Abstract
The object of this study was to test whether posaconazole, a broad-spectrum antifungal agent inhibiting ergosterol biosynthesis, exhibits synergy with the β-1,3 glucan synthase inhibitor caspofungin or the calcineurin inhibitor FK506 against the human fungal pathogen Candida albicans. Although current drug treatments for Candida infection are often efficacious, the available antifungal armamentarium may not be keeping pace with the increasing incidence of drug resistant strains. The development of drug combinations or novel antifungal drugs to address emerging drug resistance is therefore of general importance. Combination drug therapies are employed to treat patients with HIV, cancer, or tuberculosis, and has considerable promise in the treatment of fungal infections like cryptococcal meningitis and C. albicans infections. Our studies reported here demonstrate that posaconazole exhibits in vitro synergy with caspofungin or FK506 against drug susceptible or resistant C. albicans strains. Furthermore, these combinations also show in vivo synergy against C. albicans strain SC5314 and its derived echinocandin-resistant mutants, which harbor an S645Y mutation in the CaFks1 β-1,3 glucan synthase drug target, suggesting potential therapeutic applicability for these combinations in the future.
Introduction
Candida albicans is the leading Candida species causing bloodstream infections (candidemia), oral thrush, and vaginal yeast infections [1], [2]. Candidemia often results in a high mortality rate (>30%), particularly if appropriate antifungal drug treatments are delayed [3]. The increase of Candida infection is, in part, due to rising numbers of immunocompromised patients and widespread use of broad spectrum antibiotics. Azoles, echinocandins, amphotericin B, and flucytosine are current antifungal drugs for treating Candida infections. However, the implementation of these and other antifungal drugs has not kept pace with the increased incidence of drug-resistance. Therefore, either a combination of current drugs or development of novel antifungal drugs will be important for present and future therapy. Posaconazole inhibits lanosterol 14α-demethylase required for ergosterol biosynthesis and is the most recently approved triazole with broad spectrum activity against Candida, Cryptococcus, Aspergillus, zygomycetes, dermatophytes, and other fungal pathogens [4]. Caspofungin inhibits β-1,3 glucan synthesis and represents the newest class of antifungal drugs, with an excellent safety profile [5]. FK506 (tacrolimus) is an immunosuppressant for organ transplant that targets both mammalian and fungal calcineurins [6], [7].
Drug-resistant C. albicans strains have been frequently isolated from patients [8]. For example, White et al. reported a series of 17 sequential isolates associated with the emergence of azole resistance in an HIV-infected patient [9]. Furthermore, the azole resistance of the 17 isolates was correlated with increased mRNA levels of the ERG11, CDR1, and MDR1 genes in C. albicans [10]. Garcia-Effron et al. isolated C. albicans echinocandin resistant strains associated with β-1,3 glucan synthase FKS1 mutations [11]. These echinocandin resistant strains have a breakpoint at 2 µg/ml for caspofungin, and 0.5 µg/ml for micafungin and anidulafungin. However, approaches to treat these infections caused by drug resistant isolates are lacking or patients must receive the toxic polyene antifungals.
Combination therapy, also known as cocktail therapy or Highly Active Antiretroviral Therapy (HAART), was first used to inhibit HIV virus replication via multiple mechanisms [12], [13], [14], and now is one of the most successful approaches to combating infectious diseases. Therefore, it is possible that drug combinations with different mechanisms of action such as posaconazole, caspofungin, and FK506 can be deployed to manage C. albicans infection. It has been demonstrated that posaconazole alone at a dose of 2.5 mg/kg can reduce C. albicans colonization in the kidney tissues in an immunocompromised mouse model [15]. However, whether posaconazole exhibits in vivo synergy with other antifungal drugs against C. albicans drug-susceptible or drug-resistant isolates was unclear.
In this study we set out to test the potential efficacy of posaconazole when combined with either caspofungin or FK506 in treating C. albicans infection in a murine systemic infection model. We found that posaconazole exhibits in vitro synergistic antifungal activity with either caspofungin or FK506 against drug susceptible or resistant clinical C. albicans isolates. Although these combinations were not found to exhibit in vivo synergistic activity against several clinical drug-resistant isolates, they were efficacious against both C. albicans SC5314-derived echinocandin-resistant mutant YC734 and its wild-type parental isolate SC5314.
Materials and Methods
C. albians Strains used in this Study
C. albicans strains used in this study are listed in Table 1. In brief, four clinical echinocandin-resistant isolates 89, 177, 194 and 205 with different mutations in the Fks1 protein [11] were chosen to determine the efficacy of drug combination against echinocandin-resistant isolates. Meanwhile, three clinical isolates representing #1 (2–76), #9 (2–86), and #17 (12–99) of a series of 17 sequential isolates were chosen, and which are associated with the emergence of fluconazole resistance and increased expression levels of ERG11, CDR1 and MDR1 in an HIV-infected patient [9], [10]. Due to step-wise increased fluconazole resistance, isolates 2–76 and 2–86 are fluconazole-susceptible, while 12–99 is a fluconazole-resistant isolate [9]. Interestingly, these isolates were susceptible to posaconazole although 12–99 has an MIC50 ∼10 fold higher than the 2–76 or 2–86 isolate (Table 2). In addition, we generated SC5314-derived genetically engineered echinocandin resistant strains YC734 and YC736 with an S645Y mutation in the C. albicans Fks1 protein (refer to the section on strain construction for details).
Table 1. Candida albicans strains used in this study.
| Strain | Description | Background | Reference |
| SC5314 | Prototrophic wild-type SC5314 | Clinical isolate | [46] |
| 89 | Echinocandin resistant (Fks1 S645Y) | Clinical isolate | [11] |
| 177 | Echinocandin resistant (Fks1 F641S) | Clinical isolate | [11] |
| 194 | Echinocandin resistant (Fks1 S645F) | Clinical isolate | [11] |
| 205 | Echinocandin resistant (Fks1 S645P) | Clinical isolate | [11] |
| 2–76 | 1/17 azole resistant series strain | Clinical isolate | [9] |
| 2–86 | 9/17 azole resistant series strain | Clinical isolate | [9] |
| 12–99 | 17/17 azole resistant series strain | Clinical isolate | [9] |
| YC734 | Fks1 S645Y (independent isolate #1) | SC5314 | This study |
| YC736 | Fks1 S645Y (independent isolate #2) | SC5314 | This study |
Table 2. Synergistic antifungal activity between posaconazole and caspofungin or FK506 against C. albicans drug susceptible or resistant strains.
| C. albicans | PSC | CSF | FK506 | MIC100 combined | *FIC index | |||||
| MIC50 | MIC100 | MIC50 | MIC100 | MIC50 | MIC100 | (PSC,CSF) | (PSC,FK506) | PSC+CSF | PSC+FK506 | |
| SC5314# | 0.03 | >16 | 0.06 | 0.25 | >16 | >16 | 4,0.03 | 0.06,0.25 | 0.25 | 0.01 |
| 89 | 0.25 | >16 | 2 | >16 | 1 | >16 | 0.06,16 | 1,0.25 | 0.5 | 0.04 |
| 177 | 0.03 | >16 | 2 | >16 | >16 | >16 | 0.03,16 | 0.25,8 | 0.5 | 0.26 |
| 205 | 0.25 | >16 | >16 | >16 | 2 | >16 | 0.06,16 | 0.25,4 | 0.5 | 0.13 |
| 194 | 0.5 | >16 | >16 | >16 | >16 | >16 | 0.03,16 | 0.13,2 | 0.5 | 0.07 |
| 2–76 | <0.03 | >16 | 0.5 | >16 | >16 | >16 | 0.03,8 | 0.03,1 | 0.25 | 0.03 |
| 2–86 | <0.03 | >16 | 0.25 | >16 | >16 | >16 | 0.25,4 | 0.06,2 | 0.13 | 0.06 |
| 12–99 | 0.13 | >16 | 0.25 | >16 | >16 | >16 | 0.25,4 | 0.13,0.5 | 0.13 | 0.02 |
| YC734 | 0.03 | >16 | 2 | >16 | >16 | >16 | 0.13,4 | 0.06,0.5 | 0.13 | 0.02 |
| YC736 | 0.03 | >16 | 2 | >16 | >16 | >16 | 0.5,4 | 0.06,0.25 | 0.14 | 0.01 |
Orange highlighting color indicates wild-type and its derived mutants, while yellow and green highlighting colors represent echinocandin resistant and 3 (#1, #9, #17) out of 17 azole resistant isolates from the strain series, respectively.
Formula of FIC index:
[(MIC100 of drug A in combination)/(MIC100 of drug A alone)]+[(MIC100 of drug B in combination)/(MIC100 of drug B alone)].
FIC≤0.5 (Synergy); FIC >4 (Antagonism); FIC >0.5 but ≤4 (no interaction).
Determination of Minimum and Fractional Inhibitory Concentration Indices
We determined minimum inhibitory concentrations (MIC) by the Clinical and Laboratory Standards Institute (CLSI) protocol M27-A3, while fractional inhibitory concentration (drug interaction) was assessed via checkerboard titration assays. Testing was done in RPMI-1640, buffered to pH 7.0 with 0.165 M MOPS. Yeast strains were grown in YPD medium overnight, incubated at 30°C with shaking, and washed twice with dH2O. The OD600 was measured and each strain was diluted to 1 OD600/ml. This inoculum was then diluted to 0.0005 OD600/ml in RPMI-1640. 98 µl of the strain culture was added to each well in a 96 well plate format. 2 µl of serially diluted drugs were added to the wells, yielding a total volume of 100 µl per well. Posaconazole was added across the plate with the highest concentration in the left well and the lowest concentration in the right well. Posaconazole concentrations ranged from 0.03–16 µg/ml. Either caspofungin or FK506 was added from top to bottom, with the highest concentration in the top row and the lowest concentration in the bottom row. Caspofungin and FK506 concentrations ranged from 0.25–16 µg/ml. This strategy allowed for 70 different drug concentrations to be tested on one plate. The plates were incubated for ∼48 hours at 30°C, and then the OD600 of each plate was read. The in vitro drug interaction studies were performed at least twice. The minimum inhibitory concentration (MIC100) of both drugs, either alone or in combination, was defined as the lowest concentration of each drug which resulted in total inhibition of visible fungal growth [16] and produced a 99.9% of inhibition based on spectrophotometric determination at OD600 when compared to the control well. Meanwhile, MIC100 was found to be equivalent to the minimum fungicidal concentration (MFC) for C. albicans isolates [16]. The fractional inhibitory concentration (FIC) was calculated by: (MICcombined drug A/MICalone drug A)+(MICcombined drug B/MICalone drug B). A FIC index of ≤0.5 indicates synergy, >4.0 indicates antagonism, and an index between 0.5 and 4 indicates no interaction [17]. For calculation purposes, an MIC >16 was assumed to be 32.
Ethics Statement
Animals studies were conducted in the Division of Laboratory Animal Resources (DLAR) facilities at Duke University Medical Center (DUMC) in good practice as defined by the United States Animal Welfare Act and in full compliance with the guidelines of the DUMC Institutional Animal Care and Use Committee (IACUC). The vertebrate animal experiments were reviewed and approved by the DUMC IACUC under protocol number A165-11-06.
Mouse Infection and Drug Treatments
Four- to five-week-old male CD1 mice (Jackson Laboratory, ∼30 g) were used in this study. For infection, C. albicans strains were cultured in YPD broth overnight at 30°C and washed twice with sterile PBS. Cells were counted with a hemocytometer, and resuspended in sterile PBS at 5×106 cells per ml. Dilutions of the cells were plated onto YPD and incubated at 30°C for 48 hr to determine CFU and viability. Groups of 5 or 10 mice were inoculated with C. albicans via tail-vein injection of 106 cells (in 200 µl). The in vivo dosing regimens including dose level, dosing interval, and treatment duration were chosen from previous work in the literature [15], [18], [19], [20] and from our prior knowledge of pilot experiments for a susceptible C. albicans SC5314 strain. Posaconazole (NOXAFIL, Merck & Co.) was diluted with PBS and administered via oral gavage (Roboz Cat#FN-7920; 22 gauge), while placebo PBS, caspofungin (provided by Merck), and FK506 (provided by Astellas) were administered via the intraperitoneal route (in 100 µl) after 4, 24, 48, and 72 hr following Candida infection. The conditions of the mice were monitored 1 to 2 times daily, and moribund mice were euthanized with CO2. Kaplan-Meier survival curves were generated with Prism 5.03 (GraphPad software, La Jolla, CA, USA), and P values were evaluated by a Log-rank (Mantel-Cox) test. A P value of <0.05 was considered significant.
Construction of the FKS1-1 Mutants
The FKS1-1 mutant strains were constructed by direct transformation of C. albicans strain SC5314 by electroporation with 10 µg of a mutagenic 90-mer synthetic oligonucleotide JC583 (CCTTGCCAAATTGGTTGAATCTTATTTCTTCTTGACATTGTATTTGAGAGATCCTATTAGAAACTTGTCGACCATGACAATGAGATGTGT) [21], [22]. JC583 is identical to nucleotides 1893 to 1982 of the sense strand of the FKS1 ORF (orf19.2929) except at the underlined nucleotides, which introduce a C1934A (S645Y) and A1938G (silent marker; only changed in third nucleotide of the codon, but not amino acid in order to rule out spontaneous S645Y mutations occurring during selection). The PflFI site (GACnnnGTC) present in the wild-type allele was eliminated by introduction of the C1934A mutation. Transformants were selected on YPD plates containing 1 µg/ml caspofungin. Two independent echinocandin-resistant strains (YC734, YC736) derived from different transformations were obtained. These strains were confirmed by PCR of the FKS1 locus with primers JC584 (GCATCACAAACATTTACTGCC) and JC585 (CGTGGTAGCTAAAATCTTGG) and subsequent DNA sequencing (Eton Bioscience Inc.). The JC584/JC585 PCR products were also treated with ExoSAP-it and further digested with the restriction enzyme PflFI, which recognizes the GACnnnGTC sequence that is present in wild-type (SC5314) but not in the isogenic FKS1-1 mutants (YC734, YC736) and clinical isolate 89. The silent A1938G mutation was confirmed by DNA sequencing and digestion with restriction enzyme MseI.
Results
Posaconazole Exhibits Synergistic Antifungal Activity with Caspofungin in vitro and in vivo Against C. albicans SC5314
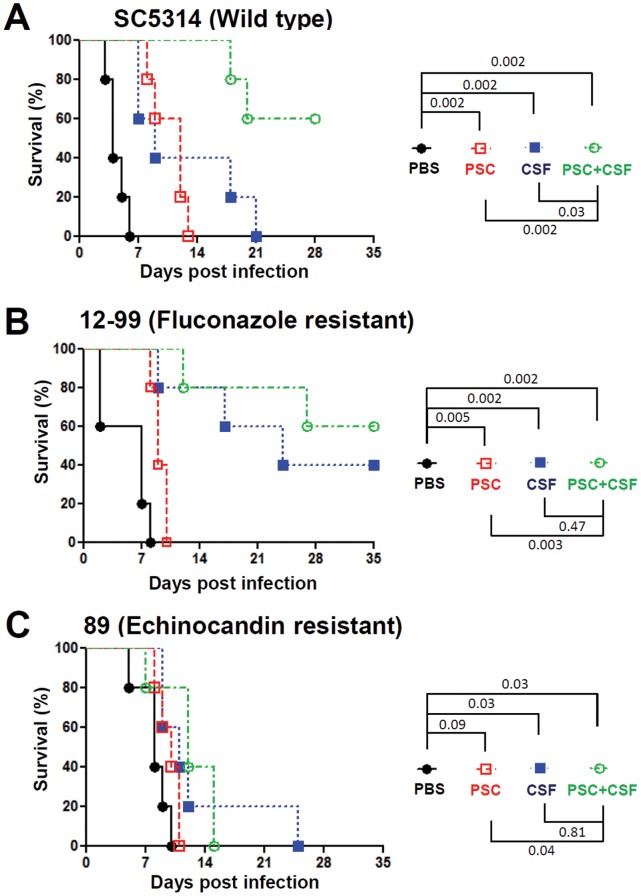
Two different antifungal drugs with distinct mechanisms for targeting pathogens can potentially exhibit synergistic antifungal activity [23], [24]. Posaconazole has been demonstrated to exhibit in vitro synergistic antifungal activity with caspofungin against human fungal pathogens, including C. albicans [25], [26], C. glabrata [27] and A. fumigatus [28]. Although an in vivo synergistic antifungal activity between posaconazole and caspofungin has been shown for A. fumigatus infection [28], it has not yet been similarly demonstrated for C. albicans. Here, we demonstrate that posaconazole exhibits in vitro synergistic antifungal activity with caspofungin against not only the C. albicans strain SC5314, but also fluconazole- or echinocandin-resistant isolates [Fractional Inhibitory Concentration (FIC)≤0.5; Table 2]. In animal infections with drug therapeutic experiments, we demonstrate that posaconazole (orally administered) alone at 2 mg/kg has therapeutic antifungal activity against C. albicans strain SC5314 (P = 0.002; log-rank test, Figure 1A) and the fluconazole resistant isolate 12–99 (P = 0.005; Figure 1B); while caspofungin (intraperitoneal administered) alone at 0.1 mg/kg exhibits therapeutic activity against C. albicans SC5314 (P = 0.002; Figure 1A), 12–99 (P = 0.002; Figure 1B), and the echinocandin-resistant isolate 89 (P = 0.03; Figure 1C). In combination drug therapy, the animals infected with C. albicans SC5314 and treated with posaconazole at 2 mg/kg and caspofungin at 0.1 mg/kg survived longer than infected groups treated with posaconazole (2 mg/kg; P = 0.002) or caspofungin (0.1 mg/kg; P = 0.03) monotherapy (Figure 1A). However, the posaconazole-caspofungin drug combination therapy did not show in vivo synergy against the drug resistant isolates 12–99 (fluconazole) or 89 (echinocandin) (Figure 1B and 1C).
Figure 1. Efficacy of posaconazole and caspofungin against C. albicans infection.
Five male CD1 mice (4–5 weeks-old) per group were infected with 106 cells of C. albicans SC5314, azole-resistant isolate 12–99, or echinocandin-resistant isolate 89, followed by treatment with placebo (PBS), posaconazole (PSC; 2 mg/kg), caspofungin (CSF; 0.1 mg/kg), or PSC (2 mg/kg) plus CSF (0.1 mg/kg). The PSC was administered via oral gavage, while PBS and CSF were administered via intraperitoneal injection at 4, 24, 48, and 72 hr post infection. Survival of the animals was monitored for up to 35 days.
Posaconazole Exhibits Synergistic Antifungal Activity with FK506 in vitro and in vivo Against C. albicans SC5314
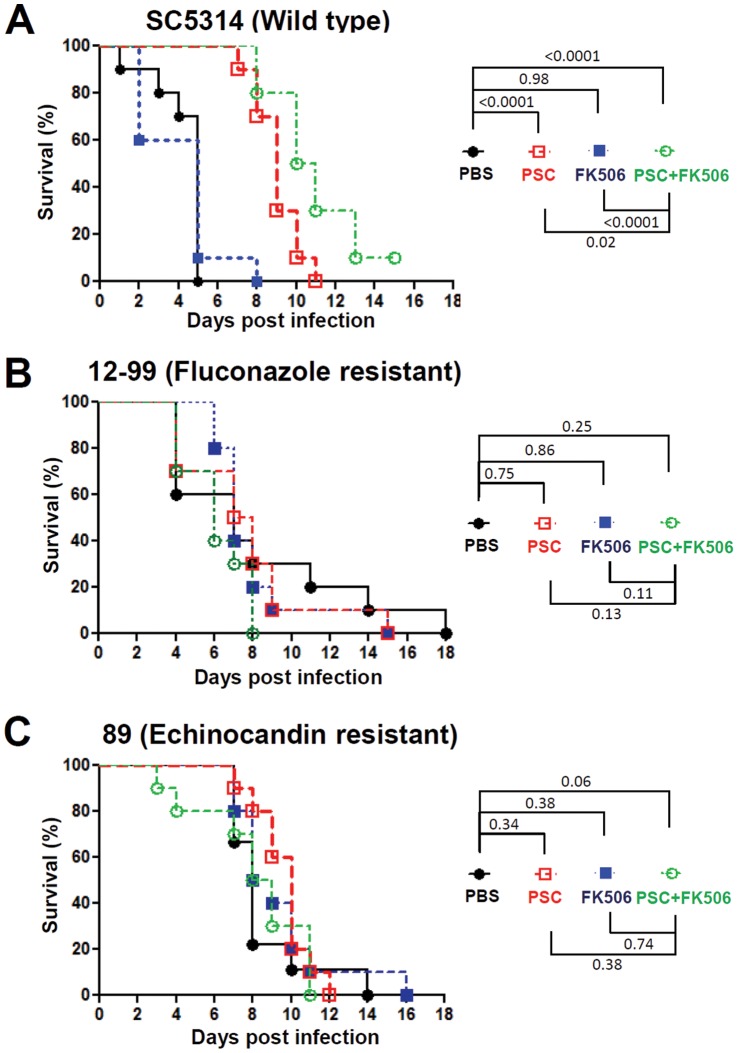
Fluconazole has been demonstrated to exhibit in vitro synergistic antifungal activity with the immunosuppressant FK506 or cyclosporin A against Candida species [29], [30], [31], [32], [33], [34], [35] but this had not yet been reported for posaconazole. Furthermore, it was unclear if posaconazole exhibits the same in vitro and in vivo synergy with FK506 against C. albicans infection. Here, we demonstrate that posaconazole exhibits in vitro synergistic antifungal activity with FK506 against not only the C. albicans SC5314 type strain, but also with the clinical azole- or echinocandin-resistant isolates (FIC<0.5, Table 2). For monotherapy with either posaconazole or FK506, we found that posaconazole at 0.5 mg/kg exhibited therapeutic activity against C. albicans SC5314, but not against fluconazole-resistant isolate 12–99 (2 mg/kg is required for therapeutic response; Figure 1B) or the echinocandin-resistant isolate 89 (Figures 2B and 2C). On the other hand, FK506 at 0.5 mg/kg had no therapeutic activity against C. albicans SC5314 or the drug-resistant strains (Figure 2). For the combination therapy of posaconazole plus FK506, we found that posaconazole exhibits modest in vivo synergy with FK506 against C. albicans SC5314 (P≤0.02; Figure 2A), but no apparent synergy against the fluconazole-resistant 12–99 or echinocandin-resistant 89 isolates (Figures 2B and 2C).
Figure 2. Efficacy of posaconazole and FK506 against C. albicans infection.
Ten male CD1 mice (4–5 weeks-old) per group were infected with 106 cells of C. albicans SC5314, azole-resistant isolate 12–99, or echinocandin-resistant isolate 89, followed by treatment with placebo (PBS), posaconazole (PSC; 0.5 mg/kg), FK506 (0.5 mg/kg), or PSC (0.5 mg/kg) plus FK506 (0.5 mg/kg). The PSC was administered via oral gavage, while PBS and FK506 were administered via intraperitoneal injection at 4, 24, 48, and 72 hr post inoculation. Survival of the animals was monitored for up to 18 days.
Serine 645 of the CaFks1 Protein is Essential for Echinocandin, but not Azole Resistance, in C. albicans
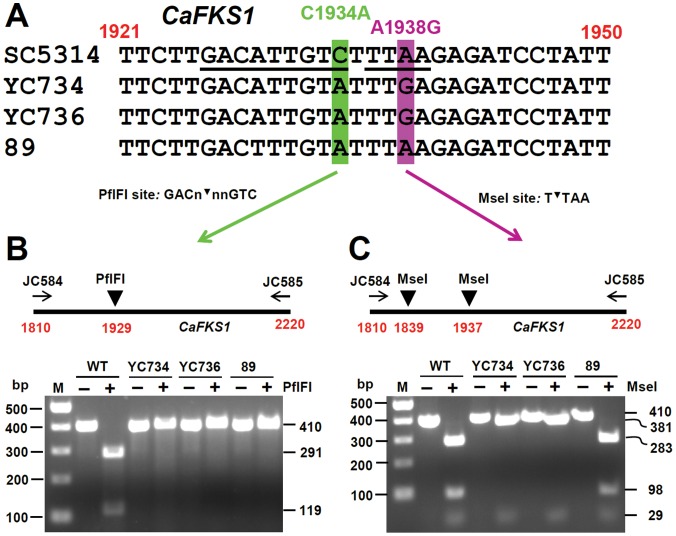
Our data show that the clinical C. albicans echinocandin resistant isolate 89, which has an S645Y mutation in the CaFks1 protein [11], exhibits caspofungin resistance (MIC100>16 µg/ml), and an 8-fold reduced susceptibility to posaconazole (MIC50 = 0.25 µg/ml) compared with SC5314 (MIC50 = 0.03 µg/ml). Because echinocandin resistant isolate 89 and SC5314 are both clinically derived, but not isogenic strains, and isolate 89 has reduced azole susceptibility along with its echinocandin resistance, the contribution of echinocandin resistance and reduced posaconazole susceptibility of isolate 89 may be due to certain strain differences, but not strictly attributable to the S645Y mutation for echinocandin resistance. To test this possibility, we introduced an S645Y mutation in the CaFks1 protein of C. albicans SC5314 via mutagenic oligonucleotide transformation. Two independent mutants (YC734 and YC736) with the C1934A (S645Y) mutation in the CaFKS1 gene were derived from different transformations (Table 1). The C1934A (S645Y) and A1938G mutations of the CaFKS1 sequences of the wild-type SC5314, YC734, YC736, and 89 strains were confirmed by DNA sequencing (Figure 3A), and two restriction enzymes (PflFI and MseI) that distinguish C1934A (S645Y) (Figure 3B) and the A1938G silent mutation (Figure 3C) from wild-type DNA sequences, respectively.
Figure 3. Confirmation of C. albicans FKS1-1 mutants with the S645Y mutation.
(A) Genomic DNA from SC5314 (wild-type), the FKS1-1 mutants (YC734 and YC736), and isolate 89 were PCR amplified with primers JC584/JC585 to detect a 410 bp (1810∼2220) product of the CaFKS1 gene, and then sequenced with primer JC584. Nucleotide 1934 of the CaFKS1 gene is labeled green. The C1934A mutation is present in FKS1-1 mutants derived from SC5314 and in the clinical isolate 89, but absent in SC5314. Nucleotide 1938 of the CaFKS1 gene is labeled purple. An A1938G silent mutation is present in the FKS1-1 mutants, but not in SC5314 or isolate 89. (B)&(C) The 410 bp PCR product of the CaFKS1 gene was amplified from genomic DNA from SC5314, FKS1-1 mutants (YC734 and YC736), and isolate 89, and digested with PflFI (B) or MseI (C) to confirm the C1934A or A1938G mutations are present and to confirm the mutations occurred in both alleles of the gene.
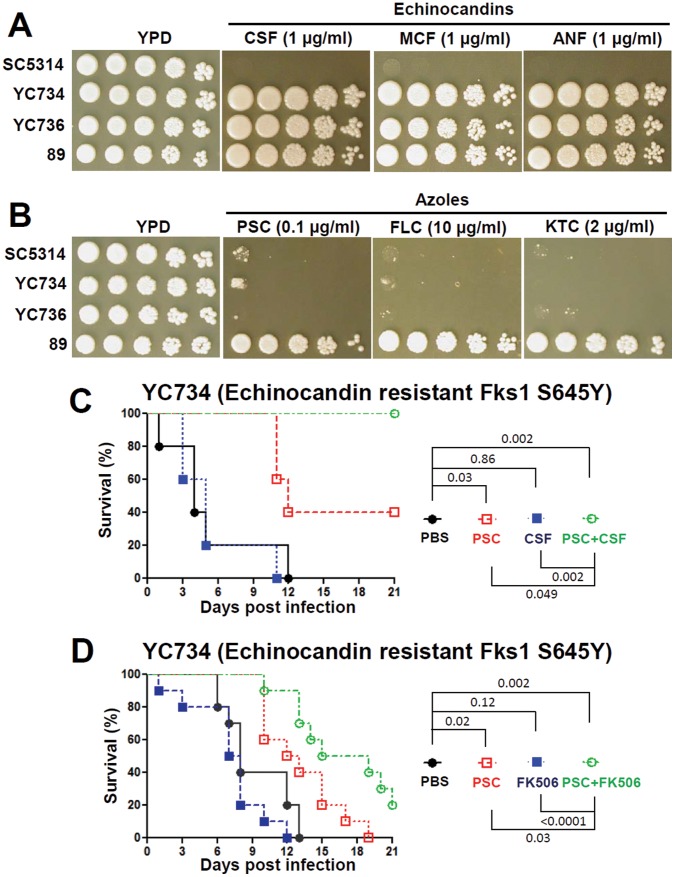
We found that YC734 and YC736 strains with the S645Y amino acid change are tolerant to 1 µg/ml of caspofungin, micafungin, or anidulafungin, while their parent strain SC5314 is susceptible to all three at this concentration (Figure 4A). These results are similar to the echinocandin resistant isolate 89 (Figure 4A), suggesting that the serine amino acid at position 645 of the CaFks1 protein is essential for echinocandin sensitivity. We further found that the echinocandin-resistant isolate 89 is tolerant to multiple azoles (including posaconazole, fluconazole, and ketoconazole) compared with the SC5314, YC734, and YC736 strains (Figure 4B). These results indicate that 1) different clinical isolates can exhibit distinct azole tolerance profiles; 2) isolate 89 may harbor additional mutation(s) altering drug pumps or multi-drug resistance or ERG11 genes that contribute to azole tolerance, and that we might not be simply measuring echinocandin-resistance in vivo when combination therapy is given.
Figure 4. In vitro phenotypes and the in vivo efficacy of posaconazole combined with either caspofungin or FK506 against C. albicans FKS1-1 mutant.
Cells were grown overnight in YPD at 30°C, 5-fold serially diluted, spotted onto YPD medium containing an echinocandin (A) or an azole (B) at the concentrations indicated, and incubated at 37°C for 48 hr. The efficacy of posaconazole (2 mg/kg) and caspofungin (0.1 mg/kg) (C) or posaconazole (0.5 mg/kg) and FK506 (0.5 mg/kg) (D) against the C. albicans FKS1-1 mutant (YC734). The experimental procedures are similar to those described in the legend to Figure 1, except that the survival of the animals was monitored for up to 21 days.
Posaconazole Exhibits in vivo Synergy with Caspofungin or FK506 against Echinocandin-resistant Mutant Generated from C. albicans SC5314
Because the clinical echinocandin-resistant isolate 89 exhibits reduced susceptibility to posaconazole compared with the C. albicans SC5314 isolate, this difference might have affected the in vitro and in vivo interaction between posaconazole and caspofungin or FK506 against the 89 and SC5314 isolates. To test this hypothesis, we determined the in vitro and in vivo efficacy of posaconazole and caspofungin or FK506 against echinocandin-resistant mutants (YC734 and YC736) generated from the isogenic SC5314 isolate. Our in vitro analyses show that, similar to isolate 89, YC734 and YC736 are tolerant to echinocandins, and exhibit similar posaconazole susceptibility profiles to SC5314, but not to isolate 89 (Figure 4 and Table 2). In checkerboard assays, we found that posaconazole exhibits in vitro synergy with caspofungin or FK506 against the YC734 and YC736 mutants (FIC<0.5), and the isolate 89 (FIC≤0.5; Table 2). Furthermore, our in vivo efficacy experiments demonstrated that either the posaconazole-caspofungin or the posaconazole-FK506 combination therapy exhibited synergistic antifungal activity (P<0.05) against echinocandin-resistant strain YC734, an isogenic derivative of SC5314 (Figures 4C and 4D).
Discussion
Our studies demonstrate that posaconazole exhibits synergistic antifungal activity with caspofungin in vitro and in vivo against the wild-type C. albicans isolate SC5314 (Table 2 and Figure 1), suggesting both posaconazole and caspofungin can be effectively combined to treat candidiasis in the murine model of systemic infection, which could possibly lead to better treatment options for patients such as those with endocarditis. While in vitro synergy was seen across the various strains, the in vivo synergy between posaconazole and caspofungin is not evident against the fluconazole-resistant 12–99 or the echinocandin-resistant 89 isolates. This is possibly due to different drug-resistance profiles and/or strain backgrounds between SC5314 and these wild type drug-resistant isolates 12–99 or 89, and demonstrates how variable but important synergy studies can be for in vivo experiments.
Graybill et al. reported that the addition of caspofungin to fluconazole does not exhibit in vivo synergistic antifungal activity in murine candidiasis [36]. On the other hand, previous studies demonstrated that posaconazole and caspofungin exhibited synergistic antifungal activity without evidence of antagonism against C. glabrata (in vitro) and A. fumigatus (in vitro and in a murine aspergillosis model) [27], [28]. Therefore, these studies support a clinical trial or occasional empirical use of these combinations because there is no antagonism found between fluconazole/posaconazole and caspofungin. Our studies have similarly found no antagonism (in vitro or in vivo) between posaconazole and caspofungin against both C. albicans SC5314, and drug-resistant clinical isolates. Interestingly, we specifically found that posaconazole exhibits in vivo synergy with caspofungin (Figure 1), which supports the hypothesis that these two drug classes with different targets are able to therapeutically synergize. The reported differences in the in vivo synergy profiles between posaconazole/caspofungin and fluconazole/caspofungin combinations may be due to 1) structural and spectrum differences between posaconazole and fluconazole [37], 2) differences in experimental protocols between labs, and/or 3) differences in C. albicans strains and/or mouse backgrounds. Previous studies had reported that patients with azole-resistant C. albicans infections can be cured with posaconazole [38], indicating a potential advantage of posaconazole against azole-resistant isolates. This feature was also supported by our finding that posaconazole treatment at a dose of 2 mg/kg (but not 0.5 mg/kg) exhibited therapeutic activity against the azole (especially fluconazole)-resistant isolate 12–99 (Figures 1B and 2B). The reason that the posaconazole-caspofungin drug combination therapy was only efficacious against the wild-type SC5314 strain, but not to fluconazole-resistant isolate 12–99 or echinocandin-resistant isolate 89, might be due to 1) insufficient doses administered to the drug-resistant isolates compared with the wild-type, or 2) high inoculum (106 cells) of drug-resistant isolate used might overwhelm the antifungal activity, or 3) cross resistance/tolerance of isolate 12–99 to caspofungin (Table 2 and data not shown) or isolate 89 to azoles (Figure 4B). For example, Schuetzer-Muehlbauer et al. demonstrated that high levels of C. albicans Cdr2 expression confer cross resistance to multiple antifungal drugs attributable to increased drug efflux [39].
Posaconazole has been demonstrated to show in vitro synergistic antifungal activity with a calcineurin inhibitor (FK506 or cyclosporin A) against zygomycetes. For example, Dannaoui et al. found that posaconazole has in vitro synergy with cyclosporin A against Mycocladus corymbiferus [40], while Narreddy et al. showed that posaconazole exhibits in vitro synergy with cyclosporin A or FK506 against Myocladus corymbifera, Cunninghamella bertholletiae, or Apophysomyces elegans [41]. Although the data of in vivo synergy between posaconazole and a calcineurin inhibitor against C. albicans are lacking, Lewis et al. recently reported that posaconazole and FK506 show in vivo synergy against Rhizopus oryzae in an experimental model of mucormycosis [42]. Furthermore, Marchetti et al. reported that fluconazole exhibits synergy with cyclosporin A against experimental endocarditis due to C. albicans [43]. Our data here showed that posaconazole exhibits synergy with FK506 against the C. albicans SC5314 strain in vitro and in vivo. However, posaconazole only exhibits in vitro and not in vivo synergy with FK506 against the drug-resistant clinical isolates 12–99 or 89 (Table 2; Figure 2). It is evident that posaconazole can be synergistic with FK506 in vitro against clinical drug susceptible or resistant C. albicans isolates (FIC<0.5; Table 2). The reason that posaconazole only exhibits modest in vivo synergistic antifungal activity with FK506 against C. albicans SC5314 or none against the drug-resistant 12–99 or 89 isolates may be attributable to immunosuppression by FK506 in the in vivo setting in addition to its antifungal activity. Another potential issue is that in vivo drug-drug interactions between posaconazole and FK506 could result in the presence of host levels of either drug that can only inhibit drug-susceptible SC5314, but not drug-resistant isolates. Previous studies have shown that posaconazole can inhibit FK506 metabolism by cytochrome P450 (CYP3A4) in cystic fibrosis lung transplant patients to result in ∼3-fold increased levels of FK506 [44], [45]. We attempted to determine if this is the case in our animal model by analyzing the pharmacokinetics of FK506 levels in mouse blood by an LC-MS/MS method. However, in our murine model we did not observe increased blood levels of FK506 when combined with posaconazole (data not shown). This finding may be due to the difficulty of tracing the dynamic changes of blood FK506 levels during our experimental protocols. Another possible explanation for the absence of in vivo synergistic antifungal activity between posaconazole and FK506 against two clinical isolates 12–99 and 89 compared with the SC5314 isolate may be attributable to different strain backgrounds and their specific drug-resistance profiles among these isolates.
In summary, we demonstrate that posaconazole exhibits in vitro synergy with caspofungin or FK506 against the C. albicans isolates tested (Table 2). Furthermore, we show that posaconazole exhibits in vivo synergy with caspofungin or FK506 against C. albicans strain SC5314, a standard wild type isolate, and a derived echinocandin-resistant mutant, YC734, of this strain. Overall, the combination of posaconazole and caspofungin or FK506 has potentially beneficial combined activity, and no apparent deleterious effects were found within host animals infected with C. albicans. These in vitro and in vivo findings clearly support the potential for combination in a clinical trial to test for improvements in therapeutic endpoints in these fragile patients with invasive candidiasis.
Acknowledgments
We thank Ted White (University of Missouri) and David Perlin (Public Health Research Institute and the University of Medicine and Dentistry of New Jersey) for fluconazole-resistant and echinocandin-resistant C. albicans strains. We acknowledge Ruthann Thomas and Tony Adinolfi for support and comments, Darrell Allen for the measurement of FK506 levels in mouse blood via LC-MS/MS analyses, and Bharathi Avula and Ikhlas Khan for the measurement of posaconazole levels in mouse blood via UPLC analyses.
Funding Statement
This work was supported by Pilot funds from Merck & Co., Inc. and Astellas Pharma, Inc., and in part by the Duke University Center for AIDS Research (CFAR) grant (2P30 AI064518-06 to Y.-L.C.) and NIH/NIAID R01 grant AI50438 (J.H.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Calderone RA (2002) Candida and Candidiasis (Chapter 1: Introduction and historical perspectives): p.3–13. American Society for Microbiology, Washington, DC. [Google Scholar]
- 2. Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, et al. (2012) Hidden killers: human fungal infections. Sci Transl Med 4: 165rv113. [DOI] [PubMed] [Google Scholar]
- 3. Morrell M, Fraser VJ, Kollef MH (2005) Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother 49: 3640–3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dolton MJ, Ray JE, Marriott D, McLachlan AJ (2012) Posaconazole exposure-response relationship: evaluating the utility of therapeutic drug monitoring. Antimicrob Agents Chemother 56: 2806–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ngai AL, Bourque MR, Lupinacci RJ, Strohmaier KM, Kartsonis NA (2011) Overview of safety experience with caspofungin in clinical trials conducted over the first 15 years: a brief report. Int J Antimicrob Agents 38: 540–544. [DOI] [PubMed] [Google Scholar]
- 6. Steinbach WJ, Reedy JL, Cramer RA Jr, Perfect JR, Heitman J (2007) Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat Rev Microbiol 5: 418–430. [DOI] [PubMed] [Google Scholar]
- 7. Chen YL, Kozubowski L, Cardenas ME, Heitman J (2010) On the roles of calcineurin in fungal growth and pathogenesis. Curr Fungal Infect Rep 4: 244–255. [Google Scholar]
- 8. Pfaller MA (2012) Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med 125: S3–13. [DOI] [PubMed] [Google Scholar]
- 9. White TC, Pfaller MA, Rinaldi MG, Smith J, Redding SW (1997) Stable azole drug resistance associated with a substrain of Candida albicans from an HIV-infected patient. Oral Dis 3 Suppl 1S102–109. [DOI] [PubMed] [Google Scholar]
- 10. White TC (1997) Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother 41: 1482–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garcia-Effron G, Park S, Perlin DS (2009) Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob Agents Chemother 53: 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hammer SM, Katzenstein DA, Hughes MD, Gundacker H (1996) Schooley RT, et al (1996) A trial comparing nucleoside monotherapy with combination therapy in HIV-infected adults with CD4 cell counts from 200 to 500 per cubic millimeter. AIDS Clinical Trials Group Study 175 Study Team. N Engl J Med 335: 1081–1090. [DOI] [PubMed] [Google Scholar]
- 13. Caliendo AM, Hirsch MS (1994) Combination therapy for infection due to human immunodeficiency virus type 1. Clin Infect Dis 18: 516–524. [DOI] [PubMed] [Google Scholar]
- 14. Markowitz M, Saag M, Powderly WG, Hurley AM, Hsu A, et al. (1995) A preliminary study of ritonavir, an inhibitor of HIV-1 protease, to treat HIV-1 infection. N Engl J Med 333: 1534–1539. [DOI] [PubMed] [Google Scholar]
- 15. Andes D, Marchillo K, Conklin R, Krishna G, Ezzet F, et al. (2004) Pharmacodynamics of a new triazole, posaconazole, in a murine model of disseminated candidiasis. Antimicrob Agents Chemother 48: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klepser ME, Ernst EJ, Ernst ME, Messer SA, Pfaller MA (1998) Evaluation of endpoints for antifungal susceptibility determinations with LY303366. Antimicrob Agents Chemother 42: 1387–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Odds FC (2003) Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52: 1. [DOI] [PubMed] [Google Scholar]
- 18. Andes D, Forrest A, Lepak A, Nett J, Marchillo K, et al. (2006) Impact of antimicrobial dosing regimen on evolution of drug resistance in vivo: fluconazole and Candida albicans . Antimicrob Agents Chemother 50: 2374–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meletiadis J, Pournaras S, Roilides E, Walsh TJ (2010) Defining fractional inhibitory concentration index cutoffs for additive interactions based on self-drug additive combinations, Monte Carlo simulation analysis, and in vitro-in vivo correlation data for antifungal drug combinations against Aspergillus fumigatus . Antimicrob Agents Chemother 54: 602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Worth LJ, Blyth CC, Booth DL, Kong DC, Marriott D, et al. (2008) Optimizing antifungal drug dosing and monitoring to avoid toxicity and improve outcomes in patients with haematological disorders. Intern Med J 38: 521–537. [DOI] [PubMed] [Google Scholar]
- 21. Moerschell RP, Das G, Sherman F (1991) Transformation of yeast directly with synthetic oligonucleotides. Methods Enzymol 194: 362–369. [DOI] [PubMed] [Google Scholar]
- 22. Moerschell RP, Tsunasawa S, Sherman F (1988) Transformation of yeast with synthetic oligonucleotides. Proc Natl Acad Sci U S A 85: 524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson MD, Perfect JR (2010) Use of antifungal combination therapy: agents, order, and timing. Curr Fungal Infect Rep 4: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Onyewu C, Afshari NA, Heitman J (2006) Calcineurin promotes infection of the cornea by Candida albicans and can be targeted to enhance fluconazole therapy. Antimicrob Agents Chemother 50: 3963–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chaturvedi V, Ramani R, Andes D, Diekema DJ, Pfaller MA, et al. (2011) Multilaboratory testing of two-drug combinations of antifungals against Candida albicans, Candida glabrata, and Candida parapsilosis . Antimicrob Agents Chemother 55: 1543–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chatzimoschou A, Katragkou A, Simitsopoulou M, Antachopoulos C, Georgiadou E, et al. (2011) Activities of triazole-echinocandin combinations against Candida species in biofilms and as planktonic cells. Antimicrob Agents Chemother 55: 1968–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oliveira ER, Fothergill AW, Kirkpatrick WR, Coco BJ, Patterson TF, et al. (2005) In vitro interaction of posaconazole and caspofungin against clinical isolates of Candida glabrata . Antimicrob Agents Chemother 49: 3544–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cacciapuoti A, Halpern J, Mendrick C, Norris C, Patel R, et al. (2006) Interaction between posaconazole and caspofungin in concomitant treatment of mice with systemic Aspergillus infection. Antimicrob Agents Chemother 50: 2587–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cruz MC, Goldstein AL, Blankenship JR, Del Poeta M, Davis D, et al. (2002) Calcineurin is essential for survival during membrane stress in Candida albicans . EMBO J 21: 546–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maesaki S, Marichal P, Hossain MA, Sanglard D, Vanden Bossche H, et al. (1998) Synergic effects of tacrolimus and azole antifungal agents against azole-resistant Candida albicans strains. J Antimicrob Chemother 42: 747–753. [DOI] [PubMed] [Google Scholar]
- 31. Marchetti O, Moreillon P, Glauser MP, Bille J, Sanglard D (2000) Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans . Antimicrob Agents Chemother 44: 2373–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen YL, Brand A, Morrison EL, Silao FG, Bigol UG, et al. (2011) Calcineurin controls drug tolerance, hyphal growth, and virulence in Candida dubliniensis . Eukaryot Cell 10: 803–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen YL, Konieczka JH, Springer DJ, Bowen SE, Zhang J, et al. (2012) Convergent evolution of calcineurin pathway roles in thermotolerance and virulence in Candida glabrata . G3 (Bethesda) 2: 675–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang J, Heitman J, Chen YL (2012) Comparative analysis of calcineurin signaling between Candida dubliniensis and Candida albicans . Commun Integr Biol 5: 122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang J, Silao FG, Bigol UG, Bungay AA, Nicolas MG, et al. (2012) Calcineurin is required for pseudohyphal growth, virulence, and drug resistance in Candida lusitaniae . PLoS One 7: e44192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Graybill JR, Bocanegra R, Najvar LK, Hernandez S, Larsen RA (2003) Addition of caspofungin to fluconazole does not improve outcome in murine candidiasis. Antimicrob Agents Chemother 47: 2373–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vazquez JA, Skiest DJ, Nieto L, Northland R, Sanne I, et al. (2006) A multicenter randomized trial evaluating posaconazole versus fluconazole for the treatment of oropharyngeal candidiasis in subjects with HIV/AIDS. Clin Infect Dis 42: 1179–1186. [DOI] [PubMed] [Google Scholar]
- 38. Firinu D, Massidda O, Lorrai MM, Serusi L, Peralta M, et al. (2011) Successful treatment of chronic mucocutaneous candidiasis caused by azole-resistant Candida albicans with posaconazole. Clin Dev Immunol 2011 (283239): 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schuetzer-Muehlbauer M, Willinger B, Krapf G, Enzinger S, Presterl E, et al. (2003) The Candida albicans Cdr2p ATP-binding cassette (ABC) transporter confers resistance to caspofungin. Mol Microbiol 48: 225–235. [DOI] [PubMed] [Google Scholar]
- 40. Dannaoui E, Schwarz P, Lortholary O (2009) In vitro interactions between antifungals and immunosuppressive drugs against zygomycetes. Antimicrob Agents Chemother 53: 3549–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Narreddy S, Manavathu E, Chandrasekar PH, Alangaden GJ, Revankar SG (2010) In vitro interaction of posaconazole with calcineurin inhibitors and sirolimus against zygomycetes. J Antimicrob Chemother 65: 701–703. [DOI] [PubMed] [Google Scholar]
- 42.Lewis RE, Ben-Ami R, Best L, Albert N, Walsh TJ, et al.. (2013) Tacrolimus Enhances the Potency of Posaconazole Against Rhizopus oryzae In vitro and In an Experimental Models of Mucormycosis. J Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marchetti O, Entenza JM, Sanglard D, Bille J, Glauser MP, et al. (2000) Fluconazole plus cyclosporine: a fungicidal combination effective against experimental endocarditis due to Candida albicans . Antimicrob Agents Chemother 44: 2932–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Berge M, Chevalier P, Benammar M, Guillemain R, Amrein C, et al. (2009) Safe management of tacrolimus together with posaconazole in lung transplant patients with cystic fibrosis. Ther Drug Monit 31: 396–399. [DOI] [PubMed] [Google Scholar]
- 45. Billaud EM, Guillemain R, Berge M, Amrein C, Lefeuvre S, et al. (2010) Pharmacological considerations for azole antifungal drug management in cystic fibrosis lung transplant patients. Med Mycol 48 Suppl 1S52–59. [DOI] [PubMed] [Google Scholar]
- 46. Gillum AM, Tsay EY, Kirsch DR (1984) Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet 198: 179–182. [DOI] [PubMed] [Google Scholar]