Abstract
Transient receptor potential vanilloid subfamily member 1 channels are polymodal sensors of noxious stimuli and integral players in thermosensation, inflammation and pain signaling. It has been shown previously that under prolonged stimulation, these channels show dynamic pore dilation, providing a pathway for large and otherwise relatively impermeant molecules. Further, we have shown recently that these nonselective cation channels, when activated by capsaicin, are potently and reversibly blocked by external application of quaternary ammonium compounds and local anesthetics. Here we describe a novel phenomenon in transient receptor potential channel pharmacology whereby their expression levels in Xenopus laevis oocytes, as assessed by the magnitude of macroscopic currents, are negatively correlated with extracellular blocker affinity: small current densities give rise to nanomolar blockade by quaternary ammoniums and this affinity decreases linearly as current density increases. Possible mechanisms to explain these data are discussed in light of similar observations in other channels and receptors.
Keywords: TRP channels, pharmacology, expression-dependent phenomena
Introduction
The transient receptor potential vanilloid subfamily member 1 (TRPV1) is a nonselective cation ion channel that is activated by vanilloids such as capsaicin and other stimuli including voltage, heat, proton concentration, thus acting a polymodal integrator of noxious stimuli.1 Given its wide tissue distribution and its implication in nociceptive pathways, TRPV1 has been identified as an important target for novel analgesic compounds.2-5 TRPV1 is a homotetramer in which each monomer contains six transmembrane segments (S1-S6), with the loop connecting S5 and S6 forming the pore domain. The activation by capsaicin is likely mediated by intracellular residues,6 proton activation is conferred by extracellular side chains,7-9 while the domain(s) and potential mechanism responsible for temperature sensitivity remains a topic of debate.10-15 In light of the distinct sites of action of the many TRPV1 stimulants, it is desirable to develop antagonists that target a domain that is common for the activation pathways of many (if not all) agonists. One obvious candidate is the pore domain, with its selectivity filter being the final integrator of the diverse stimuli acting on the ion channel, ultimately biasing the open-closed equilibrium. To this end, we and others have demonstrated extracellular TRPV1 inhibition by charged or chargeable ammonium compounds,16,17 and we have recently shown that these compounds likely target the channel pore.18
Expression-dependent extracellular block of TRPV1 channels
We have previously shown that TRPV1 channels, expressed in Xenopus leavis oocytes, are reversibly inhibited by the quaternary ammonium compound QX-314 with micromolar affinity.16 In contrast, our follow-up study demonstrated that the tertiary ammonium compound, lidocaine, and quaternary ammonium compounds such as tetraethyl ammonium (TEA) and tetramethyl ammonium (TMA) can inhibit TRPV1 channels with nanomolar affinity in Xenopus leavis oocytes.18 In the latter, and in contrast to our initial study, we had limited the whole-cell currents to a range between 0.1 and 3 μA (to limit Ca2+ overload of the cells due to large inward currents). This led us to speculate that the drastic difference in apparent affinities for ammonium inhibitors may arise from different expression levels of the TRPV1 channels.
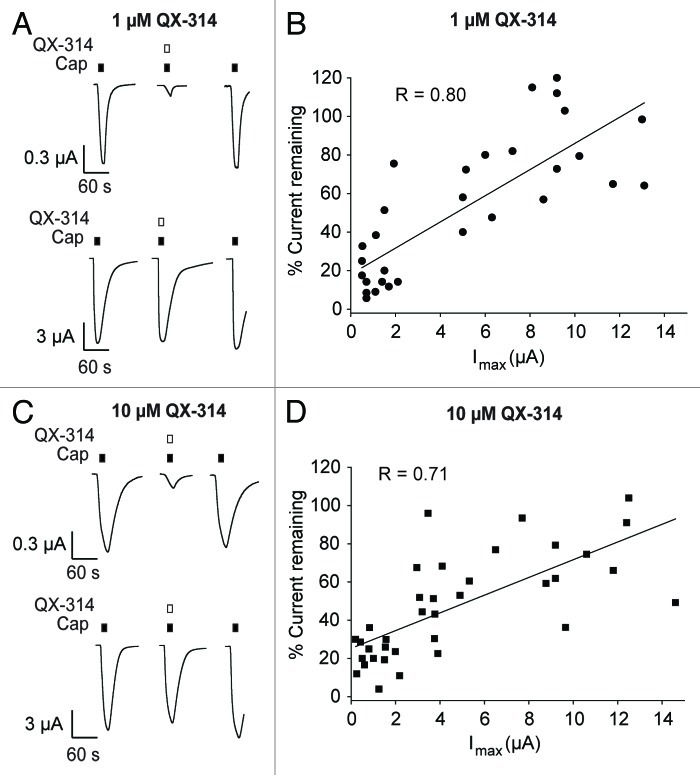
To determine if the degree of current inhibition was directly dependent on TRPV1 expression levels (as assessed by the magnitude of macroscopic currents), we chose to test TRPV1 inhibition by the quaternary ammonium compound QX-314 in Xenopus leavis oocytes. We varied both the amount of mRNA injected, as well as the incubation time (see methods for details) to obtain oocytes yielding a wide range of maximal current amplitudes (from 100 nA to 15 μA), which we assumed to roughly correlate with channel expression levels. In single drug application experiments, 1 or 10 μM QX-314 were co-applied with an approximate EC50 concentration of capsaicin (15 μM) to oocytes expressing varying levels of TRPV1 channels.16 To control for de(sensitization), each drug application was preceded and followed by an application of capsaicin alone (Fig. 1AandC). We observed a robust inverse correlation between the observed maximal currents and the level of inhibition at both 1 and 10 μM QX-314 (R values of 0.80 and 0.71, respectively, Fig. 1BandD), with increasing TRPV1 expression levels resulting in progressively less inhibition by QX-314. Similar trends were observed for all concentrations tested between 100 pM and 100 μM QX-314 (data not shown), suggesting this trend is a general phenomenon.
Figure 1.QX-314 inhibition is dependent on TRPV1 expression levels in Xenopus oocytes. Co-application of 15 μM capsaicin with different QX-314 concentrations was flanked by two applications of 15 µM capsaicin to control for (de)sensitization. Only one 10 sec drug application was performed per oocyte with 2 min washout intervals between all applications. (A and C) Representative capsaicin-evoked current traces observed before and after the co-application of (A) 1 µM and (C) 10 µM QX-314 in oocytes expressing low (top panels) or high (bottom panels) levels of TRPV1. Note the different vertical scale bars in top and bottom panels; (B and D) a strong positive correlation is observed between capsaicin-evoked TRPV1 peak current amplitudes (Imax) and the level of inhibition in the presence of 1 µM (B) or 10 µM (D) QX-314. Only oocytes with inward currents Imax > 0.1 µA and < 15 µA were included in the analysis (1 µM QX-314: n = 32; 10 µ MQX-314: n = 38).
Discussion
Before discussing the findings of our present study in more detail, it is necessary to point out a potential caveat in the interpretation of our results. It is generally assumed that with increasing amounts of injected mRNA and/or longer incubation time, the expression levels of ion channels expressed in Xenopus leavis oocytes will increase.19 However, in the present case, we cannot definitively prove that the observed macroscopic currents are linearly correlated with the expression levels of TRPV1 for two reasons. First, it has not yet been possible to identify the voltage-sensing component of TRPV1 channels, prohibiting gating current measurements, as those routinely performed on voltage-gated potassium channels, for example.20 Such gating currents would otherwise allow for assessment of surface protein expression. And second, the direct surface labeling of expressed TRPV1 would be unreliable at the very low expression levels used for many of the experiments in this study. However, given that previous studies with different ion channels expressed in Xenopus leavis oocytes have demonstrated that larger amounts of injected mRNA, as well as longer incubation times results in higher expression levels,21,22 we assume a similar correlation is true for TRPV1.
Taking into account the above mentioned caveat, we thus believe the data presented here strongly suggest that the potency of TRPV1 inhibition by the quaternary ammonium compound, QX-314, is dependent on TRPV1 expression levels as assessed by the magnitude of macroscopic currents in Xenopus leavis oocytes. At first glance, the notion of expression-dependent receptor pharmacology may seem surprising, but relevant similar observations from other ion channel types exist. First, it has long been known that some ion channels, such as ATP-gated P2X receptors, the nicotinic acetylcholine receptor and L-type calcium channels show differences in their conductance and or gating behavior, depending on whether they are studied as single molecules or in association with other channels.23-25 Similarly, density-dependent agonist-sensitivity has been found both in P2X receptors21 and glycine receptors.22,26,27 Although we did not observe significant expression-dependent changes in the agonist (capsaicin) sensitivity with TRPV1,18 we found an inverse correlation between the level of expression and the amount of inhibition by QX-314. Our mutational analysis further pointed toward two amino acids near the putative pore region of TRPV1 (E648 and F649), that may be implicated in the inhibition by QX-314.18 Interestingly, the pore region, which generally also determines channel selectivity, has been shown to be a dynamic structure in P2X receptors,28-30 acid-sensing channels31 and also voltage-gated potassium channels,32-34 which have been speculated to bear structural resemblance to TRP channels. In fact, it has recently been shown that TRPV1 can also undergo dynamic changes in the selectivity filter during agonist activation,35 and importantly, this effect was found to be expression-dependent. However, it has yet to be determined if the expression-dependent pharmacology described here is a general trait of TRP channels expressed in native and in vitro setting. Nonetheless, we interpret the data as further evidence for our finding that QX-314 inhibition of TRPV1 is likely mediated through the pore region, a protein region that likely exhibits an expression-dependent conformation,35 thereby regulating the potency of extracellular pore block through compounds such as QX-314.
How might such expression-dependent pharmacology arise? One possibility is that as more channels are present on the surface of the cell that they begin to come in contact with one another, and such channel multimerization results in architectural modification of the pore domain that is concomitant with lowered blocker affinity. Alternatively, the channel may be interacting with other molecules in the oocyte, including endogenous TRP channel subunits, which would serve to modify channel pharmacology. In this scenario, high-affinity block diminishes as more TRP channels are expressed, effectively exhausting this endogenous population, even though the actual number of TRPV1 receptors could be similar in high and low current expressing phenotypes. And while there is a clear trend with expression level (as assessed by the magnitude of macroscopic currents) and blocker affinity, there is also sizable inherent variation in blocker affinity outside of current expression levels, suggesting additional modulating factors, such as phosphorylation and Ca2+-dependent gating may also influence pore conformations and blocking ability. Regardless, the data add yet another functional twist to an already challenging array of experimental pitfalls in the study of these clinically and physiologically relevant ion channels.
Methods
An amount of 10–50 nl of TRPV1 (rat isoform) mRNA (500 ng/µl) was injected into Xenopus laevis oocytes and electrophysiological recordings were conducted 12–36 h post injection, using the two-electrode voltage-clamp technique. Glass microelectrodes were backfilled with 3 M KCl. The external solution contained (in mM): 116 NaCl, 2 KCl, 1 MgCl2, 0.5 CaCl2 and 5 HEPES; pH 7.4. Capsaicin (8-methyl-N-vanillyl-6-nonenamide) was used at 15 µM and applied (with or without QX-314) for 10 sec via a fast perfusion system in a custom-built recording chamber. Two-minute washout times were maintained between applications. All recordings were performed at room temperature and a holding potential of -60 mV. The data were acquired using an Axopatch 200B amplifier with the pCLAMP 10.0 software suite (Molecular Devices, Sunnyvale, CA) and analyzed offline using Prism 5 (GraphPad, San Diego, CA) and Sigma Plot 10 (Systat Software Inc., Chicago, IL).
Acknowledgments
We thank Dr Sebastian Brauchi, Universidad Austral de Chile, for helpful insights in reading the manuscript and for providing the rTRPV1 clone.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/23105
References
- 1.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 2.Gomtsyan A, Bayburt EK, Schmidt RG, Zheng GZ, Perner RJ, Didomenico S, et al. Novel transient receptor potential vanilloid 1 receptor antagonists for the treatment of pain: structure-activity relationships for ureas with quinoline, isoquinoline, quinazoline, phthalazine, quinoxaline, and cinnoline moieties. J Med Chem. 2005;48:744–52. doi: 10.1021/jm0492958. [DOI] [PubMed] [Google Scholar]
- 3.Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov. 2009;8:55–68. doi: 10.1038/nrd2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Premkumar LS. Targeting TRPV1 as an alternative approach to narcotic analgesics to treat chronic pain conditions. AAPS J. 2010;12:361–70. doi: 10.1208/s12248-010-9196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts LA, Connor M. TRPV1 antagonists as a potential treatment for hyperalgesia. Recent Pat CNS Drug Discov. 2006;1:65–76. doi: 10.2174/157488906775245309. [DOI] [PubMed] [Google Scholar]
- 6.Jordt SE, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. 2002;108:421–30. doi: 10.1016/S0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- 7.Jordt SE, Tominaga M, Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci U S A. 2000;97:8134–9. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu B, Yao J, Wang Y, Li H, Qin F. Proton inhibition of unitary currents of vanilloid receptors. J Gen Physiol. 2009;134:243–58. doi: 10.1085/jgp.200910255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welch JM, Simon SA, Reinhart PH. The activation mechanism of rat vanilloid receptor 1 by capsaicin involves the pore domain and differs from the activation by either acid or heat. Proc Natl Acad Sci U S A. 2000;97:13889–94. doi: 10.1073/pnas.230146497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang F, Cui Y, Wang K, Zheng J. Thermosensitive TRP channel pore turret is part of the temperature activation pathway. Proc Natl Acad Sci U S A. 2010;107:7083–8. doi: 10.1073/pnas.1000357107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao J, Liu B, Qin F. Pore turret of thermal TRP channels is not essential for temperature sensing. Proc Natl Acad Sci U S A 2010; 107:E125; author reply E6-7. [DOI] [PMC free article] [PubMed]
- 12.Yao J, Liu B, Qin F. Modular thermal sensors in temperature-gated transient receptor potential (TRP) channels. Proc Natl Acad Sci U S A. 2011;108:11109–14. doi: 10.1073/pnas.1105196108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clapham DE, Miller C. A thermodynamic framework for understanding temperature sensing by transient receptor potential (TRP) channels. Proc Natl Acad Sci U S A. 2011;108:19492–7. doi: 10.1073/pnas.1117485108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R. A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J Neurosci. 2006;26:4835–40. doi: 10.1523/JNEUROSCI.5080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cordero-Morales JF, Gracheva EO, Julius D. Cytoplasmic ankyrin repeats of transient receptor potential A1 (TRPA1) dictate sensitivity to thermal and chemical stimuli. Proc Natl Acad Sci U S A. 2011;108:E1184–91. doi: 10.1073/pnas.1114124108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rivera-Acevedo RE, Pless SA, Ahern CA, Schwarz SK. The quaternary lidocaine derivative, QX-314, exerts biphasic effects on transient receptor potential vanilloid subtype 1 channels in vitro. Anesthesiology. 2011;114:1425–34. doi: 10.1097/ALN.0b013e318216ea0c. [DOI] [PubMed] [Google Scholar]
- 17.Leffler A, Fischer MJ, Rehner D, Kienel S, Kistner K, Sauer SK, et al. The vanilloid receptor TRPV1 is activated and sensitized by local anesthetics in rodent sensory neurons. J Clin Invest. 2008;118:763–76. doi: 10.1172/JCI32751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivera-Acevedo RE, Pless SA, Schwarz SK, Ahern CA. Extracellular Quaternary Ammonium Blockade of Transient Receptor Potential Vanilloid Subtype 1 Channels Expressed in Xenopus laevis Oocytes. Mol Pharmacol. 2012;82:1129–35. doi: 10.1124/mol.112.079277. [DOI] [PubMed] [Google Scholar]
- 19.Goldin AL. Expression of Ion Channels in Xenopus Oocytes. Wiley, 2006. [Google Scholar]
- 20.Perozo E, MacKinnon R, Bezanilla F, Stefani E. Gating currents from a nonconducting mutant reveal open-closed conformations in Shaker K+ channels. Neuron. 1993;11:353–8. doi: 10.1016/0896-6273(93)90190-3. [DOI] [PubMed] [Google Scholar]
- 21.Fujiwara Y, Kubo Y. Density-dependent changes of the pore properties of the P2X2 receptor channel. J Physiol. 2004;558:31–43. doi: 10.1113/jphysiol.2004.064568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taleb O, Betz H. Expression of the human glycine receptor alpha 1 subunit in Xenopus oocytes: apparent affinities of agonists increase at high receptor density. EMBO J. 1994;13:1318–24. doi: 10.1002/j.1460-2075.1994.tb06384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding S, Sachs F. Evidence for non-independent gating of P2X2 receptors expressed in Xenopus oocytes. BMC Neurosci. 2002;3:17. doi: 10.1186/1471-2202-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hymel L, Striessnig J, Glossmann H, Schindler H. Purified skeletal muscle 1,4-dihydropyridine receptor forms phosphorylation-dependent oligomeric calcium channels in planar bilayers. Proc Natl Acad Sci U S A. 1988;85:4290–4. doi: 10.1073/pnas.85.12.4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schindler H, Spillecke F, Neumann E. Different channel properties of Torpedo acetylcholine receptor monomers and dimers reconstituted in planar membranes. Proc Natl Acad Sci U S A. 1984;81:6222–6. doi: 10.1073/pnas.81.19.6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Saint Jan D, David-Watine B, Korn H, Bregestovski P. Activation of human alpha1 and alpha2 homomeric glycine receptors by taurine and GABA. J Physiol. 2001;535:741–55. doi: 10.1111/j.1469-7793.2001.t01-1-00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pless SA, Leung AW, Galpin JD, Ahern CA. Contributions of conserved residues at the gating interface of glycine receptors. J Biol Chem. 2011;286:35129–36. doi: 10.1074/jbc.M111.269027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khakh BS, Bao XR, Labarca C, Lester HA. Neuronal P2X transmitter-gated cation channels change their ion selectivity in seconds. Nat Neurosci. 1999;2:322–30. doi: 10.1038/7233. [DOI] [PubMed] [Google Scholar]
- 29.Khakh BS, Lester HA. Dynamic selectivity filters in ion channels. Neuron. 1999;23:653–8. doi: 10.1016/S0896-6273(01)80025-8. [DOI] [PubMed] [Google Scholar]
- 30.Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A. Pore dilation of neuronal P2X receptor channels. Nat Neurosci. 1999;2:315–21. doi: 10.1038/7225. [DOI] [PubMed] [Google Scholar]
- 31.Lingueglia E, de Weille JR, Bassilana F, Heurteaux C, Sakai H, Waldmann R, et al. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem. 1997;272:29778–83. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Jurman ME, Yellen G. Dynamic rearrangement of the outer mouth of a K+ channel during gating. Neuron. 1996;16:859–67. doi: 10.1016/S0896-6273(00)80106-3. [DOI] [PubMed] [Google Scholar]
- 33.Kiss L, LoTurco J, Korn SJ. Contribution of the selectivity filter to inactivation in potassium channels. Biophys J. 1999;76:253–63. doi: 10.1016/S0006-3495(99)77194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Starkus JG, Kuschel L, Rayner MD, Heinemann SH. Ion conduction through C-type inactivated Shaker channels. J Gen Physiol. 1997;110:539–50. doi: 10.1085/jgp.110.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung MK, Güler AD, Caterina MJ. TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat Neurosci. 2008;11:555–64. doi: 10.1038/nn.2102. [DOI] [PubMed] [Google Scholar]