Abstract
Objective:
Women have worse outcomes after stroke compared to men. Since women have lower hemoglobin values, we examined whether hemoglobin levels may associate with worse stroke outcomes in women.
Methods:
We retrospectively studied 274 patients enrolled in a prospective multicenter study. We explored the relationship of hemoglobin with clinical outcome at 6 months, as measured by the modified Rankin Scale (mRS). Ordinal logistic regression was used to evaluate the independent effect of hemoglobin on clinical outcome, and to explore the influence of sex on that association.
Results:
Women had a lower mean hemoglobin level (11.7 ± 1.8 g/dL) compared to men (13.3 ± 1.7 g/dL). Low hemoglobin was associated with worse 6-month mRS outcomes in univariate analysis (p < 0.001). Lower hemoglobin remained independently associated with poor outcome after adjustment for comorbid disease, stroke severity, age, and sex. The inclusion of hemoglobin in the model attenuated the independent effect of sex on outcome.
Conclusions:
Sex differences in stroke outcome are linked to lower hemoglobin level, which is more prevalent in women. Further examination of this potentially modifiable predictor is warranted.
Women who have a stroke tend to recover less well than men,1 resulting in greater dependency, institutionalization,2–4 and poorer quality of life.5 Several reasons for the disparity have been investigated, including sex differences in clinical presentation and resource use,2,6 in acute stroke care,2,7 and biological divergence related to sex steroid hormones.1 Women with stroke are also older, which may account for some of the observed differences.8,9 In the context of an expanding elderly population, it is expected that the proportion of women with stroke will further increase,9 which amplifies the urgency of the problem.
Women also have a lower hemoglobin level than men, and previous data have shown that hemoglobin level correlates with worse neuroimaging surrogate outcomes.10 This raises the possibility that a portion of the worse outcome may be mediated by hemoglobin level. The purpose of this study was to assess whether the difference in hemoglobin level between men and women was associated with neurologic outcome, and whether this might account for a proportion of the sex difference in stroke recovery previously observed.
METHODS
Study population.
We retrospectively analyzed the clinical data collected at a single center, as part of a prospective multicenter study evaluating the utility of new CT-based neuroimaging technology to improve prediction of stroke subtype and outcome (Screening Technology and Outcome Project in Stroke [STOPStroke] Study). The STOPStroke Study enrolled consecutive patients who were evaluated by multimodal CT (noncontrast CT, CT angiography, CT perfusion) within 24 hours of symptom onset for symptoms consistent with an acute ischemic stroke. Clinical outcome status was determined using the modified Rankin Scale (mRS) obtained by telephone interview at 6 months. The study started in March 2003 and follow-up was completed in July 2006. For the present analysis, patients were eligible if they had a discharge diagnosis of stroke, had a 6-month mRS recorded, and were not treated with thrombolytic or investigational treatments. Patients with prestroke disability (mRS of >2) were excluded. All patients underwent a standardized evaluation of rehabilitation needs by the same team of physical, occupational, and speech therapists, and were given an individualized rehabilitation treatment plan before discharge.
Data collection.
Admission NIH Stroke Scale (NIHSS) score, prestroke disability, and clinical outcome at 6 months as defined by mRS were collected prospectively. Clinical characteristics including time of stroke onset (defined as last time the patient was known to be well) and demographic and medical information were ascertained for each patient through retrospective chart review. The hemoglobin level during hospitalization was abstracted retrospectively and the nadir used for analysis. Analysis was also performed using mean hemoglobin level and hemoglobin dichotomized into anemia, based on the WHO sex-specific definitions.11 The Charlson comorbidity index was calculated12 based on review of the medical record and then dichotomized around the median score. We classified stroke etiology using the Causative Classification of Stroke system.13 For a subset of patients who had the requisite laboratory values available (n = 80), an estimate of whole blood viscosity was calculated based on fibrinogen and hematocrit, using the yield shear stress (YSS) formula, YSS = 13.5 (10−6) Cf2 (Hct-6)3, where Cf is the fibrinogen concentration in gm/dL.14,15
Statistical analysis.
Differences in clinical and laboratory variables according to sex were compared using Student t, Wilcoxon rank-sum, or Fisher exact test, as appropriate. Differences in clinical and laboratory variables according to mRS score were tested using one-way analysis of variance, Kruskal-Wallis, or χ2 test, as appropriate. Because there were only 4 subjects with an mRS of 5, this group was combined with mRS 4 for all statistical analyses. Similar results were obtained in analyses when these subjects were combined with mRS of 6.
Ordinal logistic (ordered logit) regression analysis was performed with mRS as the dependent variable and the above clinical characteristics as independent variables. Models with and without hemoglobin were developed to determine whether there was attenuation of the effect of sex on outcome. The percent change in the β coefficient when hemoglobin was added was determined by log(ORunadjusted) − log(ORadjusted)/log(ORunadjusted) × 100%. NIHSS score was log-transformed prior to addition into the model. All numeric variables were expressed as mean ± SD or median and interquartile range (IQR). A level of p < 0.05 was considered statistically significant. Statistical analyses were performed using JMP 9.0 Pro and STATA statistical software (release 12, College Station, TX).
Standard protocol approvals, registrations, and patient consents.
The local institutional review board approved all aspects of the study.
RESULTS
The STOPStroke Study enrolled a total of 741 patients (580 patients at our institution) during the study period. We excluded patients who did not have a discharge diagnosis of stroke (136 patients) and those who were treated with intra-arterial thrombolysis (31), IV thrombolysis (70), or investigational treatments (8). Forty-one of the remaining subjects were lost to follow-up and 20 patients were excluded due to a prestroke mRS score >2. The remaining 274 patients comprised the final study population.
The clinical characteristics of the study cohort are presented in table 1, separated by sex. Compared to men, women were older by an average of 8 years (p < 0.001) and were significantly more likely to have a lower mean hemoglobin, by an average of 1.6 g/dL (p < 0.001). Women were also less like to have coronary artery disease (p < 0.01). At 6 months, women had worse median functional recovery (p < 0.001), which is consistent with previous reports.2,3,8
Table 1.
Clinical characteristics by sex
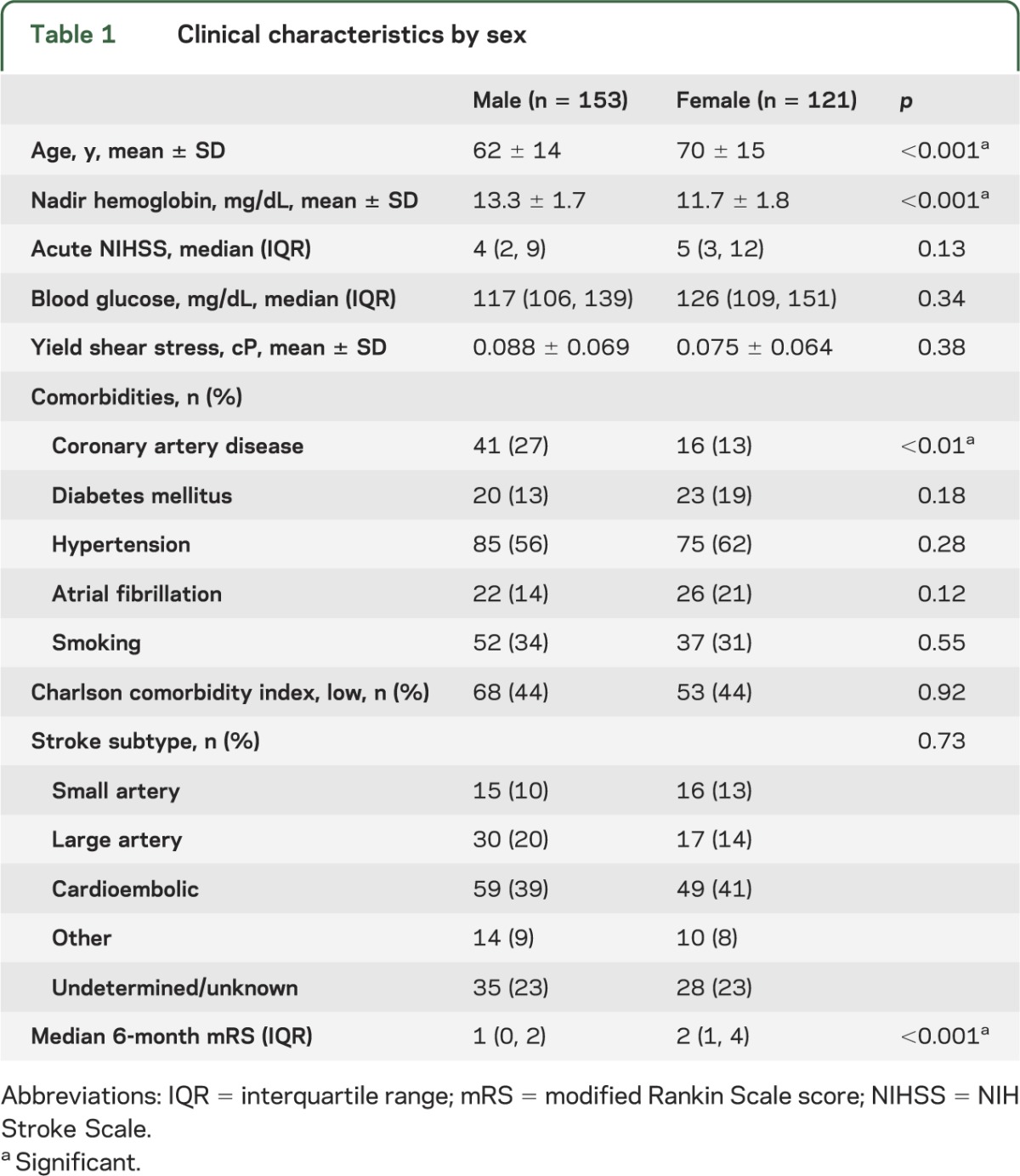
Table 2 shows the relationship of clinical characteristics to 6-month outcome, defined by the mRS score. Increased age was associated with more severe functional deficits (p < 0.001).16–18 The NIHSS also predicted 6-month outcome (p < 0.001), as did lower hemoglobin level (p < 0.001). Dichotomization of hemoglobin into WHO-defined anemia11 or mean hemoglobin yielded a similar relationship to outcome (p < 0.001 for both analyses). Women had a worse outcome compared to men (p < 0.01). Elevated blood glucose was associated with worse outcome (p < 0.01).19–21 Viscosity, which is dependent upon hematocrit and fibrinogen, has been reported to be inversely associated with cerebral blood flow,14,22 although not with neurologic outcome. In the subset of subjects in whom fibrinogen level was available (n = 80), there was no association between calculated viscosity (YSS15) and neurologic outcome.
Table 2.
Distribution of clinical characteristics according to outcomea
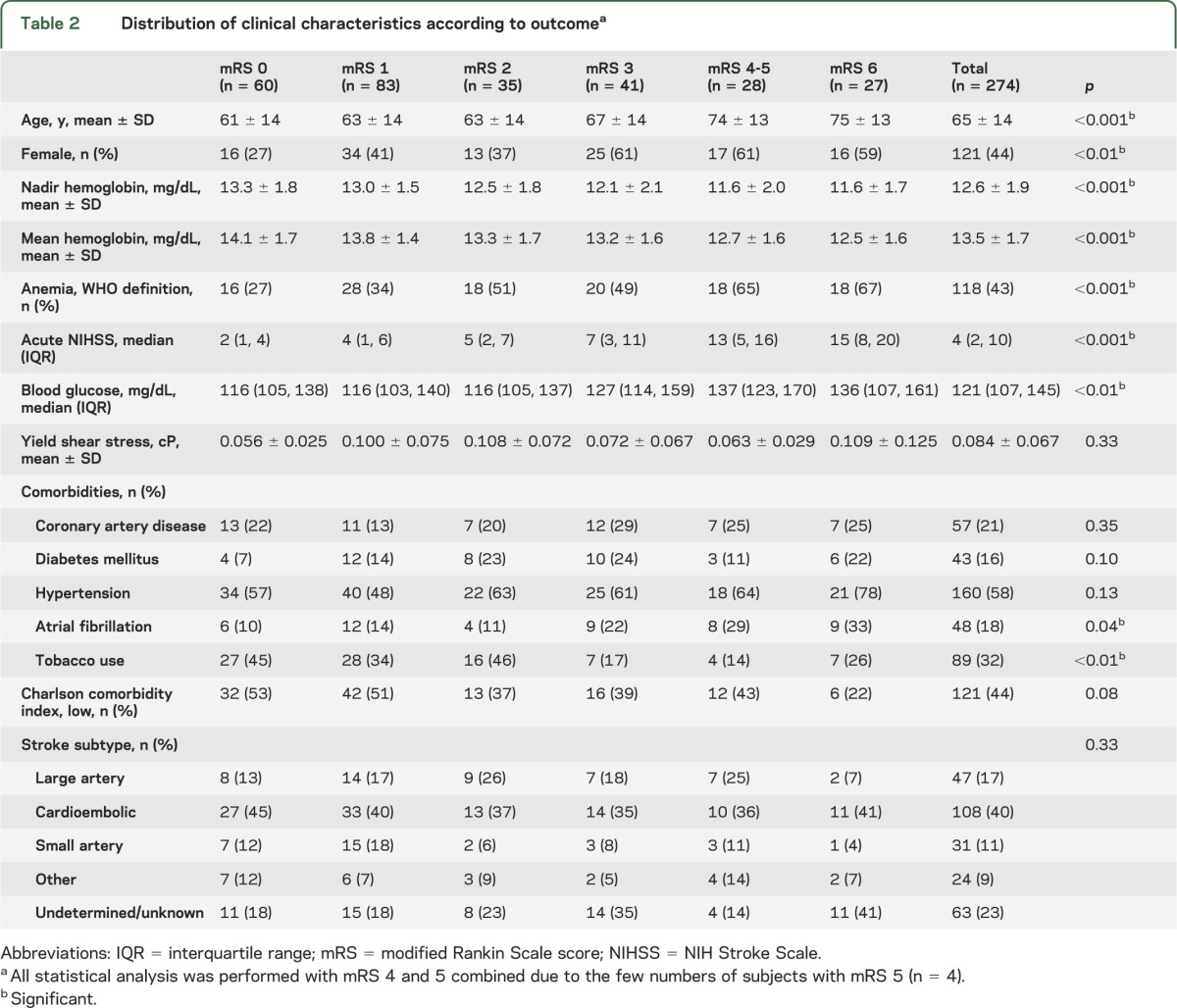
We also evaluated common comorbidities and the relationship to outcome (table 2), and found that higher rates of atrial fibrillation predicted worse recovery (p = 0.04). On the other hand, tobacco use was associated with better outcome (p < 0.01). Other individual comorbidities, a composite index of comorbidity (Charlson index), or stroke subtype were not associated with neurologic outcome.
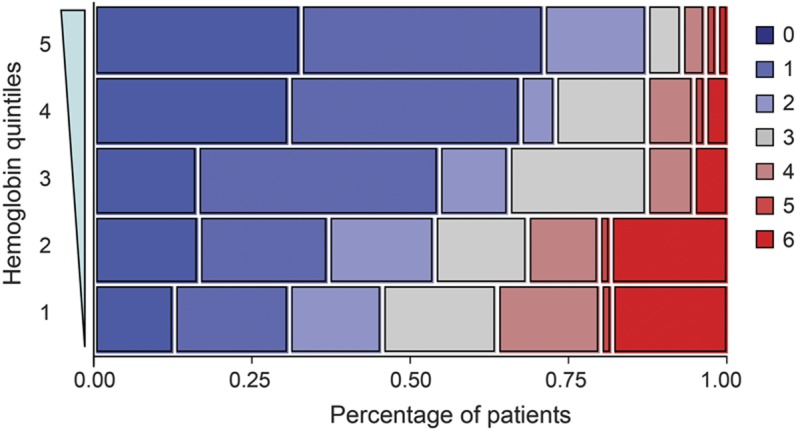
The figure graphically shows the relationship between neurologic recovery and hemoglobin divided into quintiles. The quintile with the lowest hemoglobin (9.7 g/dL ± 1.0) had a median mRS of 3 (IQR 1, 4), whereas the highest hemoglobin quintile (15.0 g/dL ± 0.5) had a median mRS of 1 (IQR 0, 2) (p <0.001, n = 55 for each quintile). The figure reveals a stepwise, dose-dependent relationship between hemoglobin quintile and functional recovery (p < 0.001). Prior studies have reported a nonlinear U-shaped relationship between admission hematocrit and outcome,23,24 whereas others did not.25 We explored this possibility in our cohort by assessing for a quadratic relationship between hemoglobin and outcome, but did not find one (p = 0.45).
Figure. Ranges of modified Rankin Scale are shown for each hemoglobin quintile (n = 55).
The independent relationship between hemoglobin and outcome was explored in multivariable ordinal logistic regression (table 3). We first modeled outcome using predictors that were significant in univariate analysis (model A). There was an independent association of hemoglobin with functional recovery (p < 0.01), with an adjusted odds ratio (OR) of 0.83 (95% confidence interval [CI] 0.73–0.96) for mRS with each increase in hemoglobin of 1 g/dL. Other independent predictors included age, NIHSS score, blood glucose, and tobacco use. Female sex was not an independent predictor in this model. We obtained similar results when we substituted mean hemoglobin or anemia into the model, although mean hemoglobin exhibited a trend toward significance (p = 0.06).
Table 3.
Predictors of 6-month modified Rankin Scale scorea
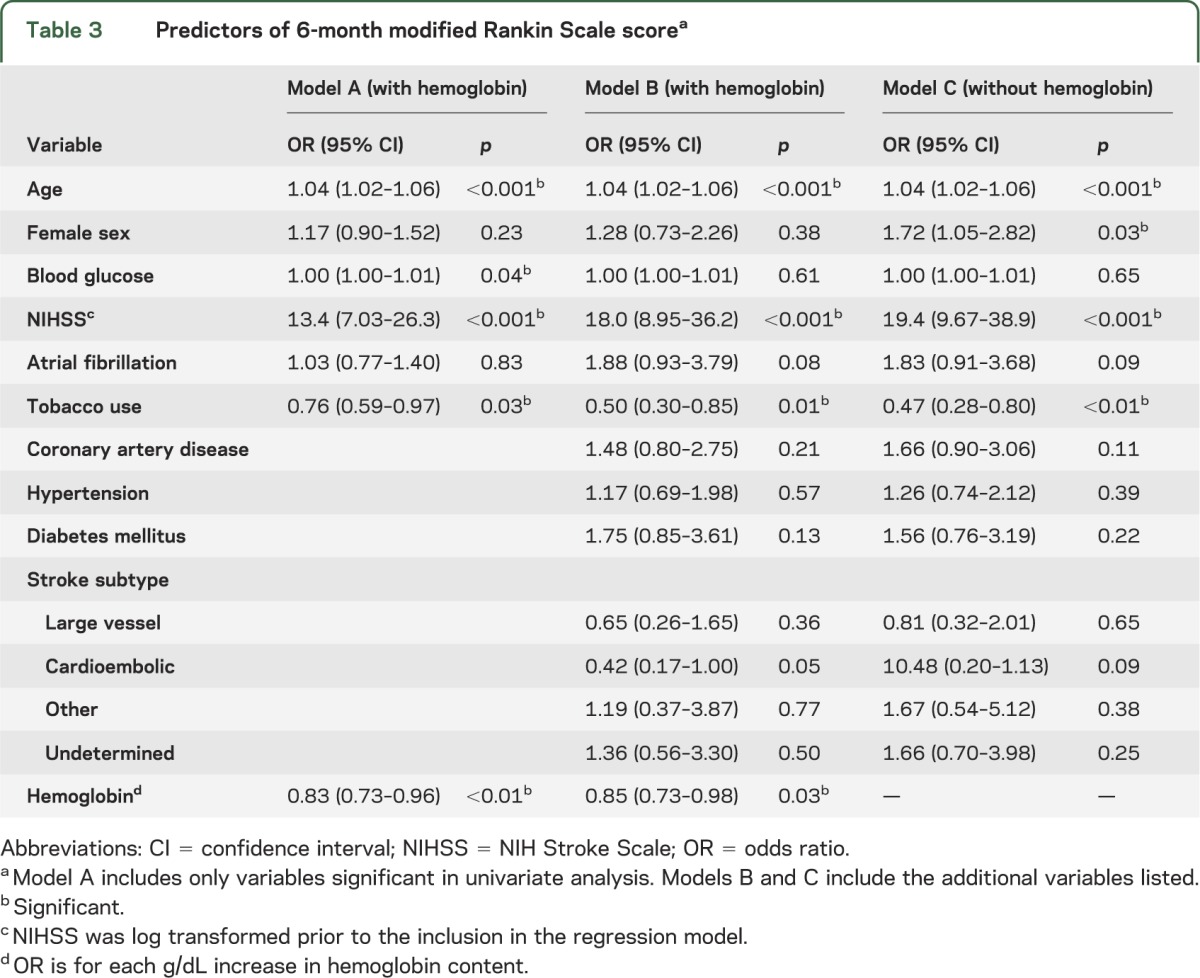
Because hemoglobin might represent a surrogate for comorbid illness, we included all past medical history variables from table 2 and stroke subtype (model B, table 3). Addition of these variables did not alter the independent effect of hemoglobin on outcome (p = 0.03), nor the ORs. Similarly, the inclusion of the Charlson comorbidity index (rather than individual past medical history) had no difference in effect. In model B, female sex was not a predictor of outcome (p = 0.38).
However, when hemoglobin was withheld from the model (model C, table 3), female sex became a predictor of clinical outcome (p = 0.03) with an adjusted OR of 1.72 (95% CI 1.05–2.82). The relative change in the OR for sex after adjustment for hemoglobin was 55%, suggesting an effect of hemoglobin on the sex effect.9 Age is also recognized as a confounder for the effect of sex on outcome.8 For illustrative comparison, when age rather than hemoglobin was withheld from the model, female sex had an adjusted OR of 1.70 (p = 0.051, 95% CI 0.99–2.93), which corresponds to similar magnitude of change (54%).
In order to explore the effect of hemoglobin and sex in greater detail, we added the interaction term of hemoglobin × sex into the final model. This addition was not significant (p = 0.30), nor did it alter the ORs of either term individually. Next, we reasoned that males with low hemoglobin might also have worse outcome, even though this may represent a smaller subset of all subjects. Subgroup analysis of male subjects demonstrated a univariate association of low hemoglobin with worse outcome (p < 0.01), which remained so in the multivariable model (p = 0.03).
DISCUSSION
The results from this study show that hemoglobin level is inversely associated with neurologic outcome after stroke. This association remains significant after adjusting for age, NIHSS score, blood glucose, stroke subtype, and several common comorbidities. Most intriguingly, our data demonstrate that the well-described effect of female sex on outcome is significantly attenuated after incorporation of hemoglobin level in the model.
Investigation into sex differences in stroke has identified a host of potential contributing factors.1 Not only do women have a greater incidence of stroke, but they tend to be older at the time of presentation.8 They also tend to have a higher prevalence of comorbid disease such as atrial fibrillation, and are less likely to receive acute treatment with alteplase.1 While some of these observations may reflect the consequence of older age at presentation for women,8 it is unlikely to account for the entirety of the difference.9 For example, there is some evidence that underlying biological reasons may also play a role, including the differential effect of estrogen on cerebrovascular reactivity and its putative role as a neuroprotectant.26,27 Our data herein raise another possible mechanism to account for sex differences in stroke outcome. As the major carrier of oxygen, variation in hemoglobin level may influence the degree of recovery in the injured ischemic brain. In turn, this may lead to reduced functional recovery. Intriguingly, in subgroup analysis only, we find hemoglobin is also associated with outcome in males, suggesting that the phenomenon is not limited to females but disproportionately affects them.
A potential confounder to the putative effect of hemoglobin is its association with comorbid disease, which may leverage an indirect penalty on neurologic recovery. We attempted to address this concern by including individual comorbidities and the Charlson comorbidity index into our model, and neither of these variables altered the independent effect of hemoglobin. Nevertheless, other unidentified comorbid factors may underlie the association that we observed with hemoglobin. On the other hand, the fact that sex-based variation itself correlates with outcome suggests that hemoglobin's effect may be independent of comorbid disease. Of course, our data do not address the question of a causative role for hemoglobin, which would require direct manipulation in a model system. Alternatively, Mendelian randomization analysis28 of hemoglobin single nucleotide polymorphisms (SNPs) could potentially provide support for a direct influence on stroke outcome.
Prior studies evaluating red blood cell physiology in stroke have focused on high hematocrit level and its effect on hemorheology.29 In patients, viscosity has been reported to be inversely associated with cerebral blood flow,14,30 but it is less clear how that affects overall net cerebral oxygen delivery.31 The role of viscosity is further clouded by negative trials of hemodilution.32 We explored the effect of viscosity on neurologic outcome in a subset of subjects with available laboratory values; however, we did not find a significant association. Some studies have identified a U-shaped relationship between hematocrit and outcome23; others have not.25 Our data did not identify a U-shaped relationship, although some of the differences may be due to differences in the timing of blood draw. On the other hand, we note that our cohort did not contain subjects with a highly elevated hematocrit (i.e., ∼50%), which may be the population most susceptible to poor outcome. Further studies are necessary to sort out the pathophysiologic mechanism that may account for our findings.
There are limitations to our analysis. This study was prospective and required informed consent for participation, and therefore might be biased toward patients with less severe stroke (median NIHSS 5). The study also used 6-month outcome by phone, which may also bias toward less severe stroke, limiting the generalizability of our findings in severe stroke populations. Secondly, although we adjusted for potential confounding comorbidities, it remains possible that we could not account for unrecognized ones that may influence the effect of hemoglobin on outcome.
Our data demonstrate that lower hemoglobin is independently associated with worse neurologic outcome and hemoglobin attenuates the independent effect of female sex on outcome. As the key oxygen-carrying molecule in the body, hemoglobin may play a direct or indirect role in influencing brain recovery and neurologic function. The association between female sex and hemoglobin identifies a new dimension to understanding why women tend to experience worse outcome after stroke. Future studies that explore sex differences should include evaluation of the role of hemoglobin.
GLOSSARY
- CI
confidence interval
- IQR
interquartile range
- mRS
modified Rankin Scale
- OR
odds ratio
- SNP
single nucleotide polymorphism
- STOPStroke
Screening Technology and Outcome Project in Stroke
- YSS
yield shear stress
AUTHOR CONTRIBUTIONS
Dr. Kimberly: study concept and design, acquisition of data, analysis and interpretation, statistical analysis, drafting of the manuscript. Dr. Lima: acquisition of data, analysis and interpretation, critical revision of the manuscript. Ms. O’Connor: drafting and critical revision of the manuscript. Dr. Furie: analysis and interpretation of data, critical revision of the data, study supervision.
STUDY FUNDING
Supported by American Academy of Neurology Foundation clinical training research fellowship (W.T.K.), the Clinical Investigator Training Program: Beth Israel Deaconess Medical Center–Harvard Medical School, in collaboration with Pfizer Inc. and Merck & Co. (W.T.K.), and by NIH 1K23NS076597 (W.T.K.) and NIH 5P50NS051343-07 and 1R01 NS064905-01A1 (K.L.F.). The original STOPStroke study was sponsored by R01HS011392-01A2.
DISCLOSURE
W.T. Kimberly reports funding from American Academy of Neurology Foundation clinical training research fellowship, the Clinical Investigator Training Program: Beth Israel Deaconess Medical Center–Harvard Medical School, in collaboration with Pfizer Inc. and Merck & Co., and by NIH 1K23NS076597-01. F. Lima reports a fellowship funded by 5P50 NS051343-07. S. O’Connor reports no disclosures. K. Furie reports funding by 5P50 NS051343-07 and 1R01 NS064905-01A1. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Reeves MJ, Bushnell CD, Howard G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol 2008;7:915–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Carlo A, Lamassa M, Baldereschi M, et al. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke 2003;34:1114–1119 [DOI] [PubMed] [Google Scholar]
- 3.Kapral MK, Fang J, Hill MD, et al. Sex differences in stroke care and outcomes: results from the Registry of the Canadian Stroke Network. Stroke 2005;36:809–814 [DOI] [PubMed] [Google Scholar]
- 4.Lai SM, Duncan PW, Dew P, Keighley J. Sex differences in stroke recovery. Prev Chronic Dis 2005;2:A13. [PMC free article] [PubMed] [Google Scholar]
- 5.Gargano JW, Reeves MJ. Sex differences in stroke recovery and stroke-specific quality of life: results from a statewide stroke registry. Stroke 2007;38:2541–2548 [DOI] [PubMed] [Google Scholar]
- 6.Holroyd-Leduc JM, Kapral MK, Austin PC, Tu JV. Sex differences and similarities in the management and outcome of stroke patients. Stroke 2000;31:1833–1837 [DOI] [PubMed] [Google Scholar]
- 7.Smith MA, Lisabeth LD, Brown DL, Morgenstern LB. Gender comparisons of diagnostic evaluation for ischemic stroke patients. Neurology 2005;65:855–858 [DOI] [PubMed] [Google Scholar]
- 8.Gall SL, Donnan G, Dewey HM, et al. Sex differences in presentation, severity, and management of stroke in a population-based study. Neurology 2010;74:975–981 [DOI] [PubMed] [Google Scholar]
- 9.Reeves MJ, Lisabeth LD. The confounding issue of sex and stroke. Neurology 2010;74:947–948 [DOI] [PubMed] [Google Scholar]
- 10.Kimberly WT, Wu O, Arsava EM, et al. Lower hemoglobin correlates with larger stroke volumes in acute ischemic stroke. Cerebrovasc Dis Extra 2011;1:44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nutritional anaemias: report of a WHO scientific group. World Health Organ Tech Rep Ser 1968;405:5–37 [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383 [DOI] [PubMed] [Google Scholar]
- 13.Ay H, Benner T, Arsava EM, et al. A computerized algorithm for etiologic classification of ischemic stroke: the Causative Classification of Stroke System. Stroke 2007;38:2979–2984 [DOI] [PubMed] [Google Scholar]
- 14.Grotta J, Ackerman R, Correia J, Fallick G, Chang J. Whole blood viscosity parameters and cerebral blood flow. Stroke 1982;13:296–301 [DOI] [PubMed] [Google Scholar]
- 15.Merrill EW, Cheng CS, Pelletier GA. Yield stress of normal human blood as a function of endogenous fibrinogen. J Appl Physiol 1969;26:1–3 [DOI] [PubMed] [Google Scholar]
- 16.Macciocchi SN, Diamond PT, Alves WM, Mertz T. Ischemic stroke: relation of age, lesion location, and initial neurologic deficit to functional outcome. Arch Phys Med Rehabil 1998;79:1255–1257 [DOI] [PubMed] [Google Scholar]
- 17.Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. The influence of age on stroke outcome: The Copenhagen Stroke Study. Stroke 1994;25:808–813 [DOI] [PubMed] [Google Scholar]
- 18.Weimar C, Konig IR, Kraywinkel K, Ziegler A, Diener HC. Age and National Institutes of Health Stroke Scale Score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: development and external validation of prognostic models. Stroke 2004;35:158–162 [DOI] [PubMed] [Google Scholar]
- 19.Weir CJ, Murray GD, Dyker AG, Lees KR. Is hyperglycaemia an independent predictor of poor outcome after acute stroke? Results of a long-term follow up study. BMJ 1997;314:1303–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott JF, Robinson GM, French JM, O'Connell JE, Alberti KG, Gray CS. Prevalence of admission hyperglycaemia across clinical subtypes of acute stroke. Lancet 1999;353:376–377 [DOI] [PubMed] [Google Scholar]
- 21.Bruno A, Biller J, Adams HP, Jr, et al. Acute blood glucose level and outcome from ischemic stroke: Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. Neurology 1999;52:280–284 [DOI] [PubMed] [Google Scholar]
- 22.Grotta J, Ostrow P, Fraifeld E, Hartman D, Gary H. Fibrinogen, blood viscosity, and cerebral ischemia. Stroke 1985;16:192–198 [DOI] [PubMed] [Google Scholar]
- 23.Tanne D, Molshatzki N, Merzeliak O, Tsabari R, Toashi M, Schwammenthal Y. Anemia status, hemoglobin concentration and outcome after acute stroke: a cohort study. BMC Neurol 2010;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diamond PT, Gale SD, Evans BA. Relationship of initial hematocrit level to discharge destination and resource utilization after ischemic stroke: a pilot study. Arch Phys Med Rehabil 2003;84:964–967 [DOI] [PubMed] [Google Scholar]
- 25.Kellert L, Martin E, Sykora M, et al. Cerebral oxygen transport failure? Decreasing hemoglobin and hematocrit levels after ischemic stroke predict poor outcome and mortality: STroke: RelevAnt Impact of hemoGlobin, Hematocrit and Transfusion (STRAIGHT): an observational study. Stroke 2011;42:2832–2837 [DOI] [PubMed] [Google Scholar]
- 26.McCullough LD, Alkayed NJ, Traystman RJ, Williams MJ, Hurn PD. Postischemic estrogen reduces hypoperfusion and secondary ischemia after experimental stroke. Stroke 2001;32:796–802 [DOI] [PubMed] [Google Scholar]
- 27.Krause DN, Duckles SP, Pelligrino DA. Influence of sex steroid hormones on cerebrovascular function. J Appl Physiol 2006;101:1252–1261 [DOI] [PubMed] [Google Scholar]
- 28.Ebrahim S, Davey Smith G. Mendelian randomization: can genetic epidemiology help redress the failures of observational epidemiology? Hum Genet 2008;123:15–33 [DOI] [PubMed] [Google Scholar]
- 29.Wood JH, Kee DB., Jr Hemorheology of the cerebral circulation in stroke. Stroke 1985;16:765–772 [DOI] [PubMed] [Google Scholar]
- 30.Kowal P, Marcinkowska-Gapinska A. Hemorheological changes dependent on the time from the onset of ischemic stroke. J Neurol Sci 2007;258:132–136 [DOI] [PubMed] [Google Scholar]
- 31.Kramer AH, Zygun DA. Anemia and red blood cell transfusion in neurocritical care. Crit Care 2009;13:R89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asplund K. Haemodilution for acute ischaemic stroke. Cochrane Database Syst Rev 2002;4:CD000103 [DOI] [PubMed] [Google Scholar]


