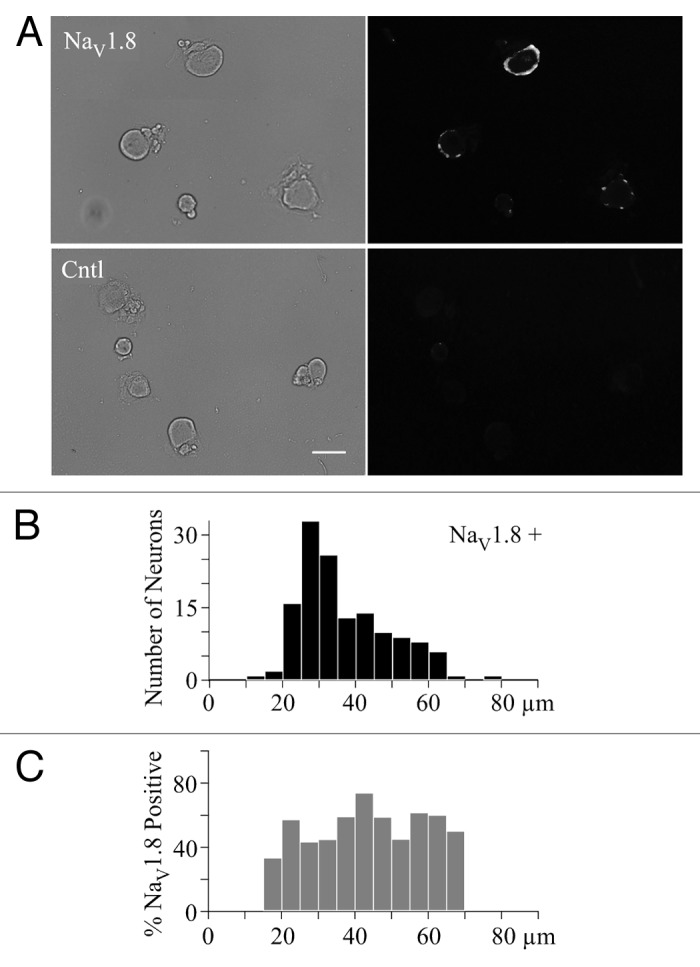
Figure 1. Large diameter sensory neurons express NaV1.8 channels. (A) The top row (NaV1.8) shows bright field (left), and NaV1.8 antibody labeling (right) images, while the bottom row (Cntl) shows the bright field (left) and NaV1.8 negative control (right) images. The white bar indicates 50 µm. (B) The distribution of NaV1.8 positive (NaV1.8+) sensory neurons vs. cell diameter (5 µm bin width). (C) The distribution of percent NaV1.8 positive neurons vs. cell diameter. The 100% values in the 10 and 75 µm bins were removed since there was only a single neuron in each bin. There is a 0% value in the 70 µm bin, which represents data from four neurons.
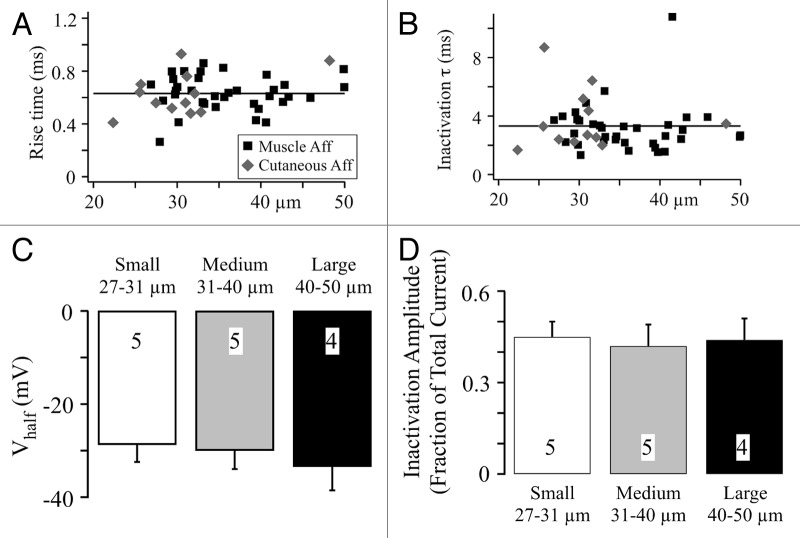
Our previous electrophysiological results from both muscle and cutaneous afferents showed that TTX-R NaV1.8 channels formed the dominant current when the holding potential was -80 mV.11 However, we did not examine the properties of this current to determine if activation or inactivation properties differed with neuron size. Here we compare the 10–90 activation rise time and inactivation time constant of NaV1.8 current at 10 mV11 vs. neuronal diameter (Fig. 2A and B). The points are clustered around the mean value line regardless of neuron diameter. This was true for both muscle and cutaneous afferent neurons with the caveat that only one large diameter cutaneous afferents was recorded (Fig. 2A and B). As a further test of potential differences, we examined the voltage dependence and magnitude of inactivation for the NaV1.8 current in muscle afferent neurons (in 300 nM TTX). The inactivation protocol generated pre- and post-pulses to 10 mV that bracketed a 100 ms inactivating step that ranged from -120 to 30 mV.16 The postpulse to prepulse current ratio was plotted vs. inactivation voltage and fit using the Boltzmann equation to determine the voltage generating half maximal inactivation (Vhalf) (Fig. 2C) and the fraction of total current inactivated (Fig. 2D). These data were grouped for small (27–31 µm), medium (31–40 µm) and large (40–50 µm) diameter muscle afferent neurons (Fig. 2C), and show that NaV1.8 current inactivation did not vary with neuron diameter, which is consistent with recent experiments using mouse sensory neurons.13
Figure 2. NaV1.8 current properties do not change with sensory neuron diameter. (A) Current activation kinetics were measured at 10 mV by the 10–90 rise time11 and the values are plotted vs. neuron diameter. The solid line represents the average value from all data. (B) The inactivation time constant (τ)11 was measured at 10 mV and is plotted vs. neuron diameter. The solid line and symbols have the same meaning as in panel A. (C and D) Vhalf and maximal inactivation were determined as described in Results. The mean ± SD for small, medium and large sensory neurons is shown. The number of neurons measured is shown in each bar.
Discussion
Previous work showed preferential expression of NaV1.8 channels in small to medium diameter (< 35 µm), nociceptive C and Aδ neurons.10 Nociceptor expression of these channels has been supported by multiple studies correlating NaV1.8 channel activity with pain.5,7,9,17-19 While NaV1.8 channels clearly play a role in nociceptor excitability, there is increasing evidence that these channels are functionally expressed in non-nociceptors, including large diameter Aβ sensory afferents that signal vibration sense.11-13,15 This includes studies from adult human DRG with 60–80% of large diameter neurons (60–80 µm) positively labeled with an NaV1.8 antibody,15 adult mice with 48% of large neurons positively stained12 and adult rats with 39% of large DRG neurons positively stained.14 In addition, studies of skin samples from humans and mice showed NaV1.8 immunoreactivity in primary Aβ afferents innervating cutaneous Meissner’s corpuscles and hair cells, which supports NaV1.8 involvement in sensory transduction of fast conducting sensory fibers.13,15 Here we showed that 33–74% of rat sensory neurons with diameters ranging from 20 to 70 µm were positively labeled by a NaV1.8 antibody. This extends our previous finding that NaV1.8 was the dominant NaV current in 86% of muscle afferent neurons (25–50 µm) and 12/13 cutaneous afferent neurons (20–50 µm).11 NaV1.8 channels appear to comprise a large fraction of the active NaV channels in the soma, and perhaps nerve terminals, of both nociceptive and non-nociceptive sensory neurons.
We also wanted to determine if there were any differences in the NaV1.8 current in these different populations of afferent neurons. Shields et al.13 recently demonstrated that NaV1.8 activation and inactivation voltage dependent properties were similar between large vs. small DRG mouse neurons. Here we demonstrate that in rat sensory neurons the activation and inactivation kinetics, as well as inactivation voltage dependence and magnitude, of NaV1.8 current are invariant with diameter. While it remains to be tested, NaV1.8 channels may play a similar role in electrogenesis of large diameter afferent neurons as they do in small diameter neurons.1-3
Methods and Materials
Sensory neurons were isolated from adult male Sprague Dawley rats obtained from Hill Top Laboratories by enzymatic digestion of lumbar dorsal root ganglia L4 and L5.11 Muscle afferent neurons were identified by retrograde labeling with DiI (1,1'-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate) injected into the right and left triceps surae muscles.11 All procedures were reviewed and approved by the Institutional Animal Care and Use Committee and followed NIH guidelines.
For immunocytochemistry experiments, isolated DRG neurons were fixed with 4% formaldehyde, permeabilized with 2% Tween 20 and exposed overnight to either the NaV1.8 antibody plus blocking solution (normal goat serum and phosphate buffered saline) (Test) or blocking solution alone (Cntl). The neurons were then washed and exposed to an Alexa Fluor secondary antibody (either Alexa Fluor 350 or 635).11
For patch clamp recordings the external solution consisted of (in mM) 45 NaCl, 100 N-methyl d-glucosamine (NMG)•Cl, 4 MnCl2, 10 Na•HEPES and 10 glucose, with pH = 7.4 and osmolarity = 320 mOsm, and the pipet solution contained (in mM) 104 NMG•Cl, 14 Creatine•PO4, 6 MgCl2, 10 NMG•HEPES, 5 Tris•ATP, 10 NMG2•EGTA and 0.3 Tris2•GTP with pH 7.4 and osmolarity = 300 mOsm. Neuronal diameter was calculated from membrane capacitance as previously described.11 NaV currents were recorded using an Axopatch 200A amplifier and analyzed using Igor Pro (WaveMetrics).
Acknowledgments
This work was funded by National Institutes of Health Grant AR059397 (K.S.E.).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/22445
References
- 1.Rush AM, Cummins TR, Waxman SG. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J Physiol. 2007;579:1–14. doi: 10.1113/jphysiol.2006.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blair NT, Bean BP. Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons. J Neurosci. 2002;22:10277–90. doi: 10.1523/JNEUROSCI.22-23-10277.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renganathan M, Cummins TR, Waxman SG. Contribution of Na(v)1.8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol. 2001;86:629–40. doi: 10.1152/jn.2001.86.2.629. [DOI] [PubMed] [Google Scholar]
- 4.Dong XW, Goregoaker S, Engler H, Zhou X, Mark L, Crona J, et al. Small interfering RNA-mediated selective knockdown of Na(V)1.8 tetrodotoxin-resistant sodium channel reverses mechanical allodynia in neuropathic rats. Neuroscience. 2007;146:812–21. doi: 10.1016/j.neuroscience.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 5.Jarvis MF, Honore P, Shieh C-C, Chapman M, Joshi S, Zhang X-F, et al. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc Natl Acad Sci U S A. 2007;104:8520–5. doi: 10.1073/pnas.0611364104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimmermann K, Leffler A, Babes A, Cendan CM, Carr RW, Kobayashi J-i, et al. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature. 2007;447:855–8. doi: 10.1038/nature05880. [DOI] [PubMed] [Google Scholar]
- 7.Thakor DK, Lin A, Matsuka Y, Meyer EM, Ruangsri S, Nishimura I, et al. Increased peripheral nerve excitability and local NaV1.8 mRNA up-regulation in painful neuropathy. Mol Pain. 2009;5:14. doi: 10.1186/1744-8069-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roza C, Laird JM, Souslova V, Wood JN, Cervero F. The tetrodotoxin-resistant Na+ channel Nav1.8 is essential for the expression of spontaneous activity in damaged sensory axons of mice. J Physiol. 2003;550:921–6. doi: 10.1113/jphysiol.2003.046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold MS, Weinreich D, Kim C-S, Wang R, Treanor J, Porreca F, et al. Redistribution of Na(V)1.8 in uninjured axons enables neuropathic pain. J Neurosci. 2003;23:158–66. doi: 10.1523/JNEUROSCI.23-01-00158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Djouhri L, Fang X, Okuse K, Wood JN, Berry CM, Lawson SN. The TTX-resistant sodium channel Nav1.8 (SNS/PN3): expression and correlation with membrane properties in rat nociceptive primary afferent neurons. J Physiol. 2003;550:739–52. doi: 10.1113/jphysiol.2003.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramachandra R, McGrew SY, Baxter JC, Kiveric E, Elmslie KS. Tetrodotoxin-resistant voltage-dependent sodium (NaV) channels in identified muscle afferent neurons. J Neurophysiol. 2012;108:2230–2241. doi: 10.1152/jn.00219.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Renganathan M, Cummins TR, Hormuzdiar WN, Waxman SG. alpha-SNS produces the slow TTX-resistant sodium current in large cutaneous afferent DRG neurons. J Neurophysiol. 2000;84:710–8. doi: 10.1152/jn.2000.84.2.710. [DOI] [PubMed] [Google Scholar]
- 13.Shields SD, Ahn H-S, Yang Y, Han C, Seal RP, Wood JN, et al. Na(v)1.8 expression is not restricted to nociceptors in mouse peripheral nervous system. Pain. 2012;153:2017–30. doi: 10.1016/j.pain.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 14.Novakovic SD, Tzoumaka E, McGivern JG, Haraguchi M, Sangameswaran L, Gogas KR, et al. Distribution of the tetrodotoxin-resistant sodium channel PN3 in rat sensory neurons in normal and neuropathic conditions. J Neurosci. 1998;18:2174–87. doi: 10.1523/JNEUROSCI.18-06-02174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coward K, Plumpton C, Facer P, Birch R, Carlstedt T, Tate S, et al. Immunolocalization of SNS/PN3 and NaN/SNS2 sodium channels in human pain states. Pain. 2000;85:41–50. doi: 10.1016/S0304-3959(99)00251-1. [DOI] [PubMed] [Google Scholar]
- 16.Fassl J, High KM, Stephenson ER, Yarotskyy V, Elmslie KS. The intravenous anesthetic propofol inhibits human L-type calcium channels by enhancing voltage-dependent inactivation. J Clin Pharmacol. 2011;51:719–30. doi: 10.1177/0091270010373098. [DOI] [PubMed] [Google Scholar]
- 17.Bulaj G, Zhang MM, Green BR, Fiedler B, Layer RT, Wei S, et al. Synthetic muO-conotoxin MrVIB blocks TTX-resistant sodium channel NaV1.8 and has a long-lasting analgesic activity. Biochemistry. 2006;45:7404–14. doi: 10.1021/bi060159+. [DOI] [PubMed] [Google Scholar]
- 18.Ekberg J, Jayamanne A, Vaughan CW, Aslan S, Thomas L, Mould J, et al. muO-conotoxin MrVIB selectively blocks Nav1.8 sensory neuron specific sodium channels and chronic pain behavior without motor deficits. Proc Natl Acad Sci U S A. 2006;103:17030–5. doi: 10.1073/pnas.0601819103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joshi SK, Mikusa JP, Hernandez G, Baker S, Shieh C-C, Neelands T, et al. Involvement of the TTX-resistant sodium channel Nav 1.8 in inflammatory and neuropathic, but not post-operative, pain states. Pain. 2006;123:75–82. doi: 10.1016/j.pain.2006.02.011. [DOI] [PubMed] [Google Scholar]