Abstract
We have previously identified Usp46, which encodes for ubiquitin-specific peptidase 46, as a quantitative trait gene affecting the immobility time of mice in the tail suspension test (TST) and forced swimming test. The mutation that we identified was a 3-bp deletion coding for lysine (Lys 92), and mice with this mutation (MT mice), as well as Usp46 KO mice exhibited shorter TST immobility times. Behavioral pharmacology suggests that the gamma aminobutyric acid A (GABAA) receptor is involved in regulating TST immobility time. In order to understand how far Usp46 controls behavioral phenotypes, which could be related to mental disorders in humans, we subjected Usp46 MT and KO mice to multiple behavioral tests, including the open field test, ethanol preference test, ethanol-induced loss of righting reflex test, sucrose preference test, novelty-suppressed feeding test, marble burying test, and novel object recognition test. Although behavioral phenotypes of the Usp46 MT and KO mice were not always identical, deficiency of Usp46 significantly affected performance in all these tests. In the open field test, activity levels were lower in Usp46 KO mice than wild type (WT) or MT mice. Both MT and KO mice showed lower ethanol preference and shorter recovery times after ethanol administration. Compared to WT mice, Usp46 MT and KO mice exhibited decreased sucrose preference, took longer latency periods to bite pellets, and buried more marbles in the sucrose preference test, novelty-suppressed feeding test, and marble burying test, respectively. In the novel object recognition test, neither MT nor KO mice showed an increase in exploration of a new object 24 hours after training. These findings indicate that Usp46 regulates a wide range of behavioral phenotypes that might be related to human mental disorders and provides insight into the function of USP46 deubiquitinating enzyme in the neural system.
Introduction
The CS mouse, an inbred strain originally established by crossing the NBC and SII strains (both now extinct), exhibits extremely low immobility time in both the tail suspension test (TST) and forced swimming test (FST). Previously, we performed quantitative trait locus (QTL) genetic analysis using an F2 population cross between CS and normal C57BL/6J mice to identify the gene responsible for the reduced immobility times. After mapping the QTL, we produced several congenic or subcongenic strains, in order to narrow the QTL interval, and focused on a candidate gene within this QTL region. We produced bacterial artificial chromosome transgenic mice, and finally rescued the phenotype. Consequently, we identified Usp46, which encodes an ubiquitin-specific peptidase, as one of the genes responsible for the shortened immobility times [1].
The Usp46 mutation that we identified has a 3-bp deletion coding for lysine (Lys 92) in the open reading frame, and mice with this mutation (MT mice) exhibit a shorter duration of loss of righting reflex caused by muscimol (gamma aminobutyric acid A [GABAA] receptor agonist) administration and a significantly lower amplitude of the muscimol-induced GABAA current in hippocampal CA1 pyramidal neurons. Additionally, hippocampal expression of the 67-kDa isoform of glutamic acid decarboxylase is decreased in these mice. Thus, this mutation is implicated to play a role in the regulation of the GABAergic system. To support these results, we examined null Usp46 (Usp46 KO) mice, and found that TST immobile behavior of the Usp46 KO mice is similar to the 3-bp deleted Usp46 mutation and that the GABAA receptor participates in the regulation of immobility times [2]. Although USP46 deubiqutinating enzyme seems to be implicated in the GABAergic system, most of the cellular functions of this enzyme are not known. To understand these issues, the identification of the target substrates of USP46 is critical, but it has not yet been determined. However, recently it is reported that many proteins interact with USP46, which seems to be necessary to regulate biological functions by this enzyme [3]–[5].
In the present study, in order to gain insight into the function of USP46 in the central nervous system, we subjected Usp46 MT and KO mice to a variety of behavioral tests, particularly taking notice of GABA-related behaviors.
Materials and Methods
Animals
We purchased C57BL/6J (WT) mice from CLEA Japan Inc. Animals (all male mice, aged 8–12 weeks) were housed under a 12 hour light/dark cycle (LD 12∶12, 7:00 on, 19:00 off), with free access to food and water in our animal facility, at a temperature maintained at approximately 24°C. Animal care and all experimental procedures were approved by the Animal Experiment Committee, Graduate School of Bioagricultural Sciences, Nagoya University (approved no. 2011030303).
Usp46 mutant mice
The Usp46 mutant mice were developed as congenic strains on a C57BL/6J (B6) genetic background using a marker-assisted breeding strategy. These mice (B6.CS-Ngu1053) contained chromosome 5 regions harboring the Usp46 of the CS mice [1].
Usp46 KO mice
The Usp46 KO mice were generated as previously described [2]. The KO mice (17-13906 Usp46 gene trapped mice, TG Resource Bank #6072) were descendants of the mouse strain generated by Trans Genic Inc. (Kumamoto, Japan) using the gene trap technique. Tested mice were the offspring of heterozygous pairings and were backcrossed to B6 a minimum of 9 times in order to remove any phenotypic variations caused by a different genomic background. To determine their genotypes, ear biopsies were performed at 4 weeks of age, and the samples were subjected to DNA detection using PCR. F1 heterozygous and B6 alleles were amplified using a neo primer pair: 5′-CTGAATGAACTGCAGGACGAG-3′ and 5′-GTCCAGATCATCCTGATCGAC-3′, and lox-SA primer pair: 5′-AGGTCGAGGGACCTATACCG-3′ and 5′-GAGGCCGCTTGTCCTCTTTG-3′. The F1 heterozygous mice were crossed between themselves to obtain the F2 generation: wild-type, heterozygous, and homozygous. To determine their genotypes, FP3 primer 5′-ATAGACTCTGCTGTTTCTCCTATGCTCC-3′, RP1 primer 5′-AATGTTGAGGCAAAGCTGCCAAGCTCAC-3′, and SA6AS primer 5′-CCGGCTAAAACTTGAGACCT-3′ were used.
Behavioral testing
Prior to behavioral testing, mice were moved to an animal room adjacent to the testing room, where their behaviors were assayed. The mice were kept there for 1 week under the same conditions as those in the animal facility. We assayed mice during the light phase (11:00–16:00), after at least 2 hours of adaptation to the test room.
Open field test
Each mouse was placed in the center of a gray plastic box (40×40×40 cm) and was allowed to freely explore for 5 min, under 40- to 50-lux fluorescent light. During the test, the time spent in the outer (7.5 cm in width) and inner zones (the rest of the outer zone) of the open field was measured using SMART software (version 2.0, Panlab, Spain). Because mice often rested in the outer zone, the nonmoving time was also measured. Additionally, the number of grooming, rearing, and climbing (standing on hind legs with forefeet on the wall) events were scored (because only a few instances of rearing were observed, rearing and climbing were combined). After the open field test, the total distance of movement in the box was calculated. At the end of the test, the mouse was returned to its home cage, and the apparatus was cleaned with 70% ethanol.
Elevated plus-maze test
The elevated plus-maze consists of 2 open arms (width, 8 cm; length, 25 cm) and 2 closed arms (width, 8 cm; height, 20 cm; length, 25 cm) that extend from a common central platform (8×8 cm) [6]–[7]. This apparatus was elevated to a height of 50 cm above the floor. Each mouse was placed on the center square, facing an open arm, and allowed to freely explore for 6 min under a 40- to 50-lux fluorescent light. The total time spent in the open arm was scored.
Ethanol preference test
Ethanol preference was measured using the standard two-bottle choice experiment [8]. Individually housed mice were presented with 2 bottles containing water for 5 days. No significant differences in total water consumption were observed between WT, MT, and KO mice (data not shown). After water consumption for 5 days, mice were presented with 2 bottles: one with water and one with 10% (v/v) ethanol solution for 5 days. The positions of the bottles were switched every 24 hours to control for any side preference. The ethanol preference ratio was calculated as the volume of ethanol consumed/total volume of fluid consumed per day.
Ethanol-induced loss of righting reflex test
Each of the mice was injected with a dose of 3.5 g/kg ethanol (20% v/v in saline intraperitoneally) and was tested for the ethanol-induced loss of righting reflex by placing the mouse on their back in a V-shaped trough [9]–[10]. The loss and recovery of the righting reflex were defined as the inability and ability of the mouse to right itself 3 times within 30 s, respectively.
Sucrose preference test
Sucrose preference was assessed using the same method as that used for ethanol preference, a two-bottle choice experiment [11]. Mice were presented with 2 bottles: one containing water and the other containing 10% (v/v) sucrose for 5 days. Sucrose preference ratio was calculated using an equation similar to the one used for the ethanol preference test. Because the latency period for drinking sucrose after presentation of the sucrose bottle was different between the genotypes, we measured this parameter on the final day of the sucrose preference test. In this test, when drinking behavior was not observed within 5 min, the latency period was defined as 5 min.
Novelty-suppressed feeding test
Individually housed mice were weighed, and food was removed from the cage before the test (water remained available ad libitum). Twenty-four hours after the food was removed, the mice were habituated to the test room for 2 hours before testing. The mouse was placed in a gray plastic box (40×40×40 cm) containing clean wood chip bedding. A white circular filter paper (90 mm in diameter) was placed in the center of the plastic box, and 2 food pellets were placed on the paper [12]. The latency time to biting the pellets, feeding time, and food consumption were recorded (the maximum latency time was 10 min). Immediately after the test, the mice were removed from the plastic box and transferred to their home cage with a pre-weighed piece of food pellet, where food consumption and feeding time were measured for 5 min under ad libitum conditions.
Marble burying test
Mice were placed individually in the center of a gray plastic box (40×40×40 cm) containing 2-cm deep clean wood chip bedding, with 25 glass marbles (1.7 cm in diameter, yellow) that were evenly distributed. Mice were observed during a 30-min test period. After the test, the number of buried marbles (defined as at least two-thirds of the surface being covered with sawdust) was recorded [13].
Novel object recognition test
On the first and second days, mice were placed in a gray plastic box (40×40×40 cm) and were allowed to explore freely for 10 min. On the third day (training), 2 identical objects (gray plastic cubes, 5×5×5 cm) were introduced into the box. The mice were again allowed to explore freely for 10 min. After training, the objects and the box were cleaned using 70% ethanol. On the final day (test), one of the cubes was replaced with a novel object (a gray plastic quadrangular pyramid, each side of the base was 5 cm). Mice were placed in the box again, and explored for 10 min. The number of instances of contact with each object and the total time spent exploring each object were recorded. To avoid any preference for place in the box, the position of each object was changed between each trial [14]–[15].
Statistics
All data are expressed as the mean+S.E.M. Statistical significance was determined by one-way ANOVA with the Fisher's PLSD test for multiple comparisons and student's t-tests for 2-group comparisons.
Results
Open field test
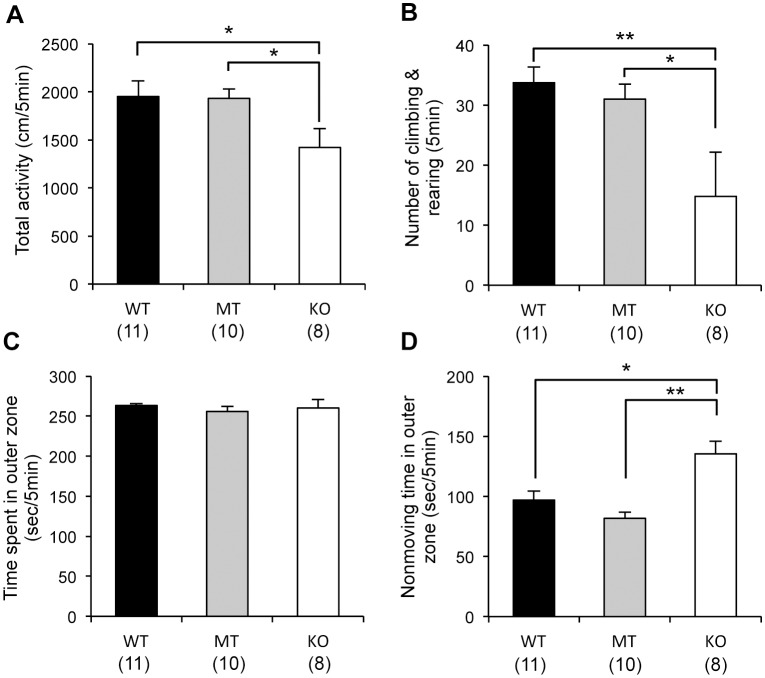
The total distance moved and the number of climbing and rearing events was significantly lower for the Usp46 KO mice than for the WT or MT mice (WT and MT vs. KO, one-way ANOVA, P<0.05, F2,26 = 3.2 for total activity; WT vs. KO, P<0.01; MT vs. KO, P<0.05, F2.26 = 4.1 for the number of climbing and rearing events) (Fig. 1-A, B). Although no significant differences in the time spent in the outer zone were observed between mice with the 3 genotypes, the KO mice exhibited significantly longer resting times in this zone (WT vs. KO, one-way ANOVA, P<0.05; MT vs. KO, P<0.01, F2,26 = 10.5) (Fig. 1-C, D).
Figure 1. Open field test.
Total distance of movement (A), number of climbing and rearing events (B), time spent in the outer zone (C), and resting time in the outer zone (D) of an open field for 5 min. Usp46 KO mice showed significantly lower values than WT and MT mice for the total distance of movement and the number of climbing and rearing events. Nonmoving time in the outer zone was significantly higher for KO mice. The number of mice used is shown within parentheses. One-way ANOVA with Fisher's PLSD test; *P<0.05, **P<0.01.
Ethanol preference test and Ethanol-induced loss of righting reflex test
Both the Usp46 MT and KO mice exhibited lower preference levels for 10% ethanol than the WT mice (WT vs. MT, WT vs. KO, one-way ANOVA, P<0.05, F2,26 = 4.9) (Fig. 2-A). The mean daily fluid consumption (ethanol+water) per body weight was significantly higher in the KO mice than the MT and WT mice (WT vs. MT or KO, one-way ANOVA, P<0.01, F2,26 = 9.8) (Fig. S1-A). In the ethanol-induced loss of righting reflex test, the duration of loss of righting reflex was significantly attenuated in both the Usp46 MT and KO mice compared to WT mice (WT vs. MT or KO, one-way ANOVA, P<0.01, F2,29 = 6.9) (Fig. 2-B). In both ethanol preference test and ethanol-induced loss of righting reflex test, the scores were not significantly different between Usp46 MT and KO mice.
Figure 2. Ethanol preference and ethanol-induced loss of righting reflex test.
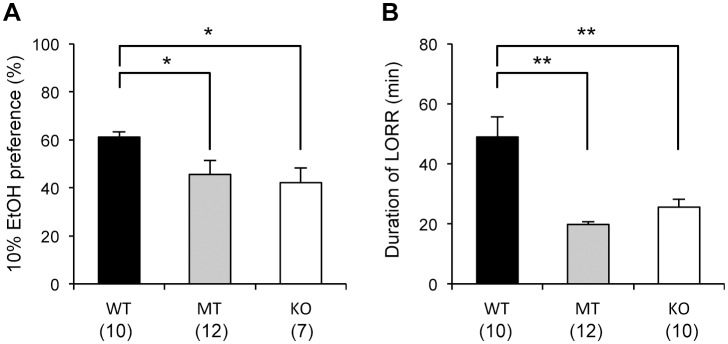
(A) Ethanol preference (10% ethanol) was significantly lower for Usp46 MT and KO mice than for WT mice. The ratio was calculated as the volume of ethanol consumed/total volume of fluid consumed per day. (B) Compared to WT mice, both Usp46 MT and KO mice showed a significantly shorter duration of loss of righting reflex after administration of 3.5 g/kg ethanol (20% v/v in saline intraperitoneally). The number of mice used is shown within parentheses. One-way ANOVA with Fisher's PLSD test; *P<0.05, **P<0.01.
Sucrose preference test
Compared to WT mice, Usp46 MT and KO mice showed a lower preference for 10% sucrose (WT vs. MT or KO, one-way ANOVA, P<0.05, F2,26 = 11.5) (Fig. 3-A). The mean daily fluid consumption (sucrose+water) per body weight was significantly lower in the MT mice than the WT mice (WT vs. MT, one-way-ANOVA, P<0.01, F2,26 = 5.5,) (Fig. S1-B). In this test, we observed that the Usp46 KO mice did not drink sucrose immediately after the bottle was presented. Therefore, to assess the response to sucrose, we measured the latency to drink sucrose on the final day of the test. The Usp46 KO mice showed a significantly longer latency to drink sucrose compared to both the Usp46 MT and WT mice (WT or MT vs. KO, one-way ANOVA, P<0.01, F2,26 = 66.2) (Fig. 3-B). However, the latency of Usp46 MT mice was not significantly different from that of the WT mice.
Figure 3. Sucrose preference test and the latency time to drink sucrose.
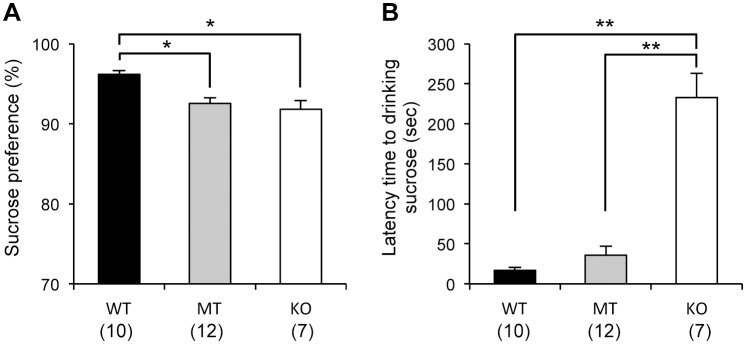
(A) Usp46 MT and KO mice showed significantly lower preference for 10% sucrose than WT mice did. The ratio was calculated as the volume of sucrose consumed/total volume of fluid consumed per day. (B) On the final day of the sucrose preference test, the latency time to drinking sucrose was measured. Usp46 KO mice showed a significantly longer latency time than either MT or WT mice did. The number of mice used is shown within parentheses. One-way ANOVA with Fisher's PLSD test; *P<0.05, **P<0.01.
Novelty-suppressed feeding test
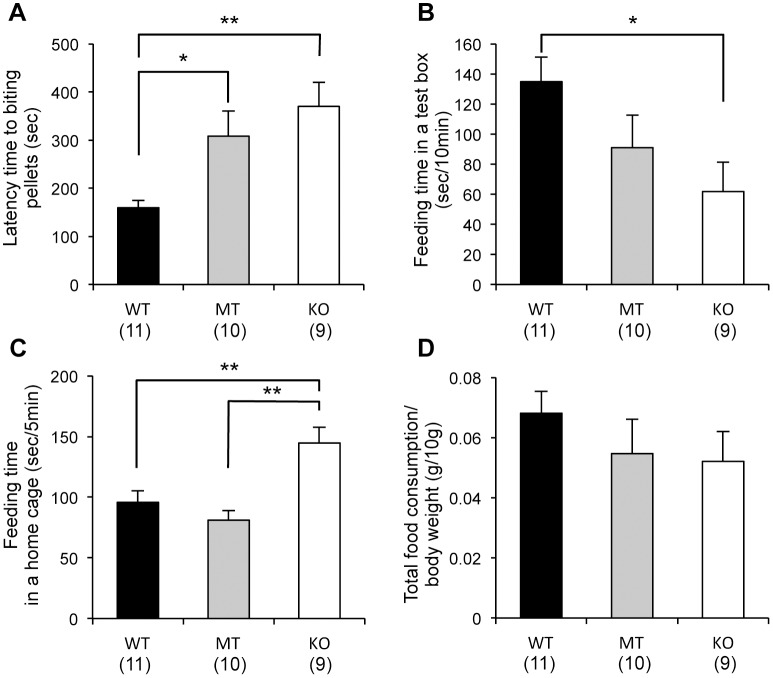
After 24 hours of food deprivation, percentage changes in bodyweight were almost the same in all genotypes of mice (Fig. S2). In the test box, both the Usp46 MT and KO mice showed a significantly longer latency to bite into pellets (WT vs. MT, P<0.05; WT vs. KO, P<0.01, one-way ANOVA, F2,27 = 9.0) (Fig. 4-A), and compared to WT mice, KO mice showed a significantly lower feeding time during the test period (10 min; WT vs. KO, one-way ANOVA, P<0.05, F2,27 = 3.6) (Fig. 4-B). However, after returning to their home cage, the KO mice spent significantly longer time feeding than either the WT or MT mice (WT or MT vs. KO, one-way ANOVA, P<0.01, F2,27 = 12.8) (Fig. 4-C). Total food consumption in the test box and home cage was not significantly different between the 3 mice groups (Fig. 4-D).
Figure 4. Novelty-suppressed feeding test.
(A) Usp46 MT and KO mice showed an increased latency time to bite pellets in a test box. (B) Feeding time in the test box for 10 min. KO mice exhibited significantly lower feeding times. (C) Feeding time in the home cage for 5 min. The KO mice showed significantly longer feeding times. (D) Total food consumption (in the test box for 10 min, and the home cage for 5 min) was not significantly different between the MT, KO, and WT mice. The number of mice used is shown within parentheses. One-way ANOVA with Fisher's PLSD test; *P<0.05, **P<0.01.
Marble burying test
Compared to WT mice, both Usp46 MT and KO mice buried significantly higher number of marbles (WT vs. MT, P<0.05; WT vs. KO, P<0.01, F2,24 = 5.2) (Fig. 5). The differences between Usp46 MT and KO mice were not significant.
Figure 5. Marble burying test.
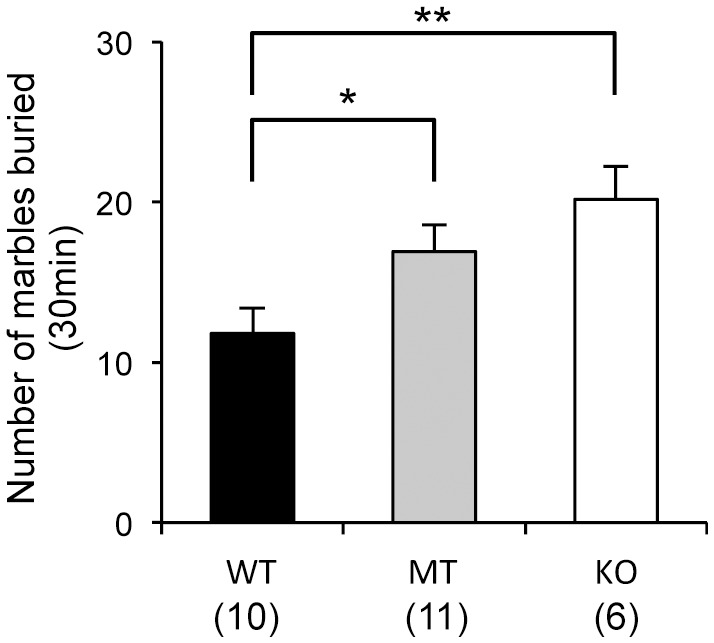
Usp46 MT and KO mice buried significantly higher number of marbles than WT mice did. The number of mice used is shown within parentheses. One-way ANOVA with Fisher's PLSD test; *P<0.05, **P<0.01.
Novel object recognition test
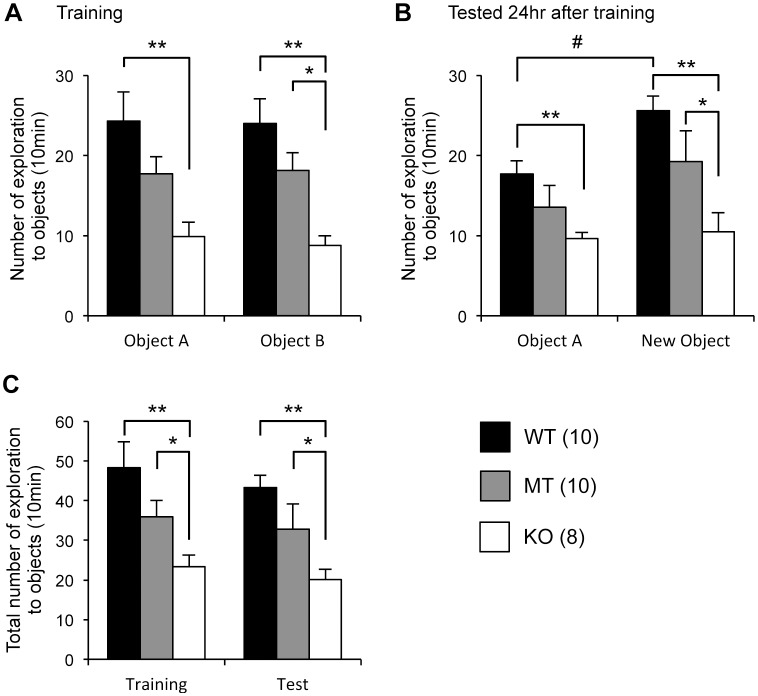
On the training day (the third day), no significant differences in the exploration numbers between the 2 objects were observed in any of the genotype groups (Fig. 6-A). However, the number of explorations for each object was significantly lower for Usp46 KO mice than for WT mice (WT vs. KO, one-way ANOVA, P<0.01, F2,25 = 7.1 for object A; WT vs. KO, P<0.01, F2,25 = 11.2 for object B). The MT mice showed an intermediate level between WT and KO mice (MT vs. KO, P<0.05 for object B). On the final day (24 hours after training), the WT mice explored the new object significantly more often than a familiar object (student's t-test, P<0.05). However, no increase in number of explorations for the new object was observed in either MT or KO mice (Fig. 6-B). The total number of exploration instances for the 2 objects was significantly lower for the KO mice than for the WT mice, and the MT mice showed an intermediate level between the WT and KO mice on both the training and test days (training; WT vs. KO, P<0.01, MT vs. KO, P<0.05, one-way ANOVA, F2,25 = 9.3: test; WT vs. KO, P<0.01, MT vs. KO, P<0.05, F2,25 = 9.6) (Fig. 6-C). When we used time of exploration as a parameter, the same trend as that for numbers of explorations was obtained (Fig. S3).
Figure 6. Novel object recognition test.
(A) Number of explorations of the 2 objects on the training day. Compared to WT mice, Usp46 KO mice showed significantly lower numbers of explorations for each object. The number of explorations for object B made by KO mice was also significantly lower than that by MT mice. (B) A familiar object was substituted by a novel object at 24 hours after the training. The number of explorations for the novel object was significantly increased for the WT mice, but not for Usp46 MT and KO mice. (C) Total number of explorations for the objects on the training day and 24 hours after the training. The number of explorations made by KO mice was significantly lower than those by WT or MT mice. The number of mice used is shown within parentheses. One-way ANOVA with Fisher's PLSD test; *P<0.05, **P<0.01. Student's t test; # P<0.05.
Discussion
In our previous study, we reported that TST immobility time for Usp46 MT mice with a 3-bp deletion coding for lysine is similar to that for Usp46 KO mice and that the GABAA receptor participates in the regulation of the immobility time [2]. In order to assess the broader functional significance of Usp46 for behavioral phenotypes that might be related to mental disorders in humans, we subjected Usp46 MT and KO mice to a variety of behavioral tests. Although behavioral phenotypes of Usp46 MT and KO mice were not always identical, Usp46 deficiency significantly affected all behaviors tested. The differences between Usp46 MT and KO mice were especially distinct with regards to the following parameters: total activity and number of climbing/rearing events, nonmoving time in the outer zone in the open field test, latency time to drinking sucrose in the sucrose preference test, and feeding time in the home cage in the novelty-suppressed feeding test. Because TST immobility time for Usp46 MT mice was similar to that for Usp46 KO mice, we thought that the 3-bp mutation of Usp46 might be a loss of function mutation, not a gain of function mutation in our previous study [2]. However, the present study demonstrated that behavioral phenotypes were not always similar between Usp46 MT and KO mice, suggesting that this mutation causes less function, not no-function. In fact, it is reported that this mutation does not eliminate total deubiquitinating enzyme activity of USP46 [16].
In the open field test, total activity and number of climbing and rearing events were lower for the Usp46 KO mice. A lower level of movement was also reflected in the increased nonmoving time in the outer zone. In general, an increased amount of time spent in the peripheral zone of an open field is thought to be an indication of higher anxiety levels [17]. To evaluate the anxiety levels, we measured the time spent in the outer zone. However, mice of all 3 genotypes spent almost the same amount of time in the outer zone, suggesting that the anxiety levels of MT and KO mice are within the normal range. These results are in agreement with those of other behavioral tests that evaluate anxiety levels: no significant differences in the time spent in the open arm of the elevated plus maze among the WT, MT and KO mice (Fig. S4-A, B) and less time spent in the dark compartment of the light-dark box test for the MT mice than the WT mice in our previous study [1].
In the ethanol preference test and ethanol-induced loss of righting reflex test, both Usp46 MT and KO mice exhibited markedly different phenotypes in comparison to WT mice. Because Usp46 deficiency results in dysfunction of GABAA receptors [1]–[2] and ethanol is known to act on GABAA receptors [18]–[20], both Usp46 MT and KO mice are expected to show altered behavioral responses to ethanol. A role for GABAA receptors in influencing ethanol preference has been previously reported in many GABAA receptor subunit KO mice (α1, α2, α5 and δ subunits) [21]–[23]. The altered duration in the ethanol-induced loss of righting reflex test found in our study has also been reported in mice deficient in genes encoding the GABA transporter and GABAA receptor subunits (α1, α2, and β2) [10] [21]–[22]. Thus, our results are consistent with the idea that Usp46 is involved in the regulation of ethanol-related functions mediated by GABAA receptors.
We performed the sucrose preference test to assess anhedonia, one of the parameters of depression-like responses in mice [24]–[26]. In this test, Usp46 MT and KO mice displayed a lower sucrose preference, indicating that these mice seem to be behaviorally in a depression-like state. In this test, we noticed that Usp46 KO mice did not immediately approach a sucrose bottle after the bottle was introduced. In fact, the latency time to drinking the sucrose for KO mice was significantly longer than for the other 2 genotypes. To evaluate the levels of anhedonia and anxiety-related behaviors in more detail, we subjected the mice to the novelty-suppressed feeding test, in which anxiety-induced hyponeophagia, the inhibition of ingestion and approach to food in a novel environment, was examined. In this test, both Usp46 MT and KO mice displayed longer latency times to start eating pellets, but their total food consumption at the end of the experiment was not significantly different from that of WT mice. These results are consistent with those of the sucrose preference test. Similar phenotypes have been observed in GABAA receptor α2 and γ2 subunit knockout mice [27]–[28]. Although our results may suggest that Usp46 is involved in the regulation of anhedonic behavior, it is also possible that other mechanisms (e.g. mechanisms regulating metabolism) are implicated in this behavior. With regard to this point, there is room for further investigation because Usp46 MT and KO mice showed several behavioral phenotypes that do not apply to depression-like behaviors; the immobility time was decreased in both the FST and TST, reflecting mania-like behavior [1], and the anxiety levels were not found to be higher in the open field test and elevated plus-maze test in the present study and in the light-dark box test in our previous study [1]. Thus, the Usp46-deficient mouse cannot be characterized as a model mouse for depressive or manic disorders with the present data. Meanwhile, Usp46-deficient mice exhibited significantly higher scores than WT mice in the marble burying test. Marble burying behavior is thought to be a pharmacological model for obsessive-compulsive disorder [29], and this behavior is also regulated by the GABAergic system [30]. Taken together, these results suggest that Usp46 controls a broad range of behavioral phenotypes, and presently, it is difficult to extrapolate the behavioral phenotype of Usp46-deficient mice to any specific mental disorders. It also needs further consideration whether different behavioral phenotypes in Usp46-deficient mice are due to alterations in GABAergic system. However, our preliminary data that protein expression of several GABAA receptor subunits are altered in several brain regions where Usp46 is expressed might provide insight into the underlying mechanisms of these behaviors (unpublished data).
Recently, a proteomic study on deubiquitinating enzymes and their associated protein complexes demonstrated that USP46 interacts with PHLPP/SCOP (PH-domain leucine-rich repeat protein phosphatase/suprachiasmatic nucleus circadian oscillatory protein), which are members of the of Ser/Thr family of protein phosphatases [3]. A different study revealed that USP46 functions as a tumor suppressor by binding to PHLPP and directly removes the polyubiquitin chains from PHLPP, resulting in inhibited Akt signaling in colon cancer [31]. Since Usp46 is intensively expressed in the hippocampus [1], and SCOP has been implicated in the formation of long-term hippocampus-dependent memory [32], we investigated the learning ability of Usp46-deficient mice using the same behavioral paradigm as reported previously [32]. In this paradigm, mice are allowed to explore a small field, in which 2 identical objects are placed. Twenty-four hours later, one of the objects is replaced with a novel object. If the mouse remembers the old object, it will spend more time approaching the new one, because mice instinctively interact more with a new object than with a familiar one. Here, we found that Usp46 MT and KO mice did not explore the new object more than they explored the familiar object. Additionally, these mice spent less time exploring objects than WT mice did on both the training day and the day after training. These results are consistent with a previous report that over-expression of SCOP in the hippocampus blocks long-term memory, but not short-tem memory [32], although we did not examine short-term memory in this study. However, we should note that our results do not necessarily indicate impairment of memory formation. It is possible that the Usp46-deficient mice are not able to recognize the object due to some problems with sensory functions, although the retinal histology is normal in these mice (unpublished data). Alternatively, the neophobia-like features of these mice may affect the approaching behavior to the new object. This neophobia-like behavior is observed not only in the novel object recognition test (decreasing approach to the object) but also in the sucrose preference test and novelty-suppressed feeding test (longer latency time to drinking sucrose and to biting pellets).
In summary, the present study has demonstrated that Usp46 is involved in the regulation of multiple of behavioral phenotypes. Elucidating the function of Usp46 at both the cellular and central nervous system level may provide further insight into the regulating mechanisms of behavioral phenotypes of Usp46-deficient mice which can extrapolate to human mental disorders.
Supporting Information
Total fluid consumption in ethanol preference and sucrose preference test. (A) Usp46 KO mice showed significantly higher daily fluid consumption (ethanol+water) per body weight (10 g) than WT or MT mice. (B) MT mice showed significantly lower daily fluid consumption (sucrose+water) per body weight (10 g) than WT mice. The number of mice used is given within parentheses. One-way ANOVA with Fisher's PLSD test; **P<0.01.
(TIF)
Weight changes after 24 hours of food deprivation in the novelty-suppressed feeding test. Percentage changes of body weight are shown. The number of mice used is given within parentheses.
(TIF)
Novel object recognition test. (A) Time of exploration of the 2 objects on the training day. Compared to WT mice, Usp46 KO mice spent significantly lesser time exploring each object. (B) A familiar object was substituted for a novel object 24 hours after the training day. Time spent exploring the novel object was significantly increased for the WT mice, but not for the Usp46 MT and KO mice. (C) Total time exploring the objects on the training day and 24 hours after the training. Exploration time for KO mice was significantly lower than that for WT mice. The number of mice used is shown within parentheses. One-way ANOVA with Fisher's PLSD test; **P<0.01. Student's t test; # P<0.05.
(TIF)
Elevated plus-maze test. (A) Time spent in the open arms during the test period of 6 min. (B) Percentage of time spent in the open arm. The number of mice used is shown within parentheses.
(TIF)
Funding Statement
This research was supported by JSPS KAKENHI Grant Number 22248033, 22658093, Takeda Science Foundation, Life Science Foundation of Japan to SE and Grant-in-Aid for JSPS Fellows (22006714) to SI. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Tomida S, Mamiya T, Sakamaki H, Miura M, Aosaki T, et al. (2009) Usp46 is a quantitative trait gene regulating mouse immobile behavior in the tail suspension and forced swimming tests. Nat Genet 41: 688–695. [DOI] [PubMed] [Google Scholar]
- 2. Imai S, Mamiya T, Tsukada A, Sakai Y, Mouri A, et al. (2012) Ubiquitin-specific peptidase 46 (Usp46) regulates mouse immobile behavior in the tail suspension test through the GABAergic system. PLoS One 7: e39084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sowa ME, Bennett EJ, Gygi SP, Harper JW (2009) Defining the human deubiquitinating enzyme interaction landscape. Cell 138: 389–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kee Y, Yang K, Cohn MA, Haas W, Gygi SP, et al. (2010) WDR20 regulates activity of the USP12×UAF1 deubiquitinating enzyme complex. J Biol Chem 285: 11252–11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Katz EJ, Isasa M, Crosas B (2010) A new map to understand deubiquitination. Biochem Soc Trans 38: 21–28. [DOI] [PubMed] [Google Scholar]
- 6. Miyamoto Y, Yamada K, Noda Y, Mori H, Mishina M, et al. (2002) Lower sensitivity to stress and altered monoaminergic neuronal function in mice lacking the NMDA receptor epsilon 4 subunit. J Neurosci 22: 2335–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mamiya T, Noda Y, Nishi M, Takeshima H, Nabeshima T (1998) Enhancement of spatial attention in nociceptin/orphanin FQ receptor-knockout mice. Brain Res 783: 236–240. [DOI] [PubMed] [Google Scholar]
- 8. Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, et al. (1999) Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKCepsilon. Nat Neurosci 2: 997–1002. [DOI] [PubMed] [Google Scholar]
- 9. Hu JH, Ma YH, Yang N, Mei ZT, Zhang MH, et al. (2004) Up-regulation of gamma-aminobutyric acid transporter I mediates ethanol sensitivity in mice. Neuroscience 123: 807–812. [DOI] [PubMed] [Google Scholar]
- 10. Cai YQ, Cai GQ, Liu GX, Cai Q, Shi JH, et al. (2006) Mice with genetically altered GABA transporter subtype I (GAT1) expression show altered behavioral responses to ethanol. J Neurosci Res 84: 255–267. [DOI] [PubMed] [Google Scholar]
- 11. Szczypka MS, Kwok K, Brot MD, Marck BT, Matsumoto AM, et al. (2001) Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron 30: 819–828. [DOI] [PubMed] [Google Scholar]
- 12. Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA (2011) Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476: 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deacon RM (2006) Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat Protoc 1: 122–124. [DOI] [PubMed] [Google Scholar]
- 14. Han S, Tai C, Westenbroek RE, Yu FH, Cheah CS, et al. (2012) Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature 489: 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13: 93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang W, Tian QB, Li QK, Wang JM, Wang CN, et al. (2011) Lysine 92 amino acid residue of USP46, a gene associated with ‘behavioral despair’ in mice, influences the deubiquitinating enzyme activity. PLoS One 6: e26297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choleris E, Thomas AW, Kavaliers M, Prato FS (2001) A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev 25: 235–260. [DOI] [PubMed] [Google Scholar]
- 18. Grobin AC, Matthews DB, Devaud LL, Morrow AL (1998) The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 139: 2–19. [DOI] [PubMed] [Google Scholar]
- 19. Koob GF (2004) A role for GABA mechanisms in the motivational effects of alcohol. Biochem Pharmacol 68: 1515–1525. [DOI] [PubMed] [Google Scholar]
- 20. Lobo IA, Harris RA (2008) GABA(A) receptors and alcohol. Pharmacol Biochem Behav 90: 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blednov YA, Jung S, Alva H, Wallace D, Rosahl T, et al. (2003) Deletion of the alpha1 or beta2 subunit of GABAA receptors reduces actions of alcohol and other drugs. J Pharmacol Exp Ther 304: 30–36. [DOI] [PubMed] [Google Scholar]
- 22. Boehm SL 2nd, Ponomarev I, Jennings AW, Whiting PJ, Rosahl TW, et al. (2004) gamma-Aminobutyric acid A receptor subunit mutant mice: new perspectives on alcohol actions. Biochem Pharmacol 68: 1581–1602. [DOI] [PubMed] [Google Scholar]
- 23. Mihalek RM, Bowers BJ, Wehner JM, Kralic JE, VanDoren MJ, et al. (2001) GABA(A)-receptor delta subunit knockout mice have multiple defects in behavioral responses to ethanol. Alcohol Clin Exp Res 25: 1708–1718. [PubMed] [Google Scholar]
- 24. Willner P, Towell A, Sampson D, Sophokleous S, Muscat R (1987) Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 93: 358–364. [DOI] [PubMed] [Google Scholar]
- 25. Pothion S, Bizot JC, Trovero F, Belzung C (2004) Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav Brain Res 155: 135–146. [DOI] [PubMed] [Google Scholar]
- 26. Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P (2004) Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology 29: 2007–2017. [DOI] [PubMed] [Google Scholar]
- 27. Vollenweider I, Smith KS, Keist R, Rudolph U (2011) Antidepressant-like properties of alpha2-containing GABA(A) receptors. Behav Brain Res 217: 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shen Q, Lal R, Luellen BA, Earnheart JC, Andrews AM, et al. (2010) gamma-Aminobutyric acid-type A receptor deficits cause hypothalamic-pituitary-adrenal axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression. Biol Psychiatry 68: 512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Albelda N, Joel D (2012) Animal models of obsessive-compulsive disorder: exploring pharmacology and neural substrates. Neurosci Biobehav Rev 36: 47–63. [DOI] [PubMed] [Google Scholar]
- 30. Egashira N, Abe M, Shirakawa A, Niki T, Mishima K, et al. (2012) Effects of mood stabilizers on marble-burying behavior in mice: Involvement of GABAergic system. Psychopharmacology (Berl) doi:10.1007/s00213-012-2904-9 [DOI] [PubMed] [Google Scholar]
- 31. Li X, Stevens PD, Yang H, Gulhati P, Wang W, et al. (2012) The deubiquitination enzyme USP46 functions as a tumor suppressor by controlling PHLPP-dependent attenuation of Akt signaling in colon cancer. Oncogene doi:10.1038/onc.2012.6632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shimizu K, Phan T, Mansuy IM, Storm DR (2007) Proteolytic degradation of SCOP in the hippocampus contributes to activation of MAP kinase and memory. Cell 128: 1219–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Total fluid consumption in ethanol preference and sucrose preference test. (A) Usp46 KO mice showed significantly higher daily fluid consumption (ethanol+water) per body weight (10 g) than WT or MT mice. (B) MT mice showed significantly lower daily fluid consumption (sucrose+water) per body weight (10 g) than WT mice. The number of mice used is given within parentheses. One-way ANOVA with Fisher's PLSD test; **P<0.01.
(TIF)
Weight changes after 24 hours of food deprivation in the novelty-suppressed feeding test. Percentage changes of body weight are shown. The number of mice used is given within parentheses.
(TIF)
Novel object recognition test. (A) Time of exploration of the 2 objects on the training day. Compared to WT mice, Usp46 KO mice spent significantly lesser time exploring each object. (B) A familiar object was substituted for a novel object 24 hours after the training day. Time spent exploring the novel object was significantly increased for the WT mice, but not for the Usp46 MT and KO mice. (C) Total time exploring the objects on the training day and 24 hours after the training. Exploration time for KO mice was significantly lower than that for WT mice. The number of mice used is shown within parentheses. One-way ANOVA with Fisher's PLSD test; **P<0.01. Student's t test; # P<0.05.
(TIF)
Elevated plus-maze test. (A) Time spent in the open arms during the test period of 6 min. (B) Percentage of time spent in the open arm. The number of mice used is shown within parentheses.
(TIF)