Abstract
Objective:
In a retrospective review to assess neuroanatomical targets of radiation-induced cognitive decline, dose volume histogram (DVH) analyses of specific brain regions of interest (ROI) are correlated to neurocognitive performance in 57 primary brain tumor survivors.
Methods:
Neurocognitive assessment at baseline included Trail Making Tests A/B, a modified Rey-Osterreith Complex Figure, California or Hopkins Verbal Learning Test, Digit Span, and Controlled Oral Word Association. DVH analysis was performed for multiple neuroanatomical targets considered to be involved in cognition. The %v10 (percent of ROI receiving 10 Gy), %v40, and %v60 were calculated for each ROI. Factor analysis was used to estimate global cognition based on a summary of performance on individual cognitive tests. Stepwise regression was used to determine which dose volume predicted performance on global factors and individual neurocognitive tests for each ROI.
Results:
Regions that predicted global cognitive outcomes at doses <60 Gy included the corpus callosum, left frontal white matter, right temporal lobe, bilateral hippocampi, subventricular zone, and cerebellum. Regions of adult neurogenesis primarily predicted cognition at %v40 except for the right hippocampus which predicted at %v10. Regions that did not predict global cognitive outcomes at any dose include total brain volume, frontal pole, anterior cingulate, right frontal white matter, and the right precentral gyrus.
Conclusions:
Modeling of radiation-induced cognitive decline using neuroanatomical target theory appears to be feasible. A prospective trial is necessary to validate these data.
Models of radiation brain injury predict the likelihood of radionecrosis,1,2 similar to normal tissue complication probability (NTCP) models for the lung,3 liver,4 and kidneys.1 However, radiation-induced cognitive decline occurs at doses far lower than what causes radionecrosis,5 making accurate predictive models elusive. Certain mechanisms contributing to radiation-induced brain injury (e.g., vascular injury,6,7 inflammation8) are, in part, a function of the dose delivered and volume of brain irradiated,9,10 yet others (e.g., progenitor cell depletion,11 disruption of brain connectivity, parallel processing reduction12) would be better explained by a model constituting functional neuroanatomical targets.
Here, we propose a neuroanatomical target theory where selective damage to specific targets can lead to cognitive impairment. Selective sparing of structures to radiation doses below a given threshold can potentially lead to improved cognitive outcome after brain irradiation. Hippocampal sparing during whole-brain irradiation (WBI) is being pursued in the Radiation Therapy Oncology Group (RTOG) 0933 prospective phase II trial,13 though limited clinical evidence exists on whether doses attainable by hippocampal-sparing WBI are sufficient to spare function.14
This retrospective study seeks to bridge putative mechanisms of radiation-induced cognitive decline and clinical radiation treatment planning by concentrating on 3 dose levels related to particular mechanistic features important to cognition. At 10 Gy, neural stem cells number is significantly reduced, and by 40 Gy white matter disease is prominent.15,16 The 60 Gy dose combined parameters of tumor location, risk of necrosis,1,2 and dose. We determined neuroanatomical target structures and what dose threshold predicted functional decline after receiving RT.
METHODS
Standard protocol approvals, registrations, and patient consents.
This retrospective review was approved by the Institutional Review Board at Wake Forest School of Medicine with a waiver of consent.
Patient population.
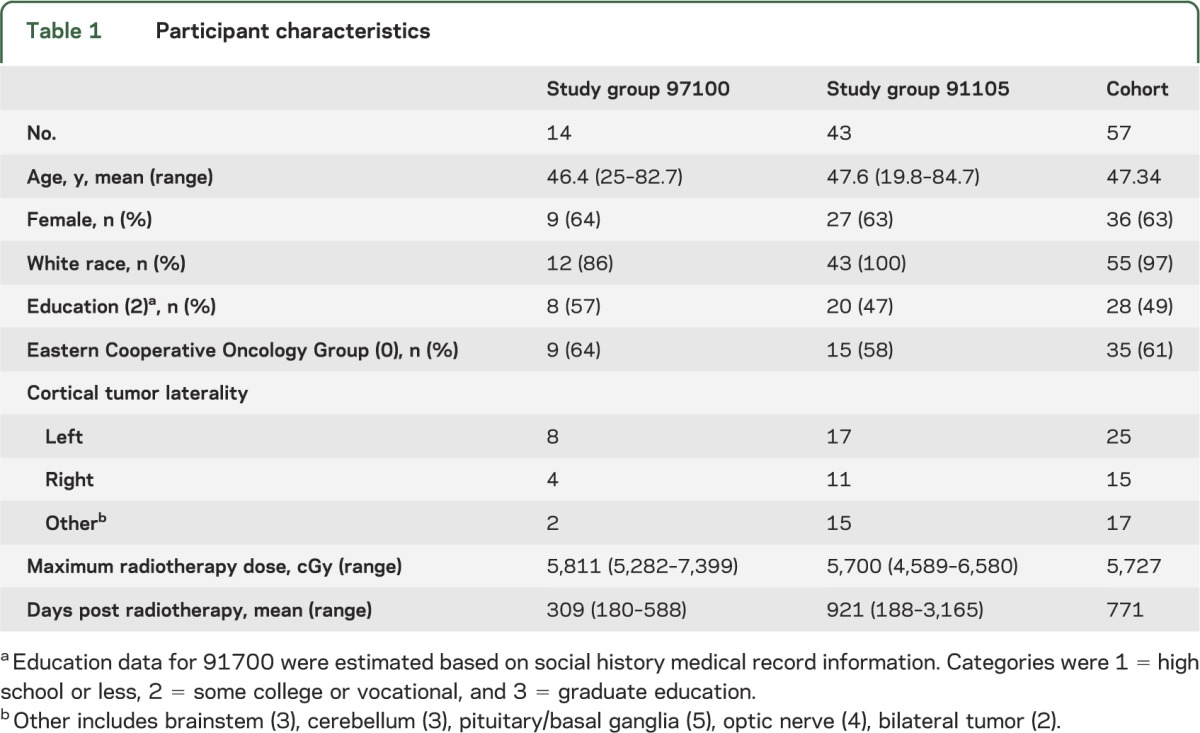
Participants were previously enrolled in a Wake Forest Community Clinical Oncology Program (CCOP) National Cancer Institute (NCI)–approved clinical trial (WFU 9710017 or 91105) assessing the use of donepezil (Aricept) in patients following radiotherapy (RT) to the brain. These trials treated patients with 24 weeks of either donepezil or placebo after a baseline neurocognitive battery that occurred ≥6 months after completion of brain irradiation. For the current study, only the pre-donepezil cognitive tests were used. Patients were included if they received conventionally fractionated (1.8–2.0 Gy/fraction) partial brain irradiation, or in the case of 2 patients, had undergone WBI with a regional boost field that created a dose gradient through the brain. From 148 potential participants, a total of 57 patients (7 glioblastoma, 11 anaplastic glioma, 4 primitive neuroectodermal tumors, and 35 benign or low-grade tumors) had an electronically recoverable treatment plan, met these criteria, and were included in this study (table 1).
Table 1.
Participant characteristics
Region of interest selection.
Selected anatomical regions of interest (ROI) represent potential functional targets of cognitive decline after RT (summarized in table e-1 on the Neurology® Web site at www.neurology.org). Briefly, the hippocampi and subventricular zone (SVZ) are regions of adult neurogenesis.11 Deficits in neurogenesis may influence cognitive outcome,18 and the hippocampus is an important structure for spatial and working memory.19 Temporal lobes were included based on data from nasopharyngeal cancer survivors, which suggest cognitive decline can be exhibited after exposure to clinical doses of radiation.20 We included the corpus callosum for its role in network connectivity of cerebral cortex,21 and thus its importance to cognition and processing speed.22 The frontal pole, anterior cingulate, and frontal white matter horn areas are associated with high-level task goals and attention, which are necessary for executive function.23 Precentral gyri and cerebellum, motor output regions,24 were also evaluated.
Region of interest delineation.
Prior to ROI delineation, the pretreatment MRI and treatment planning CT image sets were fused. Delineation and dose volume histogram (DVH) analysis of ROI were performed using the Pinnacle treatment planning system (Philips, Andover, MA). Single raters manually delineated specific ROIs on the MRI after passing intrarater reliability tests. Prior surgical resection limited delineation of some structures (left frontal, 1 participant; right frontal, 4 participants; right precentral gyrus, 1 participant), reducing the number of individuals used in that ROI analysis. ROI boundaries used outside standard anatomical landmarks are illustrated in the figure. Briefly, the frontal pole's posterior border was drawn anterior to the corpus callosum; the inferior border was superior to the orbit. A periventricular stem cell niche estimation was drawn along the caudate nucleus and ventricle. The frontal white matter horns were drawn between the inferior and superior slices of the genu of the corpus callosum. The posterior border of the anterior cingulate gyrus was drawn where the anterior medial border of the lateral ventricles appeared.
Figure. Representative region of interest delineation.
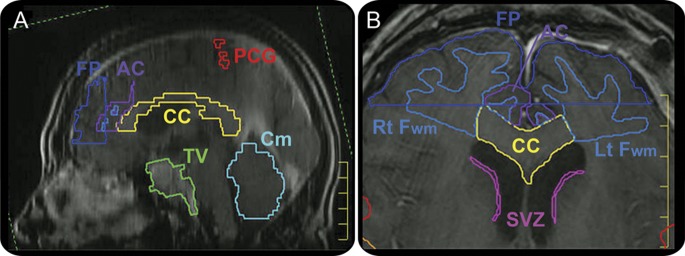
(A) T1-weighted, sagittal view MRI of the brain with corpus callosum (CC), cerebellum (Cm), tumor volume (TV), frontal pole (FP), anterior cingulate (AC), and precentral gyrus (PCG) delineated. (B) T1-weighted, axial view MRI illustrating CC, periventricular stem cell niche (SVZ), frontal white matter horns (Fwm), and FP delineation.
Dosimetry.
After ROIs were delineated, the doses from the archived treatment plan were restored. DVH analysis determined the volume each ROI received at each corresponding dose (v0, v10, v40, and v60). For the 7 individuals who did not receive 1.8 Gy fractionation, the biologic equivalent dose was assessed (e.g., 2 Gy fraction, v9.5, v38, v57). Because of variability in brain volumes and ROIs, structural dosimetry was normalized by determining the percentage of each ROI receiving 10, 40, and 60 Gy (i.e., the %v10, %v40, and %v60).
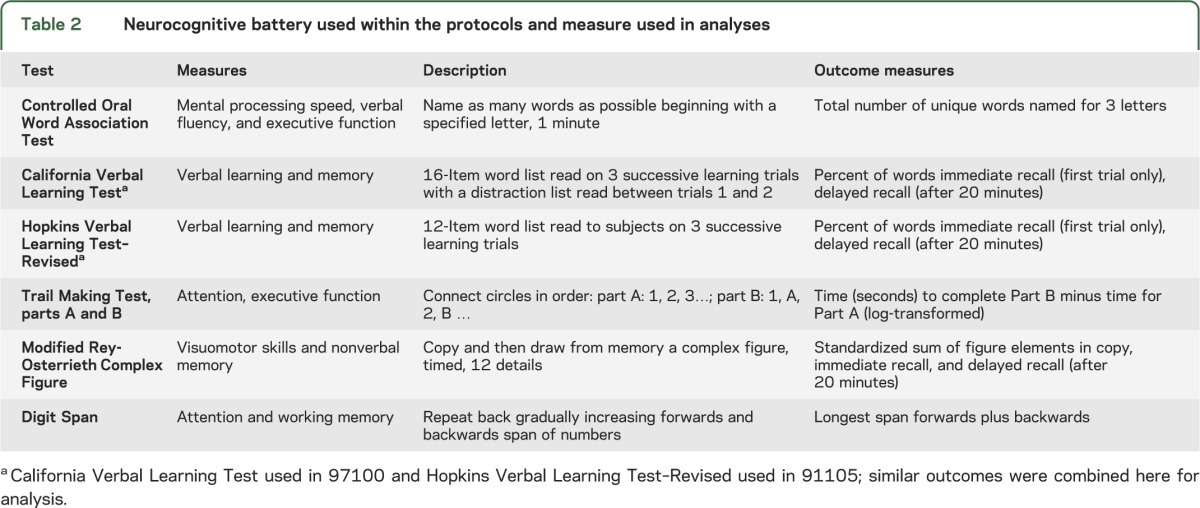
Neurocognitive test battery.
The neurocognitive battery performed on patients in each of the WFU 9710017 and 91105 trials is listed in table 2. The battery was delivered at a single time point, which was variable for each patient yet occurred at least 6 months after RT. No pretreatment battery was available for consideration. In brief, the neurocognitive tests used included the Controlled Oral Word Association (COWA),25 Trail Making Test parts A and B,26 a 24-point modified version of the Rey-Osterreith Complex Figure (ROCF),27 and Digit Span test.28 In WFU 97100, the California Verbal Learning Test (CVLT)29 was used. In WFU 91105, the CVLT was replaced by the Hopkins Verbal Learning Test–Revised.30
Table 2.
Neurocognitive battery used within the protocols and measure used in analyses
Statistics.
Stepwise regression analysis (SPSS 17.0) determined the ROI dose that predicted cognitive performance on each of the neurocognitive tests. Age and educational status were first entered into the regression to control for general cognitive abilities. DVH data were analyzed to determine the dose volume which had the greatest predictive value (p < 0.05) for each ROI on post-RT cognitive ability. Change in variance (Δr2) was reported for the addition of β3 × ROI dosage to the following regression model: outcome = β0 + β1 × age + β2 × education. Because performance is highly correlated across tests in the battery, a factor analysis (utilizing principal component methods) provided a surrogate for global cognition.
RESULTS
Older age was associated with poorer performance on all cognitive measures (r2 range 0.026–0.270). Education explained a smaller amount of variance in cognitive scores (r2 range 0–0.134) with better performance in individuals with more education. Variation in number of days post-RT testing occurred did not significantly predict performance in cognitive scores (r2 range 0–0.008) except for verbal immediate recall (r2 = 0.083, p < 0.03), where longer time intervals were weakly associated with better performance.
Index of global cognition via factors.
Factor analysis determined 2 factors that explained 72.2% of the variance across the cognitive battery. The first represented 55.1% of variance and included verbal learning and memory (verbal immediate/delayed recall), verbal fluency (COWA), attention and working memory (Digit Span), and executive functions (Trail Making part B–A, COWA). Factor 2 explained 17.1% of the remaining variance and consisted of performance on the ROCF (i.e., copy, immediate, and delayed recall), a measure of spatial ability, and nonverbal memory.
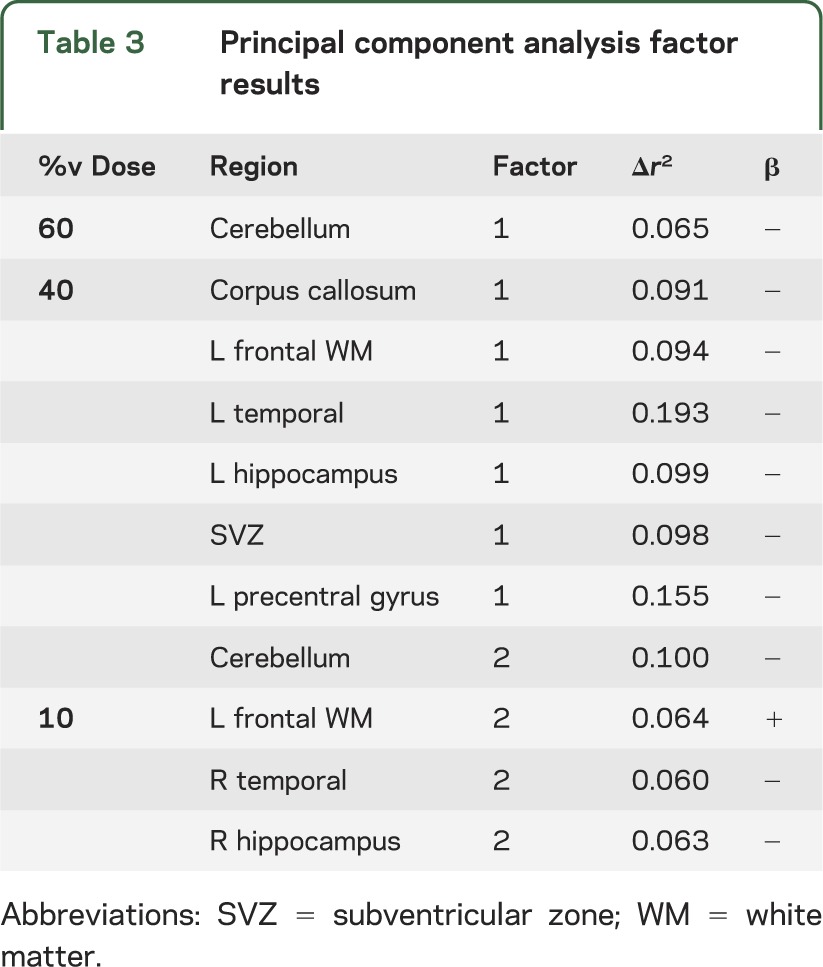
Regions predicting for global cognition.
Stepwise regression was used to determine ROIs that predicted for global cognition as represented by factor 1. Cerebellum at %v60 was predictive of poorer cognitive performance by factor 1. The %v40 for the corpus callosum, SVZ, and left frontal white matter, temporal lobe, hippocampus, and precentral gyrus also predicted factor 1. Factor 2 was predicted by the %v40 of the cerebellum and the %v10 of the right hippocampus, right temporal lobe, and left frontal white matter. Results of the regression are summarized in table 3.
Table 3.
Principal component analysis factor results
Regions for which threshold less than %v60 predict for cognition on individual tests.
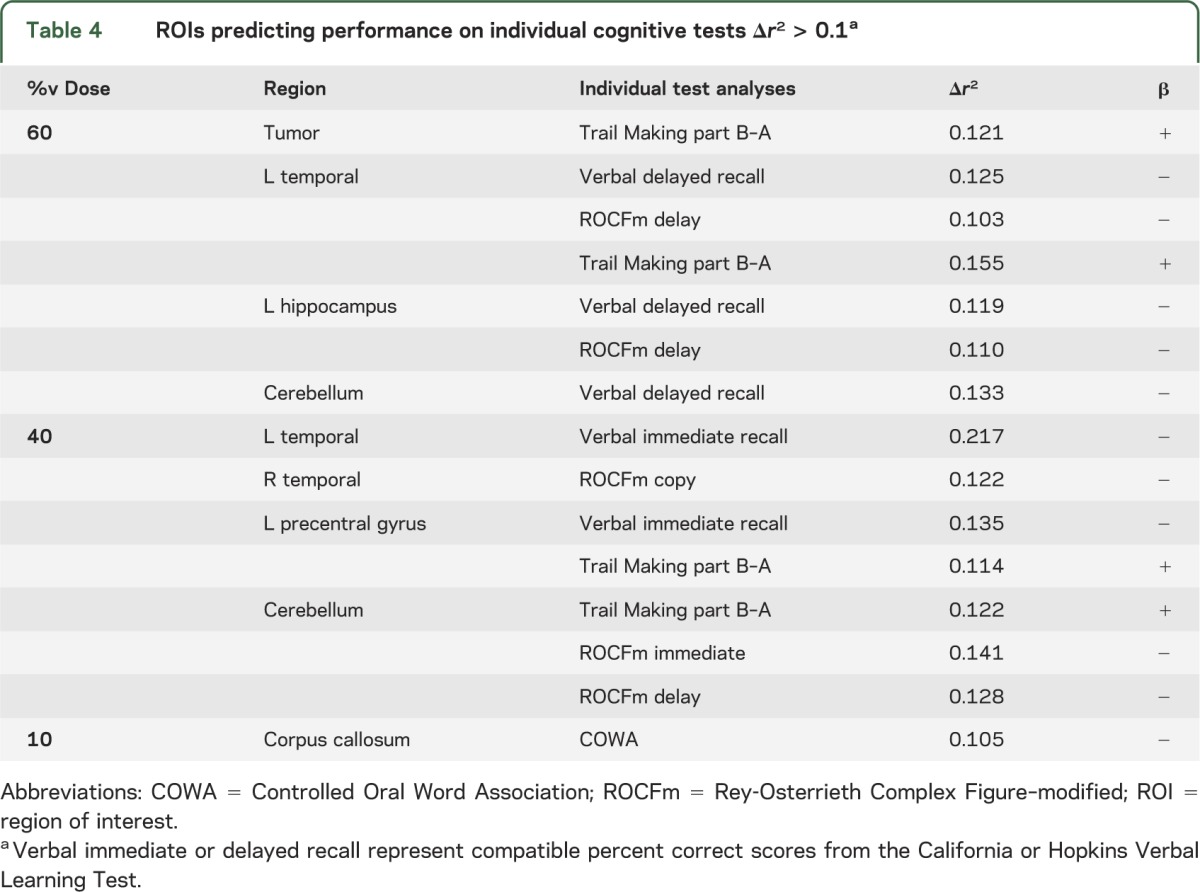
To further elucidate the results of the factor analysis, stepwise regression determined ROIs that predicted performance on individual tests. Due to the large number of contrasts, only predictors that explain >10% of the variance in cognitive ability (i.e., Δr2 > 0.10) after adjusting for age and education are presented (table 4). The %v10 of the corpus callosum predicted cognition on the COWA. The %v40 of the left precentral gyrus, bilateral temporal cortices, and cerebellum were predictive of cognition on individual tests as well. β values indicated that poorer test performance was associated with a larger percentage of ROI volume exposed to the corresponding dose. Seven other analyses revealed that the %v60 was predictive of cognition across several tests for multiple ROIs (tumor, left temporal lobe, left hippocampus, and cerebellum).
Table 4.
ROIs predicting performance on individual cognitive tests Δr2 > 0.1a
ROI that did not predict cognition.
Whole-brain DVH did not predict any cognitive outcome. ROIs that did not show a relationship with the general cognition factors included the tumor, frontal pole, anterior cingulate, right frontal white matter, and right precentral gyrus. ROIs that did not show a relationship to any individual cognitive test at the Δr2 > 0.10 threshold included frontal pole, anterior cingulate, bilateral frontal white matter, right hippocampus, SVZ, and right precentral gyrus. However, these regions did demonstrate modest but statistically significant relationships with individual test performance (predominantly the COWA) below the Δr2 > 0.10 threshold (table e-2).
DISCUSSION
Few prior attempts have been made to create an NTCP model for radiation-induced cognitive decline. If cognitive decline is indeed to be an endpoint of an NTCP model, then patients receiving partial brain irradiation (rather than WBI) would be the population of interest given its ability to spare varying amounts of brain tissue. Tumors such as gliomas and meningiomas, which are commonly treated with partial brain irradiation, have been used to study cognitive decline associated with RT. A comparison of patients with low-grade glioma, indolent lymphoproliferative malignancies (non–brain tumor control), and healthy controls showed that patients receiving partial brain irradiation trended toward worsened cognitive outcomes.31 These data, however, were unable to quantify the detrimental effect of RT. In an attempt to quantify radiation-induced cognitive decline, patients with childhood ependymoma who received RT showed a negative correlation between the volume of supratentorial brain irradiated and post-RT IQ.9 These data are unlikely translatable to adults because the mechanism of cognitive decline in children is different from that in adults given the greater degree of neurogenesis occurring in children and the impending myelination of white matter in the frontal cortex.32
Recently, a definitive cognitive decline in 17 adult patients receiving partial brain irradiation was not seen through the first 2 years after RT.33 The potential shortcoming of this study, and the likely reason it did not show a definitive decline in function, is that patients receiving partial brain irradiation were treated as a homogenous population. Partial brain irradiation and the gradients of radiation dose created by it can vary greatly between individuals, their tumors, and tumor histologies. Moreover, the brain cannot be considered a series of equivalent functional units since different structures within the brain carry out varying functions. Neuroanatomical target theory, as proposed by our current article, suggests that selective damage to various targets within the brain can lead to compromised function similar to those found in lesion studies. Further, depending on which structures are involved, different cognitive abilities will be affected. Conversely, the amount of radiation received by the brain as a whole was not predictive of cognitive outcomes in the current series. Our results suggest that a sufficient amount of radiation to the primary motor regions or to temporal cortex can lead to worsening of global cognition. The damage to these 2 regions may or may not occur by the same mechanism (e.g., vascular injury, demyelination). However, the fact that damage to these sites leads to injury implies that selective avoidance of such structures may preserve function.
Several predictors of function in this analysis derive from purported sites of adult neurogenesis (i.e., hippocampus, SVZ) and are in agreement with a recent analysis from the University of Wisconsin suggesting that hippocampal dosimetry may predict neurocognitive function after RT.34 However, the current data appear to demonstrate that different ROIs have varying importance in specific cognitive tasks. This would suggest that hippocampal-sparing alone may be an oversimplification of brain injury processes occurring post-RT and may be insufficient to spare cognitive function after brain RT.
The need for an NTCP model for radiation-induced cognitive decline is growing. Improving outcomes of patients with gliomas,35 and increasing indications for RT in patients with benign or indolent tumors,36 have made late toxicities of RT of increased interest. Technology has expanded to spare critical brain structures below certain tolerance doses or volumes. The major unresolved issue will be the determination of these threshold doses/volumes.
There are several limitations to this study. First, beyond the selection biases of retrospective studies, the contributing population of participants may have enrolled in clinical trials due to symptomatic cognitive decline. As such, the study ruled out patients who did not experience symptomatic decline in its analysis. Furthermore, this study provided a single time point along the evolution of patients' cognitive decline; it did not have pre-RT cognitive testing to allow for temporal plotting of cognitive decline. Moreover, the population was a mixture of benign and malignant tumors which have varied impact on cognitive function (e.g., having a glioma may affect cognitive status more than having had RT31). Furthermore, nearly a third of the predictive factors in this series was %v60 volumes and is confounded by the fact that it is the location of the surgical bed and where the tumor resided. As such, surgical injury and the existence of the tumor can affect performance on cognitive testing. In addition, 60 Gy is the dose which leads to a 5% likelihood of radionecrosis, which is a different mechanism by which cognitive decline occurs than the mechanisms of injury at lower doses.
Ultimately, the optimal dataset used to construct an NTCP model of radiation-induced brain injury would be derived from a prospective trial with nonvariable post-RT follow-up times. The ideal population for such a prospective trial may be patients with benign or indolent nonparenchymal brain tumors such as meningiomas and pituitary adenomas. These tumors generally lack the confounding factors that have plagued many of the previous attempts to study radiation-induced cognitive decline: they are not treated with chemotherapy, patients have a long life expectancy, and these tumors tend not to affect cognition. The RTOG is currently completing enrollment on a large phase II trial assessing the feasibility of accruing to a randomized trial for meningioma. While this trial does not collect cognitive data, the opportunity may arise for such data collection when the follow-up phase III trial is designed. Furthermore, future CCOP research base studies that may assess interventions for radiation-induced cognitive decline present opportunities to collect such data. An NTCP modeling of radiation-induced cognitive decline using neuroanatomic target theory appears to be feasible, and a prospective trial is necessary to validate these data.
Supplementary Material
GLOSSARY
- CCOP
Community Clinical Oncology Program
- COWA
Controlled Oral Word Association
- CVLT
California Verbal Learning Test
- DVH
dose volume histogram
- NCI
National Cancer Institute
- NTCP
normal tissue complication probability
- ROI
region of interest
- RTOG
Radiation Therapy Oncology Group
- ROCF
Rey-Osterrieth Complex Figure
- RT
radiotherapy
- SVZ
subventricular zone
- WBI
whole-brain irradiation
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Peiffer: study concept and design, acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision. Mr. Leyrer: acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Greene-Schloesser: acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content. Ms. Shing: acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content. Mr. Kearns: acquisition of data. Dr. Hinson: acquisition of data. Dr. Tatter: analysis and interpretation, acquisition of data. Dr. Ip: analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Rapp: acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision. Dr. Robbins: analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision. Dr. Shaw: acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision. Dr. Chan: Study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision.
STUDY FUNDING
This publication was made possible by grant R01NR009675 from NINR/NIH and U10CA 81851 from NCI/NIH. Individuals were also supported by Wake Forest School of Medicine Medical Student Research Program (C.M.L.), Louis Argenta Physician-Scientist Scholarship Fund (E.S.), NIH T32 CA113267 (D.M.G.-S.), and the Department of Radiation Oncology.
DISCLOSURE
A. Peiffer receives funding from NIH grants NR009675, AI67798, CA155293, and LRP CA154094. Research funds were also obtained from the Wake Forest School of Medicine Translational Science Institute's Ignition Fund. M. Leyrer received funding from the Wake Forest School of Medicine Medical Student Research Program. D. Greene-Schloesser received funding from NIH grant CA113267 and a travel award from the Radiation Research Society (2011). E. Shing receives funding from the Wake Forest School of Medicine Louis Argenta Physician-Scientist Scholarship. W. Kearns, W. Hinson, and S. Tatter report no disclosures. E. Ip is currently supported by a grant from Toyota Inc. He is also currently supported by NIH grants AG031827A; HL101066; AG21332; AA14007; AG017587; CA117887; HD059855, NSF grant SES-0719354. He also consults supported by a federal IES grant R305D110027. S. Rapp receives funding from NIH grants, CA081851, NO1WH44221, N01HC95165, AG033087, HHSN268200900040C, and AG022376. He is PI on NIH grant NR009675. M. Robbins received funding from NIH grants CA 112593 and 155293. He also served as PI on NIH training grant T32 CA113267, which provides stipends for postdoctoral fellows being trained in translational radiation oncology. E. Shaw receives funding from NIH grant CA081851. He also serves as a witness/consultant and provides expert testimony for legal proceedings. M. Chan received an honorarium for speaking the Elekta 2010 User's Meeting on head and neck radiosurgery. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109–122 [DOI] [PubMed] [Google Scholar]
- 2.Schultheiss TE, Kun LE, Ang KK, Stephens LC. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys 1995;31:1093–1112 [DOI] [PubMed] [Google Scholar]
- 3.Graham MV, Purdy JA, Emami B, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 1999;45:323–329 [DOI] [PubMed] [Google Scholar]
- 4.Dawson LA, Normolle D, Balter JM, McGinn CJ, Lawrence TS, Ten Haken RK. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys 2002;53:810–821 [DOI] [PubMed] [Google Scholar]
- 5.Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys 1980;6:1215–1228 [DOI] [PubMed] [Google Scholar]
- 6.Bitzer M, Topka H. Progressive cerebral occlusive disease after radiation therapy. Stroke 1995;26:131–136 [DOI] [PubMed] [Google Scholar]
- 7.Keene DL, Johnston DL, Grimard L, Michaud J, Vassilyadi M, Ventureyra E. Vascular complications of cranial radiation. Childs Nerv Syst 2006;22:547–555 [DOI] [PubMed] [Google Scholar]
- 8.Robbins ME, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int J Radiat Biol 2004;80:251–259 [DOI] [PubMed] [Google Scholar]
- 9.Merchant TE, Kiehna EN, Li C, Xiong X, Mulhern RK. Radiation dosimetry predicts IQ after conformal radiation therapy in pediatric patients with localized ependymoma. Int J Radiat Oncol Biol Phys 2005;63:1546–1554 [DOI] [PubMed] [Google Scholar]
- 10.Hsiao KY, Yeh SA, Chang CC, Tsai PC, Wu JM, Gau JS. Cognitive function before and after intensity-modulated radiation therapy in patients with nasopharyngeal carcinoma: a prospective study. Int J Radiat Oncol Biol Phys 2010;77:722–726 [DOI] [PubMed] [Google Scholar]
- 11.Barani IJ, Benedict SH, Lin PS. Neural stem cells: implications for the conventional radiotherapy of central nervous system malignancies. Int J Radiat Oncol Biol Phys 2007;68:324–333 [DOI] [PubMed] [Google Scholar]
- 12.Bosma I, Douw L, Bartolomei F, et al. Synchronized brain activity and neurocognitive function in patients with low-grade glioma: a magnetoencephalography study. Neuro Oncol 2008;10:734–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gondi V, Tome WA, Mehta MP. Why avoid the hippocampus? A comprehensive review. Radiother Oncol 2010;97:370–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gondi V, Tolakanahalli R, Mehta MP, et al. Hippocampal-sparing whole-brain radiotherapy: a "how-to" technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2010;78:1244–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu D, Leung LH, Kwong DL, Chan GC, Khong PL. Mapping radiation dose distribution on the fractional anisotropy map: applications in the assessment of treatment-induced white matter injury. Neuroimage 2006;31:109–115 [DOI] [PubMed] [Google Scholar]
- 16.Steen RG, Spence D, Wu S, Xiong X, Kun LE, Merchant TE. Effect of therapeutic ionizing radiation on the human brain. Ann Neurol 2001;50:787–795 [DOI] [PubMed] [Google Scholar]
- 17.Shaw EG, Rosdhal R, D'Agostino RB, Jr, et al. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. J Clin Oncol 2006;24:1415–1420 [DOI] [PubMed] [Google Scholar]
- 18.Monje M. Cranial radiation therapy and damage to hippocampal neurogenesis. Dev Disabil Res Rev 2008;14:238–242 [DOI] [PubMed] [Google Scholar]
- 19.Morris RG, Moser EI, Riedel G, et al. Elements of a neurobiological theory of the hippocampus: the role of activity-dependent synaptic plasticity in memory. Philos Trans R Soc Lond B Biol Sci 2003;358:773–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam LC, Leung SF, Chan YL. Progress of memory function after radiation therapy in patients with nasopharyngeal carcinoma. J Neuropsychiatry Clin Neurosci 2003;15:90–97 [DOI] [PubMed] [Google Scholar]
- 21.Schuz A, Preissl H. Basic connectivity of the cerebral cortex and some considerations on the corpus callosum. Neurosci Biobehav Rev 1996;20:567–570 [DOI] [PubMed] [Google Scholar]
- 22.Jokinen H, Ryberg C, Kalska H, et al. Corpus callosum atrophy is associated with mental slowing and executive deficits in subjects with age-related white matter hyperintensities: the LADIS Study. J Neurol Neurosurg Psychiatry 2007;78:491–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckner RL. Functional-anatomic correlates of control processes in memory. J Neurosci 2003;23:3999–4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reitan RM, Wolfson D. The significance of sensory-motor functions as indicators of brain dysfunction in children. Arch Clin Neuropsychol 2003;18:11–18 [PubMed] [Google Scholar]
- 25.Ruff RM, Light RH, Parker SB, Levin HS. Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol 1996;11:329–338 [PubMed] [Google Scholar]
- 26.Stanczak DE, Triplett G. Psychometric properties of the Mid-Range Expanded Trail Making Test: an examination of learning-disabled and non-learning-disabled children. Arch Clin Neuropsychol 2003;18:107–120 [PubMed] [Google Scholar]
- 27.Saxton J, Becker JT, Wisniewski SR. The ROCF and dementia. In: Knight JA, Kaplan E, eds. The Handbook of Rey-Osterrieth Complex Figure Usage: Clinical and Research Applications. Lutz, FL: Psychological Assessment Resources; 2003:659–682 [Google Scholar]
- 28.Berger S. The WAIS-R factors: usefulness and construct validity in neuropsychological assessments. Appl Neuropsychol 1998;5:37–42 [DOI] [PubMed] [Google Scholar]
- 29.Elwood RW. The California Verbal Learning Test: psychometric characteristics and clinical application. Neuropsychol Rev 1995;5:173–201 [DOI] [PubMed] [Google Scholar]
- 30.Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test–revised. Clin Neuropsychol 1999;13:348–358 [DOI] [PubMed] [Google Scholar]
- 31.Klein M, Heimans JJ, Aaronson NK, et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet 2002;360:1361–1368 [DOI] [PubMed] [Google Scholar]
- 32.Barnea-Goraly N, Menon V, Eckert M, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex 2005;15:1848–1854 [DOI] [PubMed] [Google Scholar]
- 33.Torres IJ, Mundt AJ, Sweeney PJ, et al. A longitudinal neuropsychological study of partial brain radiation in adults with brain tumors. Neurology 2003;60:1113–1118 [DOI] [PubMed] [Google Scholar]
- 34.Gondi V, Hermann BP, Mehta MP, Tome WA. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys 2012;83:e487–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–996 [DOI] [PubMed] [Google Scholar]
- 36.Pearson BE, Markert JM, Fisher WS, et al. Hitting a moving target: evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg Focus 2008;24:E3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


