Abstract
Objective:
To determine whether childhood obesity is a risk factor for developing pediatric multiple sclerosis (MS) or clinically isolated syndrome (CIS).
Methods:
Cases were identified through the Kaiser Permanente Southern California (KPSC) Pediatric Acquired Demyelinating Diseases Cohort between 2004 and 2010. For cases, body mass index (BMI) was obtained prior to symptom onset, for the underlying cohort BMI was obtained through the KPSC Children's health study (n = 913,097). Weight classes of normal weight, overweight, moderate obesity, and extreme obesity were assigned based on BMI specific for age and sex.
Results:
We identified 75 newly diagnosed pediatric cases of MS or CIS, the majority of which were in girls (n = 41, 55%), age 11–18 (n = 54, 72%). Obesity was associated with a significantly increased risk of MS/CIS in girls (p = 0.005 for trend) but not in boys (p = 0.93). The adjusted odds ratio and 95% confidence intervals for CIS/MS among girls was 1.58 (0.71–3.50) for overweight compared to normal weight (reference category), 1.78 (0.70–4.49) for moderately obese, and 3.76 (1.54–9.16) for extremely obese. Moderately and extremely obese cases were more likely to present with transverse myelitis compared with normal/overweight children (p = 0.003).
Conclusion:
Our findings suggest the childhood obesity epidemic is likely to lead to increased morbidity from MS/CIS, particularly in adolescent girls.
Once thought to be rare in children, multiple sclerosis (MS) and its potential precursor, clinically isolated syndrome (CIS), which encompasses optic neuritis (ON) and transverse myelitis (TM), are increasingly recognized. Pediatric MS/CIS most often affects teenage girls. While once thought to be more common in whites, one study showed that the incidence is higher in blacks compared with whites and Hispanics.1 Whether this represents increased exposure to environmental triggers that increase with age, black race, or female sex during childhood is unclear.
Over the last 30 years, the prevalence of pediatric obesity has tripled. It is well-known that obesity is characterized by a low-grade inflammatory state,2 raising the possibility that the increasing reports of pediatric MS/CIS may be due at least in part to this alarming epidemic. Yet whether obesity is a risk factor for pediatric MS/CIS is unknown. Even in adults, the relationship between obesity and MS risk is not well understood. Only 2 studies have examined this question, both of which suggest that moderate obesity at age 20 but not at other times in life double the risk of adult-onset MS in women3,4 and men.4 However, the studies are limited by retrospective self-report of body size,3,4 selection bias,3 use of volunteer controls,4 small number of obese subjects,3,4 and inability to examine the risk among extremely obese individuals.3,4
The purpose of this study was to estimate the magnitude of the association between overweight, moderate, and extreme childhood obesity and the risk of pediatric MS/CIS in our population-based, multiethnic cohort of children. Determining whether the risk of pediatric MS/CIS increases with increasing weight class is important in establishing whether a causal relationship might exist as opposed to shared causality between adolescent obesity and MS/CIS by an unmeasured confounder.
METHODS
Standard protocol approvals, registrations, and patient consents.
The institutional review board at Kaiser Permanente Southern California (KPSC) approved this study. Informed consent was waived as this was a database and chart review study only without direct patient contact.
Study design and subjects.
For this study, we used body size and demographic data on children in the KPSC pediatric acquired demyelinating diseases (ADS) cohort1 and those enrolled in the KPSC Children's Health Study between 2007 and 2009, which is described in detail elsewhere.5 KPSC is a large prepaid health maintenance organization with over 3.5 million members including over 900,000 members 18 years and younger. The KPSC pediatric membership is representative of the general pediatric population in Southern California with respect to ethnicity, age, gender, and socioeconomic status, with the exception of an under-representation of the lowest and highest ends of the socioeconomic spectrum. After exclusion of 265,241 members who did not have any medical encounters in 2007–2009, 1,030,730 patients were eligible for participation in the cohort study. Of these patients, 920,034 (89.2% of eligible patients) had at least one valid weight and height in the 3-year study period. After exclusion of pregnant patients (n = 6,856 controls, n = 0 cases), 913,172 patients were included in the final analytical cohort.
Case identification.
To identify potentially incident cases, we used the same methods described elsewhere.1 Briefly, we searched electronic databases for any mention of International Classification of Diseases–9 diagnostic codes for MS and other forms of ADS in subjects ≤18 years of age, January 1, 2004–December 31, 2010. Diagnoses were confirmed and additional clinical details were extracted through full medical records abstraction by an MS specialist (A.L.G.) according to the consensus definitions for pediatric CIS and MS.6 A first CNS demyelinating event involving the brain itself, if not accompanied by encephalopathy, was classified as CIS. Pediatric MS was defined as 2 or more episodes of CNS demyelination separated in time and space or a single such episode followed by new gadolinium-enhancing or T2 lesions on MRI scan at least 3 months after the initial event. Patients with acute disseminated encephalomyelitis6 were not included in these analyses. Idiopathic TM was defined according to proposed consensus definitions7 after exclusion of infectious, vascular, and other inflammatory causes of myelopathy.8
Body weight and height.
The most recent body weight and height prior to symptom onset in cases was abstracted from the health record. For cases and controls, body weight and height were extracted from electronic health records from the same day. BMI was calculated as weight (kilograms) divided by the square of the height (meters). For patients enrolled into the study in 2007, 2008, and 2009, the median BMI for age of all encounters in the year of study enrollment for a patient was used for analysis. Based on a validation study including 15,000 patients with 45,980 medical encounters, the estimated error rate in body weight and height data was <0.4%.9
Definitions for overweight and obesity in children and adolescents are based on the sex-specific BMI-for-age growth charts developed by the Centers for Disease Control and Prevention and WHO definitions for overweight and obesity in adults.10–12 Children were categorized as underweight (BMI for age <5th percentile), normal weight (BMI for age ≥5th and <85th percentile), overweight (BMI for age ≥85th percentile or BMI ≥25 kg/m2), moderately obese (BMI for age ≥95th percentile or a BMI ≥30 kg/m2), and extremely obese (BMI for age ≥1.2 × 95th percentile or BMI ≥35 kg/m2).9
Race/ethnicity and socioeconomic status.
Methods to assign race and ethnicity are described in detail elsewhere.13 Race and ethnicity information were obtained from health plan administrative records and birth certificates. We categorized race/ethnicity as non-Hispanic white, Hispanic white, black (regardless of ethnicity), Asian or Pacific Islander, and other or multiple race/ethnicity. A validated algorithm based on surname lists and address information derived from the US Census Bureau14–16 was used to impute missing information in the general cohort.
Statistical analysis.
Differences in the distribution of basic demographics across groups defined by weight class were assessed with the χ2 test. t Test was used to compare normally distributed variables across groups. For MS/CIS cases, age was assigned as age at symptom onset of CIS or MS diagnosis. For non-MS/CIS cases, age was assigned based on the age on July 1 of the year of study enrollment.
Multiple logistic regression models were generated to estimate odds ratios (OR) and their 95% confidence intervals (CI) for MS/CIS vs weight class (underweight/normal weight [reference], overweight, moderate obesity, extreme obesity) and adjusted for sex, age group (2–11 or 12–18 years), and race/ethnicity. All analyses were conducted using SPSS release 18.0 (SPSS Inc., Chicago, IL).
RESULTS
Similar to the general study population, the 75 children with newly diagnosed CIS or MS were more likely to be 11 years or older at diagnosis (n = 54, 72%) and Hispanic (n = 39, 52%; table 1). In contrast to the general study population, MS/CIS cases were more likely to be female (n = 41, 54.7%). Thirty-eight (50.7%) children and adolescents with MS/CIS were overweight or obese. Obesity was associated with a significantly increased risk of pediatric MS/CIS (table 2), but this risk was driven by an association in girls, not boys (figure).
Table 1.
Demographic characteristics of youth with and without multiple sclerosis or clinically isolated syndrome
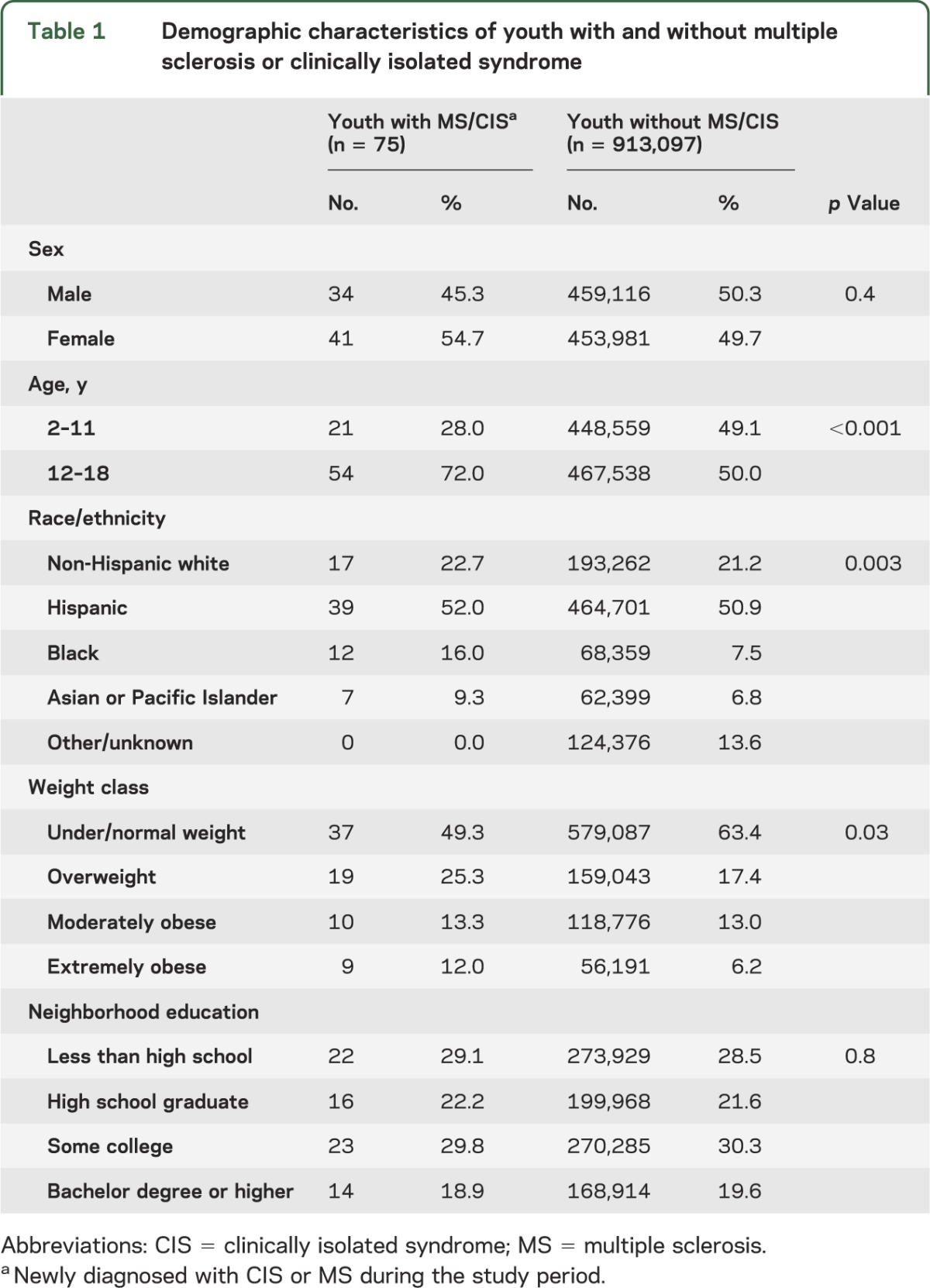
Table 2.
Predictors of pediatric multiple sclerosis and clinically isolated syndrome according to age group
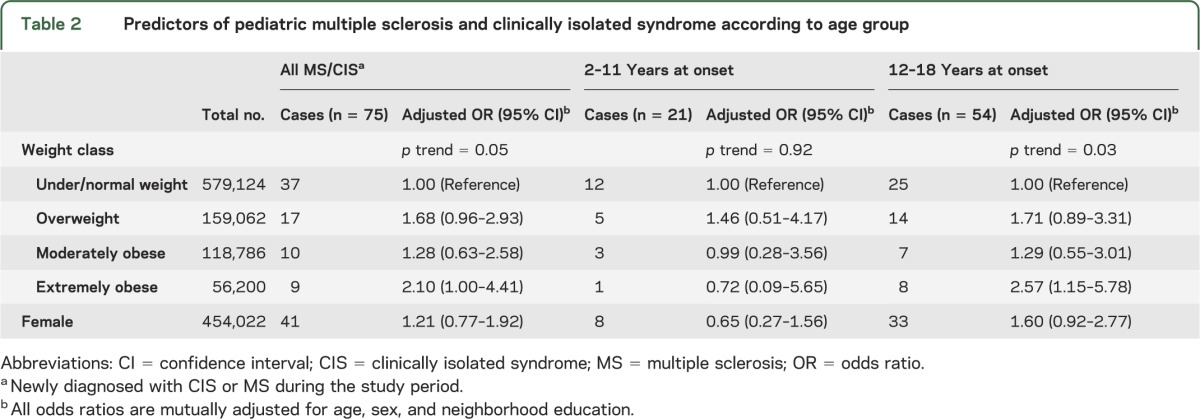
Figure. Association between weight class and pediatric multiple sclerosis/clinically isolated syndrome by sex.
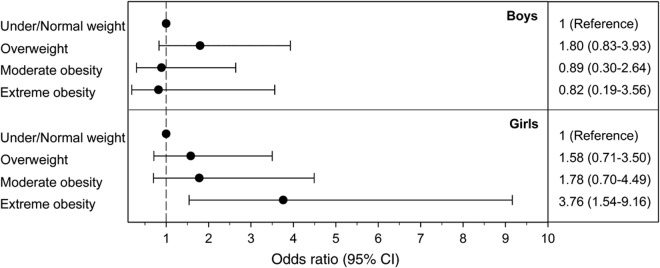
Depicted are the adjusted odds ratios (OR) and 95% confidence intervals (CI) of pediatric multiple sclerosis and clinically isolated syndrome (MS/CIS) with increasing weight class compared with normal/underweight children (reference category) stratified by sex. Increasing weight class was associated with increasingly higher OR for MS/CIS among girls (p for trend <0.005) but not boys (p for trend 0.93). OR are adjusted for age at onset and race/ethnicity.
The onset of MS/CIS was uncommon in children ages 2–11 years, particularly among young girls (table 2). We found no association between increasing weight class and MS/CIS in these young children (table 2), although in the girls, a similar trend of increasing MS/CIS risk with increasing weight class was seen that did not reach statistical significance (p = 0.13 for trend). The OR (95% CI) for under/normal weight, overweight, moderately obese, and extremely obese 2- to 11-year-old girls were 1.00, 1.35 (0.26–7.05), 1.75 (0.33–9.23), and 4.14 (0.76–22.42), respectively.
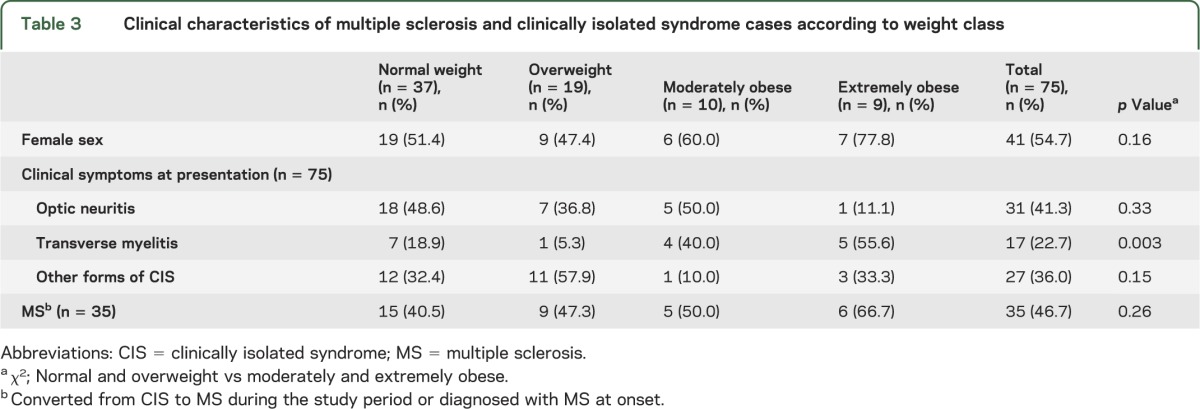
The clinical characteristics and clinical symptoms of MS/CIS by weight class are presented in table 3. The female preponderance was more striking among moderately and extremely obese children with MS/CIS than normal/overweight cases (68.4% and 50.0%, respectively, p = 0.16). Obese children were more likely to present with TM than were normal/overweight children (47.4% vs 14.3%, respectively, p = 0.003; table 3).
Table 3.
Clinical characteristics of multiple sclerosis and clinically isolated syndrome cases according to weight class
DISCUSSION
We found that childhood obesity is independently associated with an increased risk of pediatric onset MS/CIS in girls but not boys. The association between body size and pediatric MS/CIS was particularly pronounced in extremely obese adolescent girls. Consistent with our previous study,1 black (but not Hispanic) race/ethnicity and female sex were also associated with an increased risk of pediatric MS/CIS, even though childhood obesity was more common in black and Hispanic boys in our cohort. Our findings suggest that the childhood obesity epidemic is likely to lead to increased morbidity from MS/CIS, particularly in adolescent girls.
Our finding of increased risk of MS/CIS in obese adolescent girls but not in the younger age group is consistent with adult studies that found a 2-fold increased risk of adult-onset MS in women who reported moderate obesity during late adolescence3,4 but not with moderate obesity at ages 5 and 103. However, our finding that obese boys are not at increased risk of pediatric-onset MS/CIS is inconsistent with a previous report.4 That study found an increased prevalence of self-reported moderate obesity at age 20 among men with MS compared with volunteer controls (16/436 and 20/942 moderately obese men among cases and controls, respectively; OR 2.1, 95% CI 1.0–4.3).4 The most likely explanation for this discrepancy is that obesity in boys increases the risk of MS/CIS but with symptom onset delayed into adulthood.
It is also possible that sex differences of childhood obesity in the age at onset and influence on puberty may contribute to our findings. In girls, the peak age at onset of extreme obesity is 12,5 and has been attributed to a decline in physical activity during puberty.17 In boys, the peak age is earlier, at 10 years,5 and the reason is unclear. Prepubescent obesity may accelerate the age at menarche in girls, although this is controversial,18 yet delays age at puberty in boys.19 These factors should be addressed in future studies.
The increased risk of MS/CIS in moderately and extremely obese girls but not boys suggests that interactions between childhood obesity and female hormonal factors or the X chromosome may be contributing to the rising female-to-male ratio in MS.20 It is well-known that obesity is a low-grade inflammatory state in adults21 and children22 and potentially associated with increased estrogen levels in both sexes19 and lower androgen levels in males.19 We speculate that the rapid rise and high estrogenic exposure of obese, peripubescent girls in combination with the inflammatory mediators released by adipose tissue accelerate CIS/MS onset into adolescence. In obese boys, we speculate that the excess estrogenic exposure in a setting of lowered androgens takes longer to accumulate (and never approximates the estrogenic load seen in extremely obese girls), leading to increased lifetime risk of MS/CIS but with symptom onset delayed into adulthood. These hypotheses should be addressed in prospective cohort studies of children followed into adulthood.
It seems unlikely that other proposed hypotheses to explain the increased female-to-male ratio of MS in adults, like declining parity among women23 or tobacco smoke among men,24 can explain our findings. Parity in children is rare25 (and pregnant girls were excluded from our study), as is cigarette smoking. Also, obese males tend to smoke more than normal weight males,26 so if a factor, should have resulted in detecting an increased risk in obese males. Additionally, secondhand smoke exposure is an unlikely explanation as it does not differ by sex among children.27 However, a limitation of this study is that we did not measure these factors, which should be addressed in future studies.
That moderately and extremely obese children were more likely to present with symptoms of TM than normal/overweight children should be confirmed in future studies. It suggests that those caring for obese adolescents should pay careful attention in those presenting with sensory and motor symptoms referable to the spinal cord, although screening for TM in these at-risk individuals seems inefficient, given the rarity of pediatric MS/CIS.
Pediatric MS/CIS is rare, particularly in prepubertal age children. In fact, the primary limitation of this study is the small sample size, particularly of very young MS/CIS cases and males. It is possible that we may have missed an association between obesity and MS/CIS in very young children and in males for this reason. Other limitations include the use of age cutoffs rather than Tanner stage to approximate puberty, and lack of follow-up into adulthood. Another limitation of this study is reliance on coding data to identify potentially incident cases. While we used a very sensitive search algorithm for demyelinating diseases and cases were validated by chart reviews, this method relies on clinicians' abilities to recognize that a patient's symptoms may be due to demyelinating disease. Thus our estimates are conservative and likely represent an underestimation of the true risk of childhood obesity and lifetime risk of MS/CIS. Strengths of this study include the large number of children in the base population, particularly moderately and extremely obese ones, and the accuracy of the body size data.
The results of this study provide risk estimates that clearly show a strong association between increasing weight class and the risk of pediatric MS/CIS. These findings suggest that the risk of pediatric MS/CIS is highest among moderately and extremely obese teenage girls, implying that the incidence of pediatric MS/CIS is likely to increase as the childhood obesity epidemic continues.
GLOSSARY
- ADS
acquired demyelinating diseases
- BMI
body mass index
- CI
confidence intervals
- CIS
clinically isolated syndrome
- KPSC
Kaiser Permanente Southern California
- MS
multiple sclerosis
- ON
optic neuritis
- OR
odds ratios
- TM
transverse myelitis
AUTHOR CONTRIBUTIONS
Annette Magdalene Langer-Gould: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision, obtaining funding. Sonu Malik Brara: analysis or interpretation of data, acquisition of data. Brandon Emet Beaber: analysis or interpretation of data, acquisition of data. Corinna Koebnick: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision, obtaining funding.
STUDY FUNDING
Supported by the National Institute of Diabetes and Digestive and Kidney Disorders (NIDDK, R21DK085395, PI: Dr. Koebnick) and Kaiser Permanente Direct Community Benefit Funds. Neither funding source had any role in study design, data collection, analyses, manuscript preparation, or decision to submit results for publication.
DISCLOSURE
A. Langer-Gould is site principal investigator for 2 industry-sponsored phase 3 clinical trials (Biogen Idec; Hoffman-LaRoche). The other authors report no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Langer-Gould A, Zhang JL, Chung J, Yeung Y, Waubant E, Yao J. Incidence of acquired CNS demyelinating syndromes in a multiethnic cohort of children. Neurology 2011;77:1143–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calder PC, Ahluwalia N, Brouns F, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr 2011;106(suppl 3):S5–S78 [DOI] [PubMed] [Google Scholar]
- 3.Munger KL, Chitnis T, Ascherio A. Body size and risk of MS in two cohorts of US women. Neurology 2009;73:1543–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedstrom AK, Olsson T, Alfredsson L. High body mass index before age 20 is associated with increased risk for multiple sclerosis in both men and women. Mult Scler 2012;18:1334–1336 [DOI] [PubMed] [Google Scholar]
- 5.Koebnick C, Smith N, Coleman KJ, et al. Prevalence of extreme obesity in a multiethnic cohort of children and adolescents. J Pediatr 2010;157:26–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krupp LB, Banwell B, Tenembaum S. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology 2007;68:S7–S12 [DOI] [PubMed] [Google Scholar]
- 7.Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology 2002;59:499–505 [DOI] [PubMed] [Google Scholar]
- 8.Jacob A, Weinshenker BG. An approach to the diagnosis of acute transverse myelitis. Semin Neurol 2008;28:105–120 [DOI] [PubMed] [Google Scholar]
- 9.Smith N, Coleman KJ, Lawrence JM, et al. Body weight and height data in electronic medical records of children. Int J Pediatr Obes 2010;5:237–242 [DOI] [PubMed] [Google Scholar]
- 10.Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, Curtin LR. Characterizing extreme values of body mass index-for-age by using the 2000 Centers for Disease Control and Prevention growth charts. Am J Clin Nutr 2009;90:1314–1320 [DOI] [PubMed] [Google Scholar]
- 11.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 2002:1–190 [PubMed] [Google Scholar]
- 12.Organization WH Obesity: Preventing and Managing the Global Epidemic. Geneva: World Health Organization; 2000 [PubMed] [Google Scholar]
- 13.Brara SM, Koebnick C, Porter AH, Langer-Gould A. Pediatric idiopathic intracranial hypertension and extreme childhood obesity. J Pediatr 2012;161:602–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiscella K, Fremont AM. Use of geocoding and surname analysis to estimate race and ethnicity. Health Serv Res 2006;41:1482–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Word DL, Perkins RC. Building a Spanish Surname List for the 1990s: A New Approach to an Old Problem. Washington, DC: US Bureau of the Census; 1996 [Google Scholar]
- 16.Bureau of the Census Census 2000 Surname List. Washington, DC: Bureau of Census; 2009 [Google Scholar]
- 17.Kimm SY, Barton BA, Obarzanek E, et al. Racial divergence in adiposity during adolescence: the NHLBI Growth and Health Study. Pediatrics 2001;107:E34. [DOI] [PubMed] [Google Scholar]
- 18.Tolson KP, Chappell PE. The changes they are a-timed: metabolism, endogenous clocks, and the timing of puberty. Front Endocrinol 2012;3:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction 2010;140:399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koch-Henriksen N, Sorensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol 2010;9:520–532 [DOI] [PubMed] [Google Scholar]
- 21.Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol 2009;6:399–409 [DOI] [PubMed] [Google Scholar]
- 22.Tam CS, Clement K, Baur LA, Tordjman J. Obesity and low-grade inflammation: a paediatric perspective. Obes Rev 2010;11:118–126 [DOI] [PubMed] [Google Scholar]
- 23.Ponsonby AL, Lucas RM, van der Mei IA, et al. Offspring number, pregnancy, and risk of a first clinical demyelinating event: the AusImmune Study. Neurology 2012;78:867–874 [DOI] [PubMed] [Google Scholar]
- 24.Palacios N, Alonso A, Bronnum-Hansen H, Ascherio A. Smoking and increased risk of multiple sclerosis: parallel trends in the sex ratio reinforce the evidence. Ann Epidemiol 2011;21:536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamilton BE, Ventura SJ. Birth rates for U.S. teenagers reach historic lows for all age and ethnic groups. NCHS Data Brief 2012:1–8 [PubMed] [Google Scholar]
- 26.Quinn VP, Jacobsen SJ, Slezak JM, et al. Preventive care and health behaviors among overweight/obese men in HMOs. Am J Manag Care 2012;18:25–32 [PubMed] [Google Scholar]
- 27.Kabir Z, Connolly GN, Alpert HR. Secondhand smoke exposure and neurobehavioral disorders among children in the United States. Pediatrics 2011;128:263–270 [DOI] [PubMed] [Google Scholar]


