Abstract
Fabry disease (FD) results from mutations in the gene (GLA) that encodes the lysosomal enzyme α-galactosidase A (α-Gal A), and involves pathological accumulation of globotriaosylceramide (GL-3) and globotriaosylsphingosine (lyso-Gb3). Migalastat hydrochloride (GR181413A) is a pharmacological chaperone that selectively binds, stabilizes, and increases cellular levels of α-Gal A. Oral administration of migalastat HCl reduces tissue GL-3 in Fabry transgenic mice, and in urine and kidneys of some FD patients. A liquid chromatography-tandem mass spectrometry method was developed to measure lyso-Gb3 in mouse tissues and human plasma. Oral administration of migalastat HCl to transgenic mice reduced elevated lyso-Gb3 levels up to 64%, 59%, and 81% in kidney, heart, and skin, respectively, generally equal to or greater than observed for GL-3. Furthermore, baseline plasma lyso-Gb3 levels were markedly elevated in six male FD patients enrolled in Phase 2 studies. Oral administration of migalastat HCl (150 mg QOD) reduced urine GL-3 and plasma lyso-Gb3 in three subjects (range: 15% to 46% within 48 weeks of treatment). In contrast, three showed no reductions in either substrate. These results suggest that measurement of tissue and/or plasma lyso-Gb3 is feasible and may be warranted in future studies of migalastat HCl or other new potential therapies for FD.
Introduction
Fabry disease (FD, OMIM # 301500) is an X-linked lysosomal storage disorder caused by mutations in the gene (GLA; Gene/Locus MIM # 300644, Ref Seq NM_000169.2) that encodes the lysosomal hydrolase α-galactosidase A (α-Gal A, EC 3.2.1.22) [1]. Mutations in GLA that are associated with FD lead to reduced cellular α-Gal A activity [1]. Deficiency of α-Gal A results in accumulation of neutral glycosphingolipids with terminal α-galactosyl residues, primarily globotriaosylceramide (GL-3, Gb3, ceramide trihexoside), in the plasma, and in lysosomal and non-lysosomal compartments of cells of the blood vessels, skin, heart, kidney, brain, and other tissues and organs throughout the body [1], [2], [3], [4].
FD clinical manifestations include progressive renal failure, cardiac disease, cerebrovascular disease, small-fiber peripheral neuropathy, and skin lesions, among other abnormalities [1], [5]. The clinical presentation of FD spans a broad spectrum of severity, and roughly correlates with residual α-Gal A activity [1]. Males with FD who have little or no detectable α-Gal A activity are commonly referred to as “classic” Fabry patients and are most severely affected. Female Fabry patients may be mildly symptomatic or as severely affected as classic males [6]. Many individuals with FD present with a “later-onset” form, and generally have higher residual α-Gal A activity than “classic” patients [7].
Recently, the deacylated GL-3 analogue, globotriaosylsphingosine (known as lyso-Gb3), was found to be markedly increased in the plasma of “classic” male Fabry patients relative to that of normal individuals [8]. The relative excess of plasma lyso-Gb3 exceeded that of plasma GL-3 by more than an order of magnitude [8], [9], [10]. In symptomatic Fabry females, plasma lyso-Gb3 levels were clearly higher, while plasma GL-3 concentrations were in the normal range [8], [9], [10]. High levels of plasma lyso-Gb3 correlated with increased risk for cerebrovascular disease or left ventricular hypertrophy in FD males or females, respectively [9]. Greater life-time exposure to plasma lyso-Gb3 was found to correlate with disease severity in male and female patients with FD [9]. These observations suggest that plasma lyso-Gb3 is an important indicator of FD and warrants further evaluation as a marker of FD clinical severity and progression.
Currently, the only treatment available for Fabry patients is enzyme replacement therapy (ERT), with two approved products: Fabrazyme® (agalsidase beta; Genzyme Corporation, Cambridge, MA) and Replagal® (agalsidase alfa; Shire Pharmaceuticals, Cambridge, MA), generally given as regular every-other-week infusions. In humans, ERT is generally well-tolerated, and in some patients leads to lower levels of plasma, urine, and microvascular endothelial GL-3, stabilized kidney function, and improved FD-related clinical symptoms [11], [12], [13], [14], [15], [16]. Recently, reduction of plasma lyso-Gb3 levels in Fabry patients in response to ERT has been demonstrated [8], [10], [17]. Plasma lyso-Gb3 reduction was significantly lower in Fabry males who developed neutralizing antibodies towards the infused enzyme compared to those who did not [18].
A new approach to the treatment of Fabry disease, and which may serve as an alternative to ERT for some patients, is small-molecule pharmacological chaperone (PC) therapy [19], [20], [21], [22], [23]. PCs selectively bind and stabilize some mutant forms of α-Gal A in the endoplasmic reticulum, facilitate proper protein folding and trafficking, and thereby increase lysosomal enzyme activity. An investigational, orally available small molecule PC, migalastat hydrochloride (1-deoxygalactonojirimycin HCl, AT1001, GR181413A) is in Phase 3 clinical studies to evaluate its safety and efficacy as a potential treatment for FD (see ClinicalTrials.gov: NCT00925301 and NCT01218659). In pre-clinical studies, oral administration of migalastat HCl reduced GL-3 levels in plasma and disease-relevant tissues of Fabry transgenic mice (hR301Q α-Gal A Tg/KO and TgM/KO mice) [24], [25]. Furthermore, in Phase 2 clinical studies, oral administration of migalastat HCl reduced GL-3 levels in urine and in kidneys of some Fabry patients [26]. To date, the effect of migalastat HCl on plasma or tissue levels of lyso-Gb3 has not been evaluated in pre-clinical or clinical studies.
In this study, we developed methods for the detection and quantification of lyso-Gb3 in mouse tissues and human plasma using liquid chromatography-tandem mass spectrometry (LC-MS/MS). These methods were used to analyze lyso-Gb3 levels in disease-relevant tissues of GLA deficient (GLA KO) and hR301Q α-Gal A Tg/KO mice at baseline, after intravenous administration of rhα-Gal A (agalsidase beta), or after oral administration of migalastat HCl. The effects of these drug treatments on tissue lyso-Gb3 levels were compared to their effects on tissue GL-3 levels determined from the same mice. Lastly, plasma lyso-Gb3 was analyzed in six male subjects with FD who were administered migalastat HCl in Phase 2 clinical studies (see ClinicalTrials.gov: NCT00283959 and NCT00283933) [26]. Again, the effect of migalastat HCl treatment on plasma lyso-Gb3 levels was compared to the effects on urine and plasma GL-3 in the same subjects. The results show that measurement of tissue or plasma lyso-Gb3 levels, in addition to GL-3 levels, in response to ERT or oral administration of migalastat HCl is feasible and may be warranted in future pre-clinical and clinical studies.
Materials and Methods
Materials
Globotriaosylceramide (GL-3), lactosylceramide, globotriaosylsphingosine (lyso-Gb3) and plant glucopsychosine were purchased from Matreya LLC (Pleasant Gap, PA). Migalastat HCl was synthesized by Cambridge Major Laboratories (Germantown, WI). Recombinant human α-Gal A (rhα-Gal A; agalsidase beta; Fabrazyme®) was purchased from Genzyme Corporation (Cambridge, MA). Analytical grade methanol, acetonitrile, dimethysulfoxide (DMSO), acetone, sodium acetate and formic acid were purchased from Thermo Fisher Scientific (Waltham, MA). Deionized water was generated using a Mili-Q UV Plus water purifying system from Millipore (Billerica, MA).
Mice/breeding
Mice that express a mutant transgene of human α-Gal A (R301Q) on a GLA knock-out (KO) mixed background of C57BL/6 and B129Sve (hR301Q α-Gal A Tg/KO) and GLA deficient (GLA KO) mice were obtained from Dr. Robert Desnick (Mt. Sinai School of Medecine, New York, NY). Wild-type C57BL/6 mice were purchased from Taconic Farms (Germantown, NY).
Oral Administration of Migalastat HCl to hR301Q α-Gal A Tg/KO Mice
Migalastat HCl was administered orally to mice ad libitum in drinking water as described previously [25]. Briefly, migalastat HCl dosing solutions were made fresh weekly, with appropriate concentrations determined based on the average daily water consumption of hR301Q α-Gal A Tg/KO mice (∼5 mL/day per mouse) (all doses represent the free-base equivalent of the salt form). At study completion, mice were euthanized with CO2. Whole blood was drawn into lithium heparin tubes from the inferior vena cava and plasma was collected by centrifuging blood at 2,700 g for 10 minutes at 4°C. Heart, kidney, brain, and skin (shaved and removed from the lower ventral side of the neck) were quickly removed, rinsed in cold phosphate-buffered saline (PBS), blotted dry, and stored on dry ice.
Tissue Homogenate Preparation Procedure
Tissue homogenates were prepared by adding 16 µL of deionized water per mg of tissue (typically 15 to 25 mg). The mixture was homogenized with lysing matrix A/D on a FastPrep-24 homogenizer (MP Biomedicals, Solon, OH). Care was taken to ensure complete homogenization of the tissue sample.
Determination of GL-3 in Plasma or Tissue Homogenate
GL-3 was extracted from plasma (human and mouse) or mouse tissue homogenate by solid phase extraction (SPE) and analyzed via LC-MS/MS as previously described [25], [26]. Final tissue GL-3 concentrations were reported normalized to tissue weight. GL-3 in human whole urine was determined and normalized to total phosphatidylcholine (PC) as previously described [26].
Lyso-Gb3 Calibration Standard and Quality Control (QC) Sample Preparation
Stock solutions of lyso-Gb3 and the internal standard (IS) glucopsychosine were prepared by dissolving the powders in a solvent mixture of chloroform/methanol (2/1, v/v) at final concentrations of 1 mg/mL. Two different stock solutions were used to prepare calibration standard or quality control samples in two steps. In the first step, one stock solution of lyso-Gb3 was used to prepare calibration standard spiking solutions in DMSO. A separate stock solution of lyso-Gb3 was used to prepare quality control spiking solution in DMSO. In the second step, the lyso-Gb3 calibration standard or QC spiking solution was added to plasma or tissue homogenate (at a dilution of 99/1, v/v; plasma or tissue homogenate/spiking solution) to prepare 8 matrix calibration standard levels: 1, 2, 5, 10, 25, 50, 100, 250 ng/mL and up to 5 matrix QC levels: 2, 4, 40, 80, 160, 200. The matrices (normal control human or wild-type mouse plasma or tissue homogenate) were pre-screened for levels of interfering peaks to lyso-Gb3 or the IS. Blank matrices found to have low or undetectable levels of interfering lipids were used.
Lyso-Gb3 Tissue or Plasma Sample Extraction Procedure
A 50 µL aliquot of the plasma or tissue homogenate was transferred to a 13 mL silanized glass tube and 25 µL of the internal standard (500 ng/mL glucopsychosine in DMSO) was added. The mixture was further diluted with 1 mL of methanol, vortexed briefly, then sonicated at room temperature for approximately 10 minutes. After the addition of 500 µL of 1 N HCl, the mixture was shaken on medium speed (setting = 5) on a multi-tube vortexer (VWR, Radnor, PA) for approximately 30 minutes and then centrifuged at 3,220 g for 5 minutes at room temperature. The supernatant was loaded onto a pre-conditioned Oasis MCX 3 cc, 60 mg sorbent solid phase extraction cartridge (Waters, Milford, MA) and lyso-Gb3 extracted as previously described [27].
Analytical Run Composition
All analytical runs were populated with double blanks (normal control human or wild-type mouse plasma or tissue homogenate that was run to check for interfering chromatographic peaks), blanks (normal control human or wild-type mouse plasma or tissue homogenate fortified with IS and used to check the IS response), two sets of calibration standards (one at the beginning of the run, and the other at the end), and QC samples in triplicate randomly placed within the runs.
HPLC Instrumental Conditions
Chromatographic separation of lyso-Gb3 and IS was conducted using a liquid chromatography (LC) system that consisted of an HTc autosampler coupled with two LC-20AD pumps from Shimadzu (Columbia, MD). The chromatographic separation was performed at room temperature under a gradient elution profile using a Halo HILIC 2.7 µm, 75×4.6 mm silica analytical column from MAC MOD (Chadds Ford, PA). The following binary mobile phase system was used: A: 5 mM ammonium formate and 0.5% formic acid in acetonitrile/H2O (5/95, v/v), and B: 5 mM ammonium formate and 0.5% formic acid in acetonitrile/H2O (95/5, v/v). The following gradient profile was used to elute lyso-Gb3 and IS from the analytical column: 0.00 to 1.0-min/100% B, 1.01 to 4.00-min/100% to 70% B, 4.01 to 6.00-min/70% B, 6.01 to 7.50-min/60% B, 7.51 to 11∶00-min/100% B, and 11∶01-min/stop.
MS/MS Instrumental Conditions
Tandem mass (MS/MS) spectrometry detection of lyso-Gb3 and IS was performed using a 4000QTrap mass spectrometer (Applied Biosystems, Foster City, CA). All optimization was performed via FIA with the above mentioned Shimadzu LC system (see HPLC instrumental conditions) at a flow rate of 0.5 mL/minute and a 10 µL injection. Positive ion electrospray ionization (ESI+) was used with the following conditions: an ion spray voltage of +5500V, a source temperature of 500°C, a curtain gas flow of 30 psi, a Gas1 flow of 20 psi, a Gas2 flow of 60 psi, a de-clustering potential of +141V for lyso-Gb3 and +56V for the IS. Nitrogen was used as the collision gas with a pressure of 6.00 mTorr. The collision energy was set at +53V for lyso-Gb3 and +29V for the IS. Quantitative MS/MS data were collected using selected reaction monitoring (SRM) scan mode with precursor ion to product ion transitions of m/z 787 to m/z 282 for lyso-Gb3 and m/z 460 to m/z 280 for the IS. These transitions represent the neutral loss of hexose from these molecules. The total run time was 11 minutes. All MS/MS data were acquired and analyzed using Analyst version 1.4.2 (Applied Biosystems).
Determination of Lyso-Gb3 in Plasma and Tissues Samples
A linear calibration curve with weighting factor 1/x was generated by plotting the ratio of the peak area of lyso-Gb3 to that of the IS versus increasing standard actual concentrations of lyso-Gb3 in plasma or tissue homogenate. The standard curve was used to quantify lyso-Gb3 levels in the study samples.
Extraction Efficiency of Lyso-Gb3 from Plasma and Tissue Homogenates
The extraction efficiency (also referred to as “recovery”) of lyso-Gb3 from biological matrices across the dynamic range of the current method was assessed at three concentration levels in plasma or tissue homogenates. The mean % recovery of lyso-Gb3 was defined as the ratio of the mean peak areas determined from sample extracts to those determined from blank matrix extracts that were spiked with lyso-Gb3 at similar concentrations after extraction.
Data Analysis
Percent bias (% Bias) was defined as 100 times the difference between the mean found concentration and the actual concentration divided by the actual concentration [% Bias = 100×[(mean found concentration – actual concentration)/actual concentration]. Precision or percent coefficient of variation (% CV) was defined as 100 times the standard deviation divided by the mean found concentration [% CV = 100×(standard deviation/mean found concentration)].
Determinations of statistical significance were conducted using GraphPad Prism, version 5 (San Diego, CA). In the mouse studies, percent reduction (or percent change) refers to the percent of the mean difference from untreated (or control), and was calculated using Excel 2003 (Microsoft, Redmond, WA) as follows: [(mean untreated – treated) ÷ mean untreated] * 100. In the plasma samples from male FD patients, percent reduction (or percent change) refers to the percent of the difference from baseline, and was calculated using Excel 2003 as follows: [(baseline – treated) ÷ baseline] * 100.
Ethics Statement
All animal experiments including animal husbandry were conducted according to protocols approved by the Rutgers University Animal Care and Facilities Use Committee. Patients signed informed consent to future use of their samples for research related to FD. These plasma samples were obtained during two open-label, Phase 2 clinical studies (see ClinicalTrials.gov: NCT00283959 and NCT00283933), which received Ethical Committee/Institutional Review Board (IRB) approval and were conducted according to accepted standards of Good Clinical Practice (ICH-GCP) and in agreement with the Declaration of Helsinki [26].
Results
Chromatography and Calibration Curves
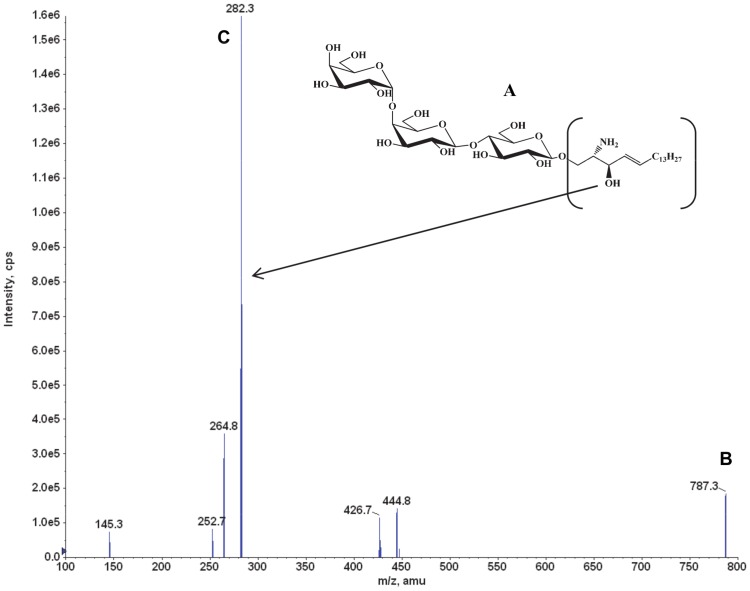
The base peak [M+H]+ at m/z = 787 was consistent with the lyso-Gb3 molecular weight of 787 ( Fig. 1A,B ). The product ion spectrum of m/z = 787 exhibited a major product ion at m/z = 282 ( Fig. 1C ). Thus, the SRM transition m/z = 787 → m/z = 282 was used to quantify lyso-Gb3 in plasma and tissue homogenates.
Figure 1. Lyso-Gb3: structure, MS, and product ion spectra.
The lyso-Gb3 molecule (A) was detected and confirmed in positive electrospray ionization mode (ESI+), where (B) shows the lyso-Gb3 [M+H]+ ion at m/z of 787, and (C) shows the most intense product ion at m/z 282. The data were collected in product ion scan mode.
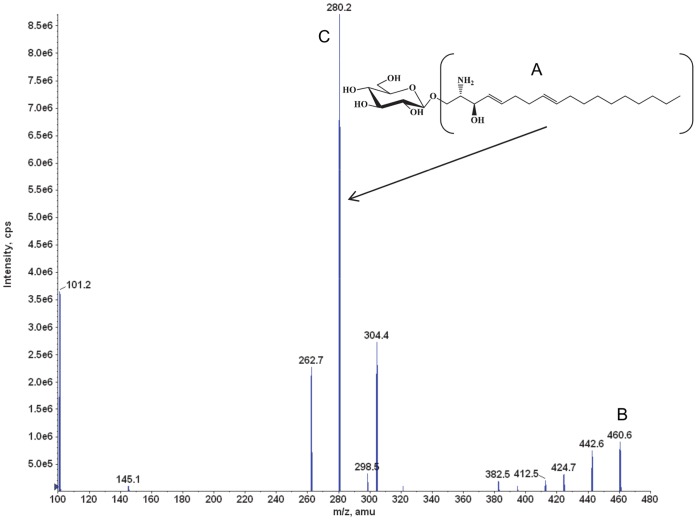
The base peak [M+H]+ at m/z = 460 was consistent with the glucopsychosine molecular weight of 460 ( Fig. 2A,B ). The product ion spectrum of m/z = 460 exhibited a major product ion at m/z = 280 ( Fig. 2C ). Therefore, the SRM transition m/z = 460 → m/z = 280 was used to quantify glucopsychosine in plasma and tissue homogenates.
Figure 2. Glucopsychosine (IS): structure, MS, and product ion spectra.
The glucopsychosine molecule (A) was detected and confirmed in positive electrospray ionization mode (ESI+), where (B) shows the glucopsychosine [M+H]+ ion at m/z of 460, and (C) shows the most intense product ion at m/z 280. The data were collected in product ion scan mode.
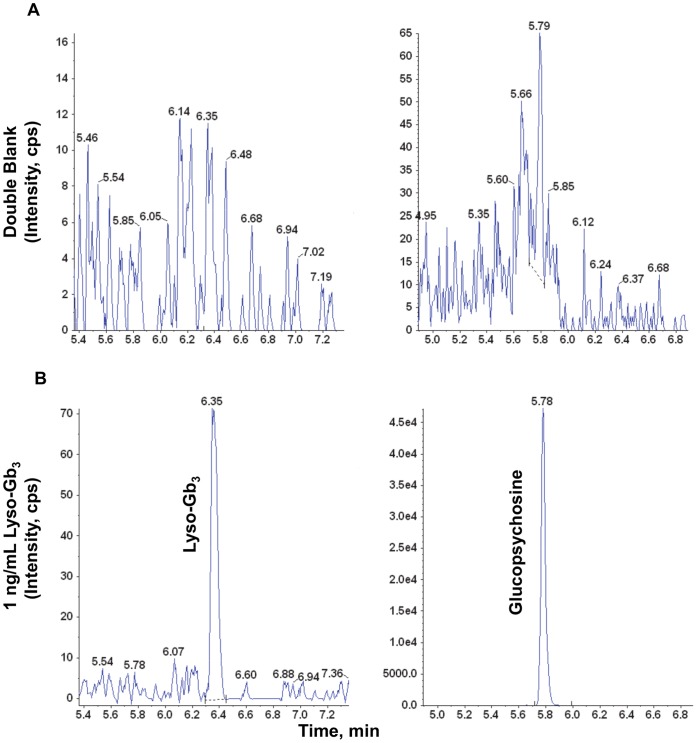
The retention times for lyso-Gb3 and the glucopsychosine IS were approximately 6.3 minutes and 5.7 minutes, respectively, with an 11-minute run time (Figure 3). There were interfering peaks due to the presence of endogenous lyso-Gb3 and glucopsychosine in both normal control plasma and tissue homogenate samples. These interfering peaks were generally ≤25% of the lyso-Gb3 peak at the LLOQ (1 ng/mL) and ≤1% of the glucopsychosine peak at the concentration of 500 ng/mL used in this assay.
Figure 3. Representative LC-MS/MS chromatograms of lyso-Gb3 and glucopsychosine extracted from human plasma.
(A) Blank human plasma without spiked lyso-Gb3 or glucopsychosine; (B) lyso-Gb3 calibration standard at LLOQ = 1 ng/mL; glucopsychosine (IS) in control human plasma.
The linear regression y = ax+b with a weighting factor of 1/x was determined to best represent the relationship of the lyso-Gb3 concentration in plasma or tissue homogenates and the detector response (defined as the peak area ratio of lyso-Gb3 to the IS). Lyso-Gb3 levels from unknown samples were determined based on this calibration curve. The overall coefficient of determination r2 of the calibration curves in all matrices was ≥0.988.
Assay Bias and Precision
QC samples were used to assess intra- and inter-assay bias and precision of the LC-MS/MS assay. QC samples were prepared in normal control human or wild-type mouse plasma or tissue homogenates at the LLOQ, low, middle, and high concentration range of the calibration curve, and were analyzed on at least two separate days. The QC samples were populated in the beginning, middle, and end of the analytical run to account for sample stability and possible instrumental drift. An intra-assay bias criterion was set, whereby the analytical run was accepted if 2/3 of the QC samples were within ±20% (±25% for the LLOQ) of the actual lyso-Gb3 concentration.
The intra-assay bias in the four mouse matrices ranged from −5.70% to 15.6% of actual values (data not shown). The intra-assay precision across all matrices was ≤16.6% (22.9% at the LLOQ) (data not shown). The inter-assay bias across the four mouse matrices ranged from −5.70% to 15.6% of actual values ( Table 1 ). The inter-assay precision across the four matrices was ≤15.8% ( Table 1 ). Inter- and intra-assay bias and precision were also assessed in quality control samples prepared in normal control human plasma, as described above. In summary, the intra-assay bias ranged from −12.8 to 15.6% of actual values (data not shown). The intra-assay precision ranged from 2.52 to 19.8% (data not shown). The inter-assay bias in human plasma ranged from −6.19 to 3.60% of actual values ( Table 2 ). The inter-assay precision ranged from 9.25 to 16.4% ( Table 2 ).
Table 1. Inter-assay % Bias and precision (% CV) of lyso-Gb3 quality control mouse tissue samples.
| QC (Actual Conc.) | Kidney | Skin | Heart | Plasma | |
| Low1(2.00 ng/mL) | Mean ± SD | 2.16±0.306 | 2.02±0.233 | 2.12±0.122 | 2.15±0.210 |
| % CV | 14.2 | 11.5 | 5.77 | 9.78 | |
| % Bias | 7.89 | 1.00 | 6.17 | 7.36 | |
| Low2(4.00 ng/mL) | Mean ± SD | 4.10±0.327 | 4.18±0.517 | 4.23±0.413 | 4.09±0.616 |
| % CV | 8.0 | 12.4 | 9.78 | 15.1 | |
| % Bias | 2.50 | 4.43 | 5.67 | 2.25 | |
| Mid(80.0 ng/mL) | Mean ± SD | 84.9±8.43 | 82.3±5.51 | 92.5±3.91 | 79.8±12.6 |
| % CV | 9.93 | 6.70 | 4.23 | 15.8 | |
| % Bias | 6.08 | 2.88 | 15.6 | −0.250 | |
| High(160 ng/mL) | Mean ± SD | 162±19.7 | 167±13.9 | 181±27.2 | 151±11.8 |
| % CV | 12.2 | 8.34 | 15.0 | 7.83 | |
| % Bias | 1.32 | 4.50 | 13.2 | −5.70 |
Quality control samples were prepared in wild-type mouse plasma or tissue homogenates at four concentration levels and analyzed on three separate days. Six to 10 replicates at each concentration level were used for inter-assay determination. Conc., concentration; SD, Standard Deviation.
Table 2. Inter-assay % Bias and precision (% CV) of lyso-Gb3 quality control human plasma samples.
| QC (actual conc.) | Low1 (2.00) | Low2 (4.00) | Mid1 (40.0) | Mid2 (80.0) | High (200) |
| Mean ± SD (ng/mL) | 2.07±0.249 | 3.75±0.617 | 41.2±5.20 | 75.0±6.94 | 200±19.7 |
| % CV | 12.0 | 16.4 | 12.6 | 9.25 | 9.84 |
| % Bias | 3.60 | −6.16 | 2.95 | −6.19 | 0.15 |
Quality control samples were prepared in normal human plasma at five concentration levels and analyzed on four separate days. Twenty replicates at each concentration level were used for inter-assay determination. Conc., concentration (ng/mL); SD, Standard Deviation.
Recovery of Lyso-Gb3 from Plasma and Tissue Homogenates
The mean % recovery of lyso-Gb3 from human plasma ranged from 76% to 104% at the three concentration levels used in this experiment ( Table 3 ). The mean % recovery in mouse plasma or tissue homogenates was greater than or equal to 65% across the actual concentrations of lyso-Gb3 assessed in the experiment ( Table 3 ).
Table 3. Recovery of lyso-Gb3 from plasma and tissue homogenates.
| % Recovery (Mean) | |||||
| Human | Mouse | ||||
| Actual Conc.(ng/mL) | Plasma | Plasma | Skin | Heart | Kidney |
| 2 | 76 | 80 | 70 | 88 | 76 |
| 10 | 94 | 76 | 64 | 66 | 71 |
| 160 | 104 | 87 | 69 | 86 | 77 |
Recovery samples were prepared in normal human plasma, and wild-type mouse tissue homogenates or plasma at three concentration levels and analyzed in quintuplicates (n = 5). % recovery (mean) = 100×(mean peak area of extracted lyso-Gb3/mean peak area of unextracted lyso-Gb3).
Determination of Lyso-Gb3 in Normal and Fabry Mouse Tissues
An α-Gal A gene knock-out (GLA KO) mouse model of FD that shows significant accumulation of GL-3 in multiple tissues including skin, heart, and kidney has been described [25], [28], [29], [30]. In addition, a new mouse model of Fabry disease that expresses a human R301Q GLA transgene transcriptionally regulated by the human GLA promoter on a GLA KO background (hR301Q α-Gal A Tg/KO) has also been shown to accumulate GL-3 in Fabry disease-relevant tissues [25]. Recently, the GLA KO mice have been shown to accumulate lyso-Gb3 in multiple tissues, as measured by o-phtaldialdehyde (OPA)-derivitization of lyso-Gb3 followed by HPLC-fluorescence detection (HPLC-FD) [8], [31]. However, lyso-Gb3 levels have not yet been characterized in the hR301Q α-Gal A Tg/KO mice. Thus, to assess whether tissue lyso-Gb3 accumulation in GLA KO mice can be reproduced using our LC-MS/MS assay, and to extend the characterization of the hR301Q α-Gal A Tg/KO mouse model, the baseline levels of lyso-Gb3 in heart, kidney, and skin tissues were measured in 12-week old male wild-type (C57BL/6), hR301Q α-Gal A Tg/KO, and GLA KO mice by LC-MS/MS ( Fig. 4A ). GL-3 levels were also measured by LC-MS/MS in the same tissue samples for comparison ( Fig. 4B ).
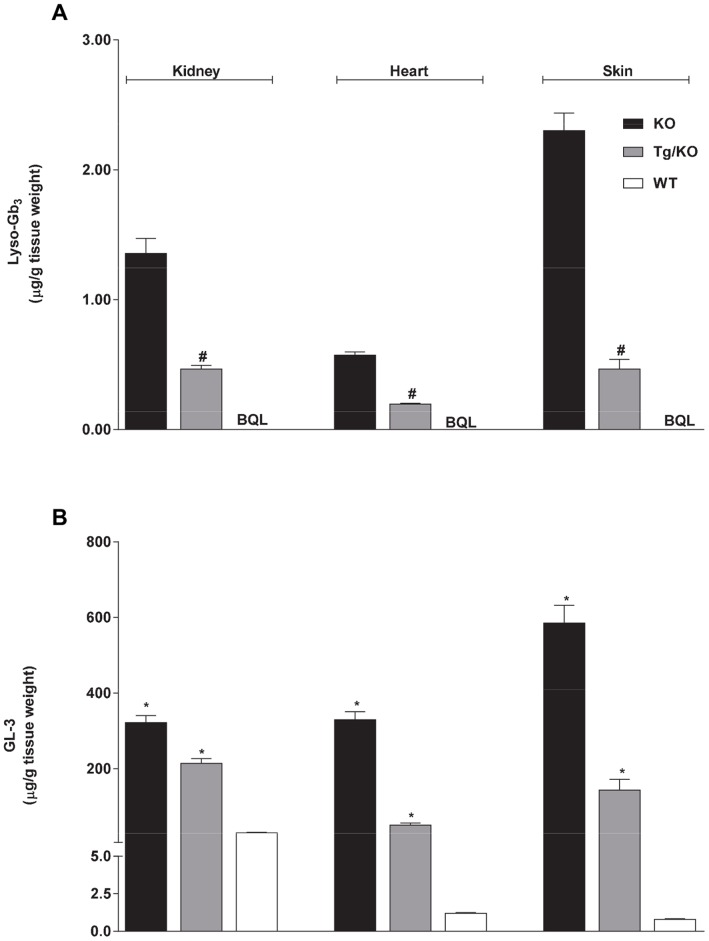
Figure 4. Baseline levels of lyso-Gb3 and GL-3 in normal and Fabry mouse tissues.
Baseline levels of (A) lyso-Gb3 and (B) GL-3 were measured in kidney, heart, and skin tissues of twelve-week-old male wild-type (C57BL/6; WT), hR301Q α-Gal A Tg/KO (Tg/KO), and GLA KO (KO) mice. *p<0.05 compared to WT, t-test; #p<0.05 compared to KO, t-test; WT contained non-detectable levels of lyso-Gb3. BQL = Below Quantitation Limit <0.034 ug/g tissue weight; the lyso-Gb3 and GL-3 data represent the mean ± SEM of 5–10 mice/group.
Lyso-Gb3 levels were below the lower limit of quantification (BQL <1 ng/mL) in wild-type mouse tissues, but were markedly elevated in hR301Q α-Gal A Tg/KO and GLA KO mice. GLA KO mice showed significantly higher tissue levels of lyso-Gb3 (2.9 to 4.9-fold) compared to those found in hR301Q α-Gal A Tg/KO mouse tissues. The trend seen for lyso-Gb3 levels (i.e., GLA KO mice>hR301Q α-Gal A Tg/KO mice>wild-type mice) was similar for GL-3 levels determined in the same tissues of these transgenic and wild-type mice.
Administration of rhα-Gal A Decreases lyso-Gb3 and GL-3 Levels in GLA KO Mice
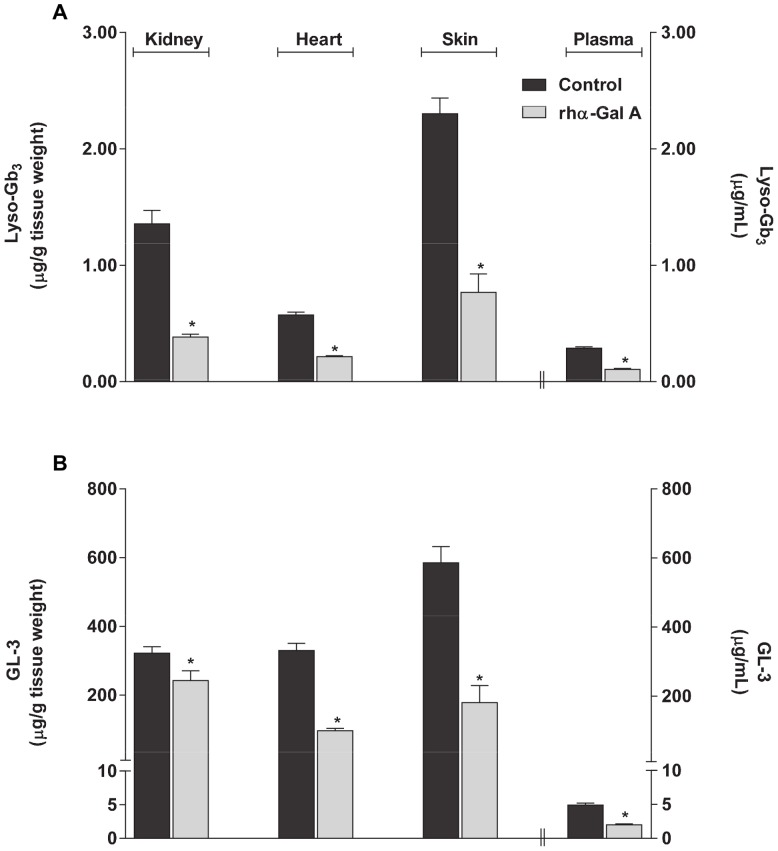
Intravenous administration of rhα-Gal A (agalsidase beta, 1 mg/kg body weight) to GLA KO mice has been shown to result in both decreased lyso-Gb3 and GL-3 levels as measured by non-LC-MS/MS methods (e.g., HPLC-FD of OPA-derivitized lyso-Gb3 and high performance thin layer chromatography with immunostaining and luminescent imaging of GL-3) [31]. In that study, the observed relative decrease in kidney lyso-Gb3 was greater than the decrease in kidney GL-3. Thus, to assess whether a similar pattern of decrease can be reproduced using LC-MS/MS, lyso-Gb3 and GL-3 levels were determined in heart, kidney, skin, and plasma of GLA KO mice seven days after a single intravenous administration of rhα-Gal A (1 mg/kg agalsidase beta; Fig. 5 ). Lyso-Gb3 and GL-3 levels in all tissues and plasma were significantly (p<0.05) lower in the rhα-Gal A-treated animals compared to untreated animals. The reductions in lyso-Gb3 levels (mean ± std. err.) were −72±2%, −63±2%, −67±7%, and −63±3% in kidney, heart, skin and plasma, respectively. The reductions in GL-3 levels were −25±9%, −71±2%, −70±8%, and −59±3% in kidney, heart, skin, and plasma, respectively. Thus, consistent with previous observations, rhα-Gal A administered to GLA KO mice in this study generally resulted in similar reductions of lyso-Gb3 and GL-3 levels in most FD-relevant tissues and plasma, except in kidney where the reduction in lyso-Gb3 (−72%) was substantially greater than that seen for GL-3 (−25%).
Figure 5. Lyso-Gb3 and GL-3 reductions in GLA KO mice administered rhα-Gal A.
Twelve-week old male GLA KO mice were used as control or administered 1 mg/kg rhα-Gal A via bolus tail vein injection. Kidney, heart, skin, and plasma were harvested 7 days post-administration for the determination of (A) lyso-Gb3 and (B) GL-3 levels. The lyso-Gb3 and GL-3 data represent the mean ± SEM of 5 mice/group. *p<0.05 compared to untreated; t-test.
Administration of Migalastat HCl Decreases Tissue Lyso-Gb3 Levels in hR301Q α-Gal A Tg/KO Mice
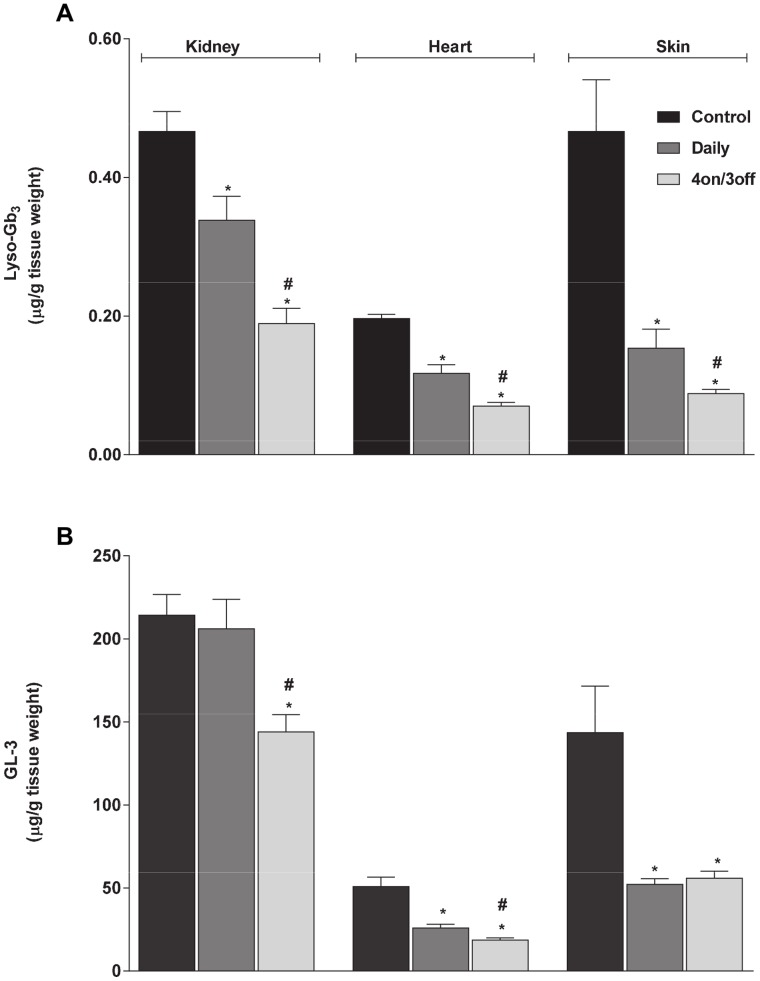
Migalastat HCl is a pharmacological chaperone that can selectively bind, stabilize, and increase cellular levels of α-Gal A [20], [23], [32], [33], [34], [35]. Recently, oral administration of migalastat HCl was shown to reduce GL-3 levels in tissues of hR301Q α-Gal A Tg/KO mice [25]. In that study, dose optimization revealed that an even greater reduction in GL-3 was achieved using less-frequent administration as compared to daily administration of migalastat HCl. Thus, in the current study, we assessed the effect of migalastat HCl on tissue lyso-Gb3 levels in these mice using our LC-MS/MS assay, and compared these results to the effect observed on tissue GL-3 as determined from the same tissue samples. To this end, hR301Q α-Gal A Tg/KO mice were administered migalastat HCl (100 mg/kg) ad libitum in drinking water either daily or less frequently (four consecutive days with drug followed by three consecutive days with drinking water only; i.e., 4 on/3 off) for 28 days.
Daily and less-frequent administration of migalastat HCl to hR301Q α-Gal A Tg/KO mice resulted in significant reductions in lyso-Gb3 levels in kidney, heart, and skin (p<0.05 for all three tissues using either regimen) ( Fig. 6A ). Furthermore, significantly greater reductions in tissue lyso-Gb3 levels were seen with the “4 on/3 off” regimen compared to daily administration. Lyso-Gb3 reductions (mean ± std. err.) of −27±7%, −40±6%, and −67±6% were seen in kidney, heart, and skin, respectively, with daily migalastat HCl administration; reductions of −59±5%, −64±3%, and −81±1%, respectively, were seen with the less-frequent regimen. In general, similar effects were seen on GL-3 levels determined from the same tissues. GL-3 reductions of −4±8%, −49±4%, and −64±2% were seen in kidney, heart, and skin, respectively, with daily migalastat HCl administration; reductions of −33±5%, −63±3%, and −61±3%, respectively, were seen with the less-frequent regimen. In general, GL-3 reduction was greater with the less-frequent regimen ( Fig. 6B ), consistent with previous findings [25]. In addition, reduction of kidney lyso-Gb3 in hR301Q α-Gal A Tg/KO mice with either migalastat HCl regimen was substantially greater than the reductions in kidney GL-3, and was consistent with the greater reduction of lyso-Gb3 compared to GL-3 seen in GLA KO mouse kidney after administration of rhα-Gal A.
Figure 6. Lyso-Gb3 and GL-3 reduction in hR301Q α-Gal A Tg/KO mice administered migalastat HCl.
Eight-week old male Fabry hR301Q α-Gal A Tg/KO mice were administered either water or migalastat HCl (100 mg/kg ad libitum in drinking water) daily or less frequently (4 on/3 off) for 28 days. Kidney, heart, and skin were subsequently harvested and analyzed for (A) lyso-Gb3 and (B) GL-3 levels. The lyso-Gb3 and GL-3 data represent the mean ± SEM of 10 mice/group. *p<0.05 compared to control; #p<0.05 compared to daily; t-test.
The Effects of Migalastat HCl on lyso-Gb3 in Plasma of FD Patients
In two open-label, Phase 2 clinical studies (see ClinicalTrials.gov: NCT00283959 and NCT00283933) a total of nine male FD patients were administered 150 mg migalastat HCl orally every other day (QOD) for up to 48 weeks. Increases in peripheral blood mononuclear cell (PBMC), skin, and kidney α-Gal A activity of at least 50% were seen in 6 of 9 patients. GL-3 decreases were also seen in skin, urine, and kidney. In urine, all nine patients had elevated GL-3 levels prior to administration of migalastat HCl. In 5 of the 9 patients, urine GL-3 levels were lower by at least 20% at the last measured time point compared to baseline [26].
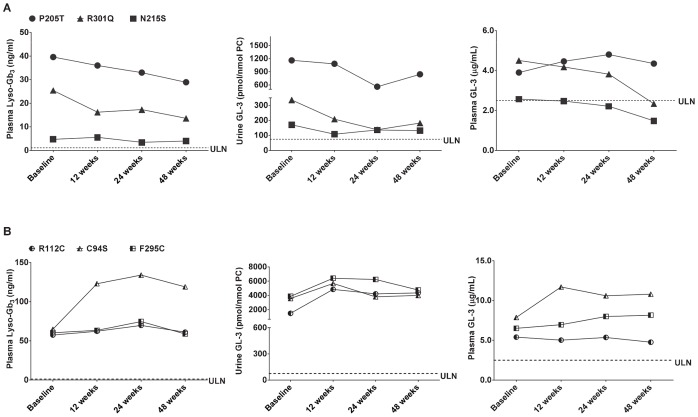
In the current study, the effects of treatment with migalastat HCl on plasma lyso-Gb3 levels were retrospectively assessed in a subset of male FD patients from these Phase 2 clinical studies. The effects on plasma lyso-Gb3 were compared to the effects on other potential biomarkers, such as plasma and urine GL-3, in the same patients. To this end, lyso-Gb3 and GL-3 levels were analyzed in plasma samples collected at baseline, as well as after 12, 24, and 48 weeks of migalastat HCl administration to six male FD patients during the Phase 2 studies. These six patients signed informed consent to future use of their samples for research related to FD. Furthermore, three of these FD patients had the GLA missense mutations p.N215S, p.P205T, and p.R301Q, and had shown increased PBMC α-Gal A activity and decreased urine GL-3 levels after migalastat HCl administration. The other three had GLA missense mutations, p.C94S, p.R112C and p.F295C, had shown increase in PBMC α-Gal A activity with no decrease in urine GL-3 levels after migalastat HCl administration at 150 mg QOD for up to 48 weeks [26].
The baseline plasma lyso-Gb3 levels of the six FD patients ranged from 4.70 to 64.9 ng/mL ( Fig. 7A, B ; left panels). These levels were markedly elevated above normal (normal level <2.00 ng/mL in the current study (n = 6), consistent with the previously reported normal range of 0.12 to 1.12 ng/mL determined from 178 healthy control plasma samples; [36]). In the three FD patients with p.P205T, p.N215S, and p.R301Q mutations who had shown urine GL-3 changes ranging from −20% to −59% after 24 or 48 weeks on migalastat HCl ( Fig. 7A ; middle panel), plasma lyso-Gb3 levels were also reduced with changes ranging from −15% to −46% at the same time points ( Fig. 7A ; left panel). In the three FD patients with p.C94S, p.R112C, and p.F295C mutations who had shown changes in urine GL-3 ranging from +7% to +192% after 24 or 48 weeks on drug ( Fig. 7B ; middle panel), plasma lyso-Gb3 levels showed changes of −2.6% to +106% ( Fig. 7B ; left panel).
Figure 7. Plasma lyso-Gb3, as well as urine and plasma GL-3 levels in male FD patients after oral administration of migalastat HCl.
(A) Male FD patients who showed urine GL-3 reductions after oral administration of migalastat HCl; (B) male FD patients who did not show urine GL-3 reductions after oral administration of migalastat HCl; ULN, upper limit of normal. The ULN was determined for urine GL-3, and the value is 74.6 pmol/nmol PC. The same acronym denotes the upper range of normal for plasma lyso-Gb3 (value is 1.12 ng/mL; [36]) and plasma GL-3 (value is 2.50 µg/mL).
In contrast to the marked baseline elevations seen for urine GL-3 and plasma lyso-Gb3, baseline plasma GL-3 levels were either moderately elevated or in the high-normal range in these six FD patients (range: 2.57 to 7.87 µg/mL compared to 1.15 to 2.50 µg/mL in 6 different normal plasma samples that were assessed in this study, and compared to 7 µg/mL upper limit of normal reported previously [37]). These results were consistent with the male FD patient plasma GL-3 ranges previously reported in multiple independent investigations [8], [9], [17]. In addition, plasma GL-3 changes after migalastat HCl administration ( Fig. 7A, B ; right panels) were not consistent with the changes seen in urine GL-3 or plasma lyso-Gb3 for some of the patients.
Discussion
Lyso-Gb3, a deacylated analogue of GL-3, the primary substrate which accumulates in FD, has recently been shown to be elevated in the plasma of Fabry patients and is an important new indicator of FD [8]. In the current study, an LC-MS/MS method was developed that allows accurate and quantitative measurement of lyso-Gb3 in human plasma as well as in mouse skin, kidney, heart, and plasma. Preliminary assessment indicates that the method can also be applied to lyso-Gb3 quantification in mouse brain (data not shown).
Previous studies of lyso-Gb3 in mouse tissues have used non-LC-MS/MS methods with lower sensitivity (∼10 ng/mL LLOQ) that have required lengthy and complicated preparation procedures [8], [31]. These same methods were used to discover the presence of elevated lyso-Gb3 in the plasma of FD patients [8]. More recently, high sensitivity (∼ 2 ng/mL LLOQ in human plasma), rapid LC-MS/MS methods for measurement of lyso-Gb3 in human urine and plasma have been developed [27], [36], [38], [39]. One of these human plasma methods used a non-commercially available glycine derivative of lyso-Gb3 as an internal standard. Our method utilizes commercially-available plant glucopsychosine as the internal standard, the same internal standard used by Auray-Blais et al. for the measurement of lyso-Gb3 in human urine [27]; and by Boutin et al. for the measurement of lyso-Gb3 in human plasma [39]. A previously stated concern about the use of plant glucopsychosine as an internal standard was the risk of interference with the glucopsychosine measurement depending on patient nutrition status or consumption of a plant-rich diet [36], [38]. Thus, in our assays the concentration of the internal standard (500 ng/mL) that was used achieved peak area counts in a range where the contribution of endogenous lipid interference was limited to 1% or less of the internal standard response. In addition, the previously reported methods have not been evaluated in mouse tissues or plasma. Thus, the LC-MS/MS method we have developed has equivalent sensitivity, accuracy, precision, reliability, and simplicity, and works for a variety of sample and tissue types in mice and humans while not requiring access to specialty reagents.
We report the first LC-MS/MS analysis of lyso-Gb3 levels, with comparison to GL-3 levels, determined in multiple tissues from two different Fabry mouse models at baseline, after intravenous administration of rhα-Gal A, or after oral administration of the PC, migalastat HCl. The results reproduced previous findings of elevated baseline lyso-Gb3 in GLA KO mice as measured by non-LC-MS/MS methods [8], [31]. Interestingly, hR301Q α-Gal A Tg/KO mice showed intermediate levels of lyso-Gb3 and GL-3 in tissues compared to GLA KO and wild-type mice, perhaps due to the low but significant residual activity of the transgene [20], [25], [33], [40]. As shown previously [31], intravenous administration of rhα-Gal A to GLA KO mice reduced lyso-Gb3 and GL-3 levels to similar extents in multiple tissues, except kidney in which the lyso-Gb3 reduction was greater. Furthermore, similar reductions in lyso-Gb3 and GL-3 were seen across most tissues, except in kidney, of the transgenic mice after daily or less-frequent oral administration of migalastat HCl. In both Fabry mouse models, kidney GL-3, but not lyso-Gb3, consistently showed less reduction than other tissues in response to the different treatments and regimens. Less kidney GL-3 reduction in the transgenic mice after oral administration of miglastat HCl has been reported previously [25]; and may be due to differences in the turnover rates of the affected cell types in the various tissues, differences in the tissue concentrations and rates of clearance of the PC, or the possibility of GL-3 re-uptake into kidney from the urine [25]. In addition, it is interesting to note that our lyso-Gb3 and GL-3 results were from tissues of male mice. Unlike normal humans or FD patients [41], [42], mature male wild-type and GLA KO mice have markedly elevated urine and kidney GL-3 levels as compared to mature female wild-type and GLA KO mice [43]. This murine-specific sex difference is thought to be due to a testosterone-induced form of GL-3 that is secreted into the urine in multi-lamellar bodies [44], [45], [46], which appears to be inaccessible to exogenously administered rhα-Gal A [30]. Moreover, incomplete clearance of kidney GL-3 in male GLA KO mice has been reported in response to other forms of therapy [47], [48], [49], [50]. However, in one study of female TgM/KO mice expressing hR301Q under a β-actin promoter (a different hR301Q mouse model than used in the current studies), oral administration of migalastat HCl (3 mg/kg ad libitum in drinking water for 4 weeks) again led to an incomplete reduction (−46%) in kidney GL-3 levels [24], although GL-3 reduction in other tissues was not assessed. Thus, less GL-3 reduction in kidney as compared to other tissues of male Fabry mice reported here is consistent with previous results and may be due to a number of factors related to the kidney tissue, the drug treatments, and/or to the sex of the animals used in the studies. Importantly, the consistently greater reductions in kidney lyso-Gb3 as compared to GL-3 seen in the current studies suggest that kidney lyso-Gb3 may be a more sensitive indicator of increased kidney α-Gal A activity in situ in pre-clinical studies of male Fabry mice.
We also report the first analysis of lyso-Gb3 levels in plasma from male Fabry patients after oral administration of migalastat HCl, an investigational PC that is currently in Phase 3 studies for the treatment of FD (see ClinicalTrials.gov: NCT00925301 and NCT01218659). This retrospective analysis of samples obtained during Phase 2 clinical studies (see ClinicalTrials.gov: NCT00283959 and NCT00283933) [26] showed that the baseline plasma lyso-Gb3 levels in all six patients studied (6 to 83 nM) were markedly elevated above normal (normal <1.4 nM). The baseline plasma lyso-Gb3 range was lower, but overlapped with the previous reported plasma lyso-Gb3 range seen in males from a larger cohort of patients with “classic” FD (range: 51 to 489 nM; n = 37 [9]).
Three FD patients in the Phase 2 clinical studies had GLA missense mutations (p.N215S, p.P205T, and p.R301Q), and had shown increased PBMC α-Gal A activity and decreased urine GL-3 levels after migalastat HCl administration [26]. Based on the current study, these three FD patients also showed decreased plasma lyso-Gb3 levels after oral administration of migalastat HCl (150 mg QOD for up to 48 weeks). The other three FD patients had GLA mutations corresponding to p.C94S, p.R112C, and p.F295C, and had not shown increased PBMC α-Gal A activity or decreased urine GL-3 levels after migalastat HCl administration [26]. Based on the current study, these three FD patients also did not show decreased plasma lyso-Gb3 levels after oral administration of migalastat HCl. These results indicate that in this limited number of male FD patients, the change in plasma lyso-Gb3 was consistent with the change in urine GL-3 after oral administration of migalastat HCl. In contrast, plasma GL-3 changes from baseline were not consistent with the changes seen in urine GL-3 or plasma lyso-Gb3 for some of the patients. Furthermore, the baseline plasma GL-3 levels were not clearly elevated in all of the patients, consistent with high-normal plasma GL-3 ranges previously reported in male FD patients [8], [9], [10], [17].
In the three FD patients that showed plasma lyso-Gb3 reductions, the magnitudes of the changes (−15% to −47%) after 24 to 48 weeks of migalastat HCl administration were lower than those (e.g., −68%, n = 22) seen in male classic FD patients after 48 weeks of treatment with different ERT regimens [8], [10], [17]. However, in those previous studies, the baseline plasma lyso-Gb3 levels in the ERT-treated male patients were markedly greater (102 to 397 nM, ∼250 to 450 nM, and ∼75 nM respectively). It is possible that greater plasma lyso-Gb3 reductions may be dependent, in part, on greater elevation at baseline; further investigation is warranted.
For more than a decade, monitoring plasma or urine GL-3 levels as a diagnostic tool for FD and as a marker of treatment efficacy has been evaluated in numerous independent investigations [51], [52]. Urine GL-3 was more consistently elevated as compared to plasma GL-3, particularly in females with FD. However, for some females and a few exceptional males with certain ‘later-onset’ disease-associated mutations (e.g., N215S and M296I), urine GL-3 was in the normal range, limiting its diagnostic value for such patients [53]. More recently, plasma lyso-Gb3 has shown greater sensitivity than plasma GL-3 as an indicator of FD in females [9]. However, a direct and thorough comparison of the sensitivity of plasma lyso-Gb3 to that of urine GL-3 is not yet available. The current study provides an initial retrospective assessment of plasma lyso-Gb3 and urine GL-3 levels at baseline and in response to oral administration of migalastat HCl in samples from the same FD patients. In the small number of male FD patients tested, plasma lyso-Gb3 and urine GL-3 showed comparable results. Future clinical investigation to extend the comparison of urine GL-3 and plasma lyso-Gb3 to larger FD patient cohorts, preferably matched for age, sex, disease severity, and GLA genotype is warranted.
In conclusion, we have developed a sensitive and robust method for the detection of lyso-Gb3 levels in mouse tissues and human plasma using LC-MS/MS. We have found that lyso-Gb3 is elevated in disease-relevant tissues of a transgenic mouse model of Fabry disease. We have shown significant reductions in the transgenic mouse tissue lyso-Gb3 levels in response to daily or less-frequent oral administration of migalastat HCl. The tissue lyso-Gb3 reductions were generally equal to or greater than those of GL-3 determined from the same mice. The LC-MS/MS method was also used to retrospectively analyze plasma lyso-Gb3 levels in six male subjects orally administered migalastat HCl (150 mg QOD) in Phase 2 clinical studies [26]. Importantly, the results show for the first time that migalastat HCl can lower plasma lyso-Gb3 levels in some FD patients treated with this PC. Thus, the LC-MS/MS method developed here represents a single approach that enables accurate measurement of lyso-Gb3 in pre-clinical and clinical studies of investigational new therapies for the treatment of FD, as well as in new studies of currently available ERTs. As migalastat HCl is in Phase 3 clinical studies to further assess its safety and efficacy for the treatment of FD, the results from the current studies suggest that monitoring of lyso-Gb3, in addition to other assessments, is feasible and warranted in future prospective or retrospective studies.
Acknowledgments
The authors thank the Fabry patients and their families for their dedication and cooperation, and for their participation in the Phase 2 clinical studies. The authors also thank all the Phase 2 study site personnel.
Funding Statement
Amicus Therapeutics funded the research and the publication fees. Amicus Therapeutics designed and performed the study, collected and analyzed the data, made the decision to publish, and prepared the manuscript. No external funding was used for this study.
References
- 1.Desnick R, Ioannou Y, Eng C (2001) α-Galactosidase A deficiency: Fabry disease. In: Scriver C, Beaudet A, Sly W, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. New York, New York, USA: McGraw-Hill. 3733–3774.
- 2. Askari H, Kaneski CR, Semino-Mora C, Desai P, Ang A, et al. (2007) Cellular and tissue localization of globotriaosylceramide in Fabry disease. Virchows Arch 451: 823–834. [DOI] [PubMed] [Google Scholar]
- 3. Brady OR, Gal AE, Bradley RM, Martensson E, Warshaw AL, et al. (1967) Enzymatic defect in Fabry's disease: ceramide trihexosidase deficiency. N Engl J Med 276: 1163–1167. [DOI] [PubMed] [Google Scholar]
- 4. Sweeley CC, Klionsky B (1963) Fabry's disease: classification as a sphingolipidosis and partial characterization of a novel glycolipid. J Biol Chem 238: PC3148–PC3150. [PubMed] [Google Scholar]
- 5.Germain DP (2010) Fabry disease. Orphanet J Rare Dis doi:10.1186/1750-1172-5-30. [DOI] [PMC free article] [PubMed]
- 6. Wilcox WR, Oliveira JP, Hopkin RJ, Ortiz A, Banikazemi M, et al. (2008) Females with Fabry disease frequently have major organ involvement: Lessons from the Fabry Registry. Mol Genet Metab 93: 112–128. [DOI] [PubMed] [Google Scholar]
- 7. von Scheidt W, Eng CM, Fitzmaurice TF, Erdmann E, Hubner G, et al. (1991) An atypical variant of Fabry's disease with manifestations confined to the myocardium. N Engl J Med 324: 395–399. [DOI] [PubMed] [Google Scholar]
- 8. Aerts JM, Groener JE, Kuiper S, Donker-Koopman WE, Strijland A, et al. (2008) Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Nat Acad Sci USA 105: 2812–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rombach SM, Dekker N, Bouwman MG, Linthorst GE, Zwinderman AH, et al. (2010) Plasma globotriaosylsphingosine: diagnostic value and relation to clinical manifestations of Fabry disease. Biochim Biophys Acta 1802: 741–748. [DOI] [PubMed] [Google Scholar]
- 10. Togawa T, Kodama T, Suzuki T, Sugawara K, Tsukimura T, et al. (2010) Plasma globotriaosylsphingosine as a biomarker of Fabry disease. Mol Genet Metab 100: 257–261. [DOI] [PubMed] [Google Scholar]
- 11. Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, et al. (2001) Safety and efficacy of recombinant human α-galactosidase A replacement therapy in Fabry's disease. N Engl J Med 345: 9–16. [DOI] [PubMed] [Google Scholar]
- 12. Hughes DA (2008) Early therapeutic intervention in females with Fabry disease? Acta Paediatr 97: 41–47. [DOI] [PubMed] [Google Scholar]
- 13. Schiffmann R, Floeter MK, Dambrosia JM, Gupta S, Moore DF, et al. (2003) Enzyme replacement therapy improves peripheral nerve and sweat function in Fabry disease. Muscle Nerve 28: 703–710. [DOI] [PubMed] [Google Scholar]
- 14. Schiffmann R, Kopp JB, Austin Iii HA, Sabnis S, Moore DF, et al. (2001) Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA 285: 2743–2749. [DOI] [PubMed] [Google Scholar]
- 15. West M, Nicholls K, Mehta A, Clarke JTR, Steiner R, et al. (2009) Agalsidase alfa and kidney dysfunction in Fabry disease. J Am Soc Nephrol 20: 1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Warnock DG, Ortiz A, Mauer M, Linthorst GE, Oliveira JP, et al. (2012) Renal outcomes of agalsidase β treatment for Fabry disease: role of proteinuria and timing of treatment initiation. Nephrol Dial Transplant 27: 1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Breemen MJ, Rombach SM, Dekker N, Poorthuis BJ, Linthorst GE, et al. (2011) Reduction of elevated plasma globotriaosylsphingosine in patients with classic Fabry disease following enzyme replacement therapy. Biochim Biophys Acta 1812: 70–76. [DOI] [PubMed] [Google Scholar]
- 18.Rombach SM, Aerts JMFG, Poorthuis BJ, Groener JEM, Donker-Koopman WE, et al. (2012) Long-term effect of antibodies against infused α-galactosidase A in Fabry disease on plasma and urinary lyso-Gb3 reduction and treatment outcome. PLoS ONE 7(10) doi:10.1371/journal.pone.0047805. [DOI] [PMC free article] [PubMed]
- 19. Fan J-Q, Ishii S (2003) Cell-based screening of active-site specific chaperone for the treatment of Fabry disease. Methods Enzymol 363: 412–420. [DOI] [PubMed] [Google Scholar]
- 20. Fan J-Q, Ishii S, Asano N, Suzuki Y (1999) Accelerated transport and maturation of lysosomal α-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nature Med 5: 112–115. [DOI] [PubMed] [Google Scholar]
- 21. Valenzano KJ, Khanna R, Powe AC, Boyd R, Lee G, et al. (2011) Identification and characterization of pharmacological chaperones to correct enzyme deficiencies in lysosomal storage disorders. Assay Drug Dev Technol 9: 213–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yam GH, Bosshard N, Zuber C, Steinmann B, Roth J (2006) Pharmacological chaperone corrects lysosomal storage in Fabry disease caused by trafficking-incompetent variants. Am J Physiol Cell Physiol 290: C1076–1082. [DOI] [PubMed] [Google Scholar]
- 23. Yam GH, Zuber C, Roth J (2005) A synthetic chaperone corrects the trafficking defect and disease phenotype in a protein misfolding disorder. FASEB J 19: 12–18. [DOI] [PubMed] [Google Scholar]
- 24. Ishii S, Chang H-H, Yoshioka H, Shimada T, Mannen K, et al. (2009) Preclinical efficacy and safety of 1-deoxygalactonojirimycin in mice for Fabry disease. J Pharmacol Exp Ther 328: 723–731. [DOI] [PubMed] [Google Scholar]
- 25. Khanna R, Soska R, Lun Y, Feng J, Frascella M, et al. (2010) The pharmacological chaperone 1-deoxygalactonojirimycin reduces tissue globotriaosylceramide levels in a mouse model of Fabry disease. Mol Ther 18: 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Germain DP, Giugliani R, Hughes DA, Mehta A, Nicholls K, et al. (2012) Safety and pharmacodynamic effects of the pharmacological chaperone migalastat hydrochloride on α-galactosidase A activity and globotriosylceramide clearance in Fabry disease: report from two phase 2 clinical studies. Orphanet J Rare Dis 7: 91. Available: http://www.ojrd.com/content/7/1/91 Accessed 2013 Jan 17. [DOI] [PMC free article] [PubMed]
- 27. Auray-Blais C, Ntwari A, Clarke JTR, Warnock DG, Oliveira JP, et al. (2010) How well does urinary lyso-Gb3 function as a biomarker in Fabry disease? Clin Chim Acta 411: 1906–1914. [DOI] [PubMed] [Google Scholar]
- 28. Ohshima T, Murray GJ, Swaim WD, Longenecker G, Quirk JM, et al. (1997) α-galactosidase A deficient mice: a model of Fabry disease. Proc Natl Acad Sci USA 94: 2540–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Benjamin ER, Khanna R, Schilling A, Flanagan JJ, Pellegrino LJ, et al. (2012) Co-administration with the pharmacological chaperone AT1001 increases recombinant human α-galactosidase A tissue uptake and improves substrate reduction in Fabry mice. Mol Ther 20: 717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ioannou YA, Zeidner KM, Gordon RE, Desnick RJ (2001) Fabry disease: preclinical studies demonstrate the effectiveness of α-galactosidase A replacement in enzyme-deficient mice. Am J Hum Genet 68: 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Togawa T, Kawashima I, Kodama T, Tsukimura T, Suzuki T, et al. (2010) Tissue and plasma globotriaosylsphingosine could be a biomarker for assessing enzyme replacement therapy for Fabry disease. Biochem Biophys Res Comm 399: 716–720. [DOI] [PubMed] [Google Scholar]
- 32. Asano N, Ishii S, Kizu H, Ikeda K, Yasuda K, et al. (2000) In vitro inhibition and intracellular enhancement of lysosomal α-galactosidase A activity in Fabry lymphoblasts by 1-deoxygalactonojirimycin and its derivatives. Eur J Biochem 267: 4179–4186. [DOI] [PubMed] [Google Scholar]
- 33. Benjamin E, Flanagan J, Schilling A, Chang H, Agarwal L, et al. (2009) The pharmacological chaperone 1-deoxygalactonojirimycin increases α-galactosidase A levels in Fabry patient cell lines. J Inherit Metab Dis 32: 424–440. [DOI] [PubMed] [Google Scholar]
- 34. Shin S-H, Murray GJ, Kluepfel-Stahl S, Cooney AM, Quirk JM, et al. (2007) Screening for pharmacological chaperones in Fabry disease. Biochem Biophys Res Comm 359: 168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Germain DP, Fan JQ (2009) Pharmacological chaperone therapy by active-site-specific chaperones in Fabry disease: in vitro and preclinical studies. Int J Clin Pharmacol Ther 47: S111–117. [PubMed] [Google Scholar]
- 36. Krüger R, Tholey A, Jakoby T, Vogelsberger R, Mönnikes R, et al. (2012) Quantification of the Fabry marker lyso-Gb3 in human plasma by tandem mass spectrometry. Journal of Chromatography B 883–884: 128–135. [DOI] [PubMed] [Google Scholar]
- 37. Wilcox WR, Banikazemi M, Guffon N, Waldek S, Lee P, et al. (2004) Long-term safety and efficacy of enzyme replacement therapy for Fabry disease. Am J Hum Genet 75: 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Auray-Blais C, Boutin M, Gagnon R, Dupont FO, Lavoie P, et al. (2012) Novel urinary lyso-Gb3-related biomarkers for Fabry disease targeted by metabolomics. Anal Chem 84: 2745–2753. [DOI] [PubMed] [Google Scholar]
- 39.Boutin M, Gagnon R, Lavoie P, Auray-Blais C (2012) LC–MS/MS analysis of plasma lyso-Gb3 in Fabry disease [published online ahead of print October 2, 2012]. Clin Chim Act doi: 10.1016/j.cca.2012.09.026. [DOI] [PubMed]
- 40. Wu X, Katz E, Valle CD, Mascioli K, Flanagan J, et al. (2011) A pharmacogenetic approach to identify mutant forms of α-galactosidase A that respond to a pharmacological chaperone for Fabry disease. Human Mutat 32: 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Auray-Blais C, Cyr D, Ntwari A, West ML, Cox-Brinkman J, et al. (2008) Urinary globotriaosylceramide excretion correlates with the genotype in children and adults with Fabry disease. Mol Genet Metab 93: 331–340. [DOI] [PubMed] [Google Scholar]
- 42. Boyd B, Lingwood C (1989) Verotoxin receptor glycolipid in human renal tissue. Nephron 51: 207–210. [DOI] [PubMed] [Google Scholar]
- 43. Durant B, Forni S, Sweetman L, Brignol N, Meng X-L, et al. (2011) Sex differences of urinary and kidney globotriaosylceramide and lyso-globotriaosylceramide in Fabry mice. J Lipid Res 52: 1742–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gross SK, Daniel PF, Evans JE, McCluer RH (1991) Lipid composition of lysosomal multilamellar bodies of male mouse urine. J Lipid Res 32: 157–164. [PubMed] [Google Scholar]
- 45. McCluer RH, Deutsch CK, Gross SK (1983) Testosterone-responsive mouse kidney glycosphingolipids: developmental and inbred strain effects. Endocrinology 113: 251–258. [DOI] [PubMed] [Google Scholar]
- 46. McCluer RH, Williams MA, Gross SK, Meisler MH (1981) Testosterone effects on the induction and urinary excretion of mouse kidney glycosphingolipids associated with lysosomes. J Biol Chem 256: 13112–13120. [PubMed] [Google Scholar]
- 47. Park J, Murray GJ, Limaye A, Quirk JM, Gelderman MP, et al. (2003) Long-term correction of globotriaosylceramide storage in Fabry mice by recombinant adeno-associated virus-mediated gene transfer. Proc Nat Acad Sci USA 100: 3450–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ogawa K, Hirai Y, Ishizaki M, Takahashi H, Hanawa H, et al. (2009) Long-term inhibition of glycosphingolipid accumulation in Fabry model mice by a single systemic injection of AAV1 vector in the neonatal period. Mol Genet Metab 96: 91–96. [DOI] [PubMed] [Google Scholar]
- 49. Ohshima T, Schiffmann R, Murray GJ, Kopp J, Quirk JM, et al. (1999) Aging accentuates and bone marrow transplantation ameliorates metabolic defects in Fabry disease mice. Proc Natl Acad Sci USA 96: 6423–6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Takenaka T, Murray GJ, Qin G, Quirk JM, Ohshima T, et al. (2000) Long-term enzyme correction and lipid reduction in multiple organs of primary and secondary transplanted Fabry mice receiving transduced bone marrow cells. Proc Natl Acad Sci USA 97: 7515–7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Aerts J, Kallemeijn W, Wegdam W, Joao Ferraz M, van Breemen M, et al. (2011) Biomarkers in the diagnosis of lysosomal storage disorders: proteins, lipids, and inhibodies. J Inherit Metab Dis 34: 605–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bekri S, Lidove O, Jaussaud R, Knebelmann B, Barbey F (2006) The role of ceramide trihexoside (globotriaosylceramide) in the diagnosis and follow-up of the efficacy of treatment of Fabry disease: a review of the literature. Cardiovasc Hematol Agents Med Chem 4: 289–297. [DOI] [PubMed] [Google Scholar]
- 53.Young E, Mills K, Morris P, Vellodi A, Lee P, et al.. (2005) Is globotriaosylceramide a useful biomarker in Fabry disease? Acta Paediatr 94: 51–54; discussion 37–58. [DOI] [PubMed]