Abstract
P2X receptors are calcium permeable ligand-gated ion channels activated by ATP. Their role as cell surface receptors for extracellular ATP released physiologically by mammalian cells is well established. However, the cellular function of P2X receptor subtypes that populate the membranes of intracellular compartments is not defined. An initial report described how intracellular P2X receptors control the function of the contractile vacuole, an osmoregulatory organelle in Dictyostelium and other protists, and that genetic disruption of P2X receptors severely impaired cell volume control during hypotonic stress. However, later studies refuted a functional role of intracellular P2X receptors in Dictyostelium. Here we provide evidence that the discrepancies reported between the studies are due to the laboratory strain of Dictyostelium employed, which display different phenotypes in response to hypotonic stress and a varied dependency upon P2X receptors for osmoregulation. We use the recent discovery that intracellular P2X receptors are novel calcium release channels to provide some mechanistic insight in an effort to explain why the strain variance may exist.
Keywords: Calcium signaling, P2X receptor, ATP, ion channel, intracellular, Dictyostelium
Introduction
P2X receptors (P2XRs) comprise a family of cation-selective ligand-gated ion channels activated by micromolar adenosine 5′-triphosphate (ATP).1 Functional receptors assemble as trimers of pore-forming units of which the human genome encodes seven (P2X1–7). Homo- and heteromeric assembly of receptor is documented and allows fine-tuning of biophysical and cellular responses to ATP. Initially cloned in mammals, the dogmatic view of P2XRs is that of cell surface receptors serving to respond to extracellular ATP secreted by cells in processes of cell stress, pain, inflammation and chemotransduction. ATP is omnipresent in biological systems with a major role as an energy source and substrate for enzymatic reactions. Despite the wide distribution of ATP its role as a signaling molecule appears to be somewhat restricted, represented by the unusual phylogeny of P2XRs.2 Phylogenetic analysis of P2XRs outside mammals reveals expression by amoeba;3 single-celled green algae;4 tick5 and schistosome6 yet P2XRs homologs are not present in Drosophila, C. elegans, yeast or higher plants.2
In addition to a cell surface residency, some P2XR subtypes are localized to intracellular compartments of mammalian and other eukaryotic cells, including lysosomes7-9 and phagosomes.10 In 2007 we cloned the first P2XR from a unicellular organism, from the amoeba Dictyostelium.3 In contrast to the recognized cell surface role of P2XRs, the receptors of Dictyostelium are exclusively intracellular.3,11,12 Dictyostelium P2XRs (P2XA-E) are localized to the contractile vacuole (CV), an osmoregulatory organelle and acidic calcium store. The receptors are orientated such that the receptor is positioned to sense changes in luminal not cytosolic ATP.11,12 Our initial study3 demonstrated that genetic disruption of the P2XA receptor compromised osmoregulatory function and loss of regulated cell volume decrease (RVD) in response to hypotonic swelling.3 These data suggested a functional role for intracellular P2XRs. However, a consequent study by Ludlow et al. (2009) revealed near normal osmoregulation in cells void of P2XRs. This controversy has left the functional role of intracellular P2XRs unclear. The factors underlying the discrepancy between the two studies is important to address in an effort to: (1) validate Dictyostelium as a model with which to study intracellular P2XR signaling; and (2) to further study how ATP dependent signaling evolved. One striking difference between the study of Fountain et al. (2007) and Ludlow et al. (2009) is the use of laboratory strain, AX4 and AX2, respectively. Although one might expect phenotypic differences between laboratory strains to be subtle, extensive differences have been previously reported13-15 with genetic variability a likely contributing factor.16
Our recent study12 has gone someway to demonstrate strain variance as a factor between the two studies, but here we provide a direct and definitive analysis of the effect of strain on the dependency for P2XRs during normal osmoregulation.
Results
Proficiency of osmoregulation varies between laboratory strains of Dictyostelium
Hypotonic challenge caused cell swelling in both wild-type AX2 and AX4 laboratory strains (Fig. 1). Time to peak and the magnitude of peak swelling was similar between strains. However, regulated cell volume decrease (RVD) was markedly different with AX2 recovering much greater volume compared with swollen AX4 cells (Fig. 1). RVD in AX4 was approximately 2-fold less than AX2 strain cells. This direct comparison of wild-type AX2 and AX4 demonstrates distinct strain variance in response to hypotonic challenge and suggests AX2 cells are far more adept at volume recovery following swelling (Fig. 1).
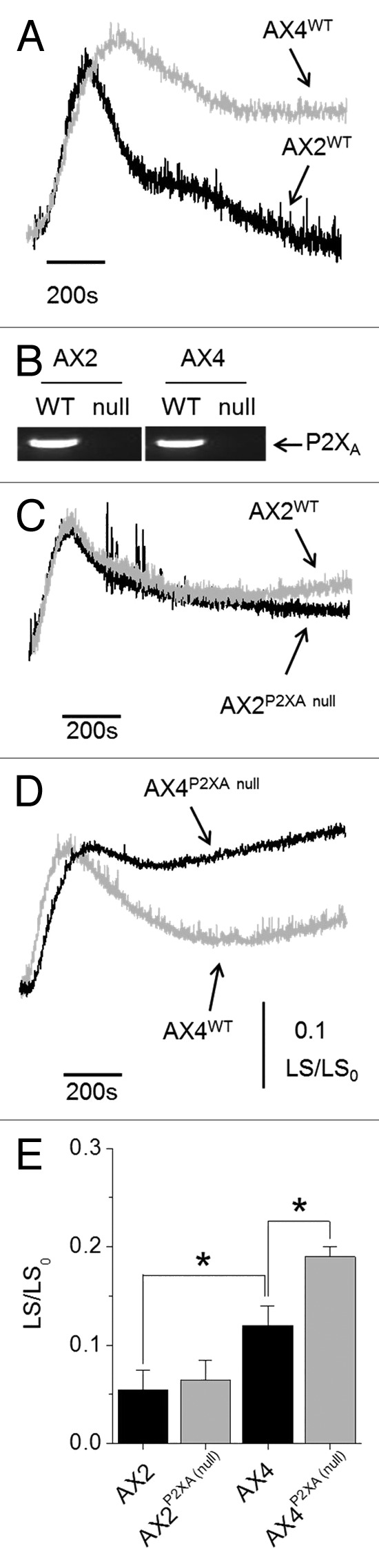
Figure 1. Dependency on P2XAR for normal osmoregulation is strain variant. (A) Time-dependent changes in cell volume following hypotonic challenge for wild-type AX2 and AX4 strain Dictyostelium (n = 10). (B) Generation of P2XA null cells verified by RT-PCR. (C and D) Effect of P2XA knockout on AX2 and AX4 hypotonic phenotype (n = 8–10). (E) Average light scatter for each cell type 800s after hypotonic challenge (n = 8–10; *p < 0.05).
Differences in P2XR dependency and ATP evoked vacuolar Ca2+ release
Blasticidin resistant clones were identified following transformation with the P2XA receptor targeting vector.11 P2XA null cells were verified by RT-PCR (Fig. 1). AX2 P2XA null cells behaved as wild-type (Fig. 1) exhibiting no differences in neither peak swelling nor RVD. In stark contrast, disruption of P2XA in AX4 ablated RVD (Fig. 1) with cells exhibiting persistent swelling after peak. Highly purified vacuoles isolated from AX2 and AX4 wild-type cells both released calcium into the extravacuolar space in response to 4 mM ATP (Fig. 2). The magnitude of calcium release was significantly smaller in vacuoles isolated from AX2 cells vs. AX4 cells (Fig. 2). The magnitude of release was approximately 2-fold less in AX2 cells. Knockout of P2XA significantly reduced ATP evoked calcium release in vacuoles isolated from both AX2 and AX4 strains (Fig. 2).
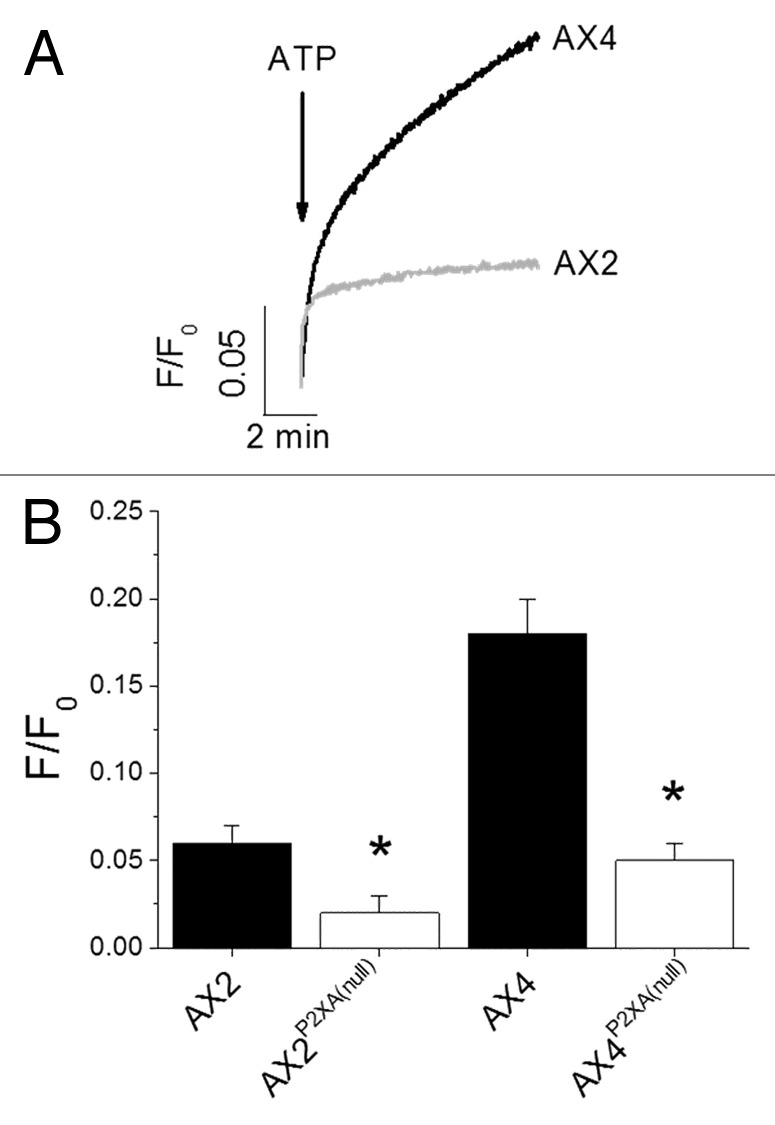
Figure 2. Comparison of ATP evoked vacuolar Ca2+ release in different strains. (A) ATP (4 mM) evoked calcium release measured in highly purified contractile vacuoles isolated from wild-type AX2 and AX4 Dictyostelium. (B) Mean peak ATP evoked vacuolar Ca2+ release for both wild-type strains and P2XA null strains n = 6–8; *p < 0.01).
Discussion
This study demonstrates that two commonly used laboratory strains of Dictyostelium display different phenotypes in response to hypotonic stress. We also provide direct evidence that the magnitude of CV calcium released in response to ATP differs substantially between AX2 and AX4, with the magnitude of release being significantly smaller in AX2 cells. Our data agree with those of Ludlow et al. (2009) in that AX2 cells are not dependent upon the P2XA receptor for normal volume regulation. However this current study, which employs the P2XA receptor targeting vector described by Ludlow et al. (2009), substantiates our original findings3 that intracellular P2XRs are required for normal osmoregulation in AX4 cells. However the molecular basis for the differences in proficiency of osmoregulation displayed between AX2 and AX4 wild-type strains remains unclear, and is likely to be a fruitful line of investigation to fully understand the role of intracellular P2XR function in cell volume control. One apparent difference is in the magnitude of ATP evoked calcium release from the CV, the organelle underlying RVD. We recently described that intracellular P2X receptors mediate calcium release in response to ATP, and this is true for both AX2 and AX4 strains.12 Indeed the P2XA receptor contributes around 20–30% of total calcium release in response to ATP in both strains.12 However, one striking difference is the magnitude of ATP evoked calcium release observed in CVs isolated from AX2 and AX4 strains, with AX2 vacuoles release significantly (approximately 2-fold) less calcium in response to ATP. Genetic disruption of P2XA significantly reduced ATP evoked calcium release in AX2 and AX4, approximately 70% for both strains. Interestingly, P2XA disruption does not ablate ATP evoked calcium release as for disruption of all P2XRs (P2XA-P2XE),12 and suggests P2XA is the major component of calcium release in both Dictyostelium strains. CV calcium is important for normal osmoregulation as depleting it results in total loss of RVD.12 If RVD in Dictyostelium was completely dependent upon P2XR-dependent calcium release from the CV one might expect that AX2 would be less adept at osmoregulation than AX4 cells, owing to the smaller magnitude of ATP evoked calcium release. CV calcium is important for normal osmoregulation as depleting it results in total loss of RVD.12 One interpretation is that P2XR-dependent calcium release is less important for RVD or redundant in AX2, and that another calcium release pathways exist and predominant. Other signals such as calmodulin antagonism, arachidonic and calcium itself mobilise CV calcium.17 The presence of P2XR redundant mechanisms in AX2 cells and how different signaling pathways interact merits further investigation. This current study and our previous study12 supports a role for intracellular P2XRs as novel calcium release channels which release stored calcium in response to elevated luminal ATP. We also validate Dictyostelium as a genetically amenable model eukaryote with which to study signaling by intracellular P2XRs, with the hope to understand how P2XRs may regulate the function of intracellular compartments in mammalian cells.
Methods
Cell culture and gene disruption
Wild-type AX2 (Rob Kay laboratory strain) and wild-type AX4 (Chris Thompson laboratory strain) were cultivated in shaking culture at 21°C in HL5 medium containing glucose. Cells were maintained at a density less than 1 × 106 cells/mL. P2XA knockouts were generated using the targeting vector used previously by Ludlow et al. (2009). Briefly, cells were transformed by electroporation followed by selection with 10μg/mL blasticidin for 14 d. Loss of P2XA was confirmed by RT-PCR using 5′-GCAGTCGATTTACATGGTTAC-3′sense and 5′-AGTTTGGAAATGGAAAGAACC-3′′ antisense primers.
Vacuole purification and calcium release assay
Purification and real-time measurement of calcium release were performed as described previously.12 Calcium release was followed using membrane impermeable Fluo-3 (ex λ 505-nm; em λ 526-nm).
Osmoregulation assay
All cells were suspended in fresh HL5 medium for 2 h prior to experimentation in an effort to avoid any adverse effects of conditioned media on cell performance. Changes in cell size were measured by right-angled scatter of light at 600nm using a Hitachi F-2000 spectrophotometer. Hypotonic stress was induced by replacing HL5 medium with distilled water (1 × 106 cells/mL).
Statistics
Average results are expressed as mean ± SE from the number of experiments indicated. Hypothesis testing employed unpaired two-tailed Student’s t-test.
Acknowledgments
This work was supported by the Biotechnology and Biological Sciences Research Council (BBSRC). S.J.F is a BBSRC David Phillips Fellow. We thank Dr. Steve Ennion (University of Leicester) for provision of the P2XA receptor targeting vector and parental AX2 cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/22737
References
- 1.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–67. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 2.Fountain SJ, Burnstock G. An evolutionary history of P2X receptors. Purinergic Signal. 2009;5:269–72. doi: 10.1007/s11302-008-9127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fountain SJ, Parkinson K, Young MT, Cao L, Thompson CR, North RA. An intracellular P2X receptor required for osmoregulation in Dictyostelium discoideum. Nature. 2007;448:200–3. doi: 10.1038/nature05926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fountain SJ, Cao L, Young MT, North RA. Permeation properties of a P2X receptor in the green algae Ostreococcus tauri. J Biol Chem. 2008;283:15122–6. doi: 10.1074/jbc.M801512200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bavan S, Farmer L, Singh SK, Straub VA, Guerrero FD, Ennion SJ. The penultimate arginine of the carboxyl terminus determines slow desensitization in a P2X receptor from the cattle tick Boophilus microplus. Mol Pharmacol. 2011;79:776–85. doi: 10.1124/mol.110.070037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agboh KC, Webb TE, Evans RJ, Ennion SJ. Functional characterization of a P2X receptor from Schistosoma mansoni. J Biol Chem. 2004;279:41650–7. doi: 10.1074/jbc.M408203200. [DOI] [PubMed] [Google Scholar]
- 7.Qureshi OS, Paramasivam A, Yu JC, Murrell-Lagnado RD. Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J Cell Sci. 2007;120:3838–49. doi: 10.1242/jcs.010348. [DOI] [PubMed] [Google Scholar]
- 8.Stokes L, Surprenant A. Dynamic regulation of the P2X4 receptor in alveolar macrophages by phagocytosis and classical activation. Eur J Immunol. 2009;39:986–95. doi: 10.1002/eji.200838818. [DOI] [PubMed] [Google Scholar]
- 9.Toulme E, Garcia A, Samways D, Egan TM, Carson MJ, Khakh BS. P2X4 receptors in activated C8-B4 cells of cerebellar microglial origin. J Gen Physiol. 2010;135:333–53. doi: 10.1085/jgp.200910336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuehnel MP, Rybin V, Anand PK, Anes E, Griffiths G. Lipids regulate P2X7-receptor-dependent actin assembly by phagosomes via ADP translocation and ATP synthesis in the phagosome lumen. J Cell Sci. 2009;122:499–504. doi: 10.1242/jcs.034199. [DOI] [PubMed] [Google Scholar]
- 11.Ludlow MJ, Durai L, Ennion SJ. Functional characterization of intracellular Dictyostelium discoideum P2X receptors. J Biol Chem. 2009;284:35227–39. doi: 10.1074/jbc.M109.045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sivaramakrishnan V, Fountain SJ. A mechanism of intracellular P2X receptor activation. J Biol Chem. 2012;287:28315–26. doi: 10.1074/jbc.M112.372565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.May T, Blusch J, Sachse A, Nellen W. A cis-acting element responsible for early gene induction by extracellular cAMP in Dictyostelium discoideum. Mech Dev. 1991;33:147–55. doi: 10.1016/0925-4773(91)90081-G. [DOI] [PubMed] [Google Scholar]
- 14.Jain R, Gomer RH. A developmentally regulated cell surface receptor for a density-sensing factor in Dictyostelium. J Biol Chem. 1994;269:9128–36. [PubMed] [Google Scholar]
- 15.Deery WJ, Gao T, Ammann R, Gomer RH. A single cell density-sensing factor stimulates distinct signal transduction pathways through two different receptors. J Biol Chem. 2002;277:31972–9. doi: 10.1074/jbc.M204539200. [DOI] [PubMed] [Google Scholar]
- 16.Bloomfield G, Tanaka Y, Skelton J, Ivens A, Kay RR. Widespread duplications in the genomes of laboratory stocks of Dictyostelium discoideum. Genome Biol. 2008;9:R75. doi: 10.1186/gb-2008-9-4-r75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malchow D, Lusche DF, De Lozanne A, Schlatterer C. A fast Ca2+-induced Ca2+-release mechanism in Dictyostelium discoideum. Cell Calcium. 2008;43:521–30. doi: 10.1016/j.ceca.2007.08.002. [DOI] [PubMed] [Google Scholar]


