Abstract
The main keratinase (kerA) gene from the Bacillus licheniformis S90 was optimized by two codon optimization strategies and expressed in Pichia pastoris in order to improve the enzyme production compared to the preparations with the native kerA gene. The results showed that the corresponding mutations (synonymous codons) according to the codon bias in Pichia pastoris were successfully introduced into keratinase gene. The highest keratinase activity produced by P. pastoris pPICZαA-kerAwt, pPICZαA-kerAopti1 and pPICZαA-kerAopti2 was 195 U/ml, 324 U/ml and 293 U/ml respectively. In addition, there was no significant difference in biomass concentration, target gene copy numbers and relative mRNA expression levels of every positive strain. The molecular weight of keratinase secreted by recombinant P. pastori was approx. 39 kDa. It was optimally active at pH 7.5 and 50°C. The recombinant keratinase could efficiently degrade both α-keratin (keratin azure) and β-keratin (chicken feather meal). These properties make the P. pastoris pPICZαA-kerAopti1 a suitable candidate for industrial production of keratinases.
Introduction
Keratins are major component of cuticulum i.e. feathers, wool, scales, hair, hoofs, nails and so on. Due to intensive cross-linking by hydrogen, hydrophobic and cystine disulfide bonds, keratins are recalcitrant and poorly degraded by most proteinases, e.g. papain, collagenase, pepsin and trypsin [1]–[2]. In spite of the considerably stability, keratin can be efficiently degraded by many microorganisms because of the secretion of keratinases [2]–[3].
Keratinases are metallo or serine proteinases which can degrade the insoluble structure forming keratin substrates. The keratinases obtained from various sources have been widely used in medicine, detergent, cosmetics, leather and biodegradable plastic manufacture [3]–[4]. Keratinases were also used to degrade the prion for treatment of bovine spongiform encephalopathy, scrapie and human Creutzfeldt-Jakob disease [5]. Moreover, keratinases have been developed by poultry and feed industries to improve the properties of poultry diets [6]–[7]. Therefore, the requirement for keratinases has been recently increased. A myriad of bacteria, fungi and actinomycetes were found to naturally secrete keratinases [2]–[4].
Bacillus licheniformis has been known as potential host to produce efficient keratinolytic enzymes for industrial applications [8]–[10]. The keratinase (kerA) gene of B. licheniformis has been sequenced and cloned [11]–[12]. The molecular mass of mature keratinase is about 30–33 kDa [8]–[9]. The keratinase presents an optimum activity around 50–60°C [8]–[9], [13]. The isoelectric point and optimum pH of keratinase are 7.25 and 7.5, respectively [8]–[9]. Due to relatively high enzyme production costs, the industrial applications of keratinase produced by Bacillus licheniformis are still not extensive. Moreover, the keratinases secreted by B. licheniformis are usually contaminated by other enzymes (e.g. amylase, mannase, cellulolytic enzyme, etc.), which complicate the purification process.
Recently, production of keratinases using heterologous expression systems such as fungi and bacteria have attracted considerable research interests due to high level of protein yield and high purity. One of the most attractive expression systems is the yeast Pichia pastoris. It combines many advantages of the eukaryotic protein expression systems including protein posttranslational modification, processing, folding and so on, meanwhile its manipulation is as easy as E. coli expression system. Compared to other eukaryotic protein expression systems, the P. pastoris expression system is easier, faster, less expensive and higher expression [13]–[14]. Many proteases from bacteria, fungi, actinomycetes and mammal have been successfully expressed in P. pastoris system [15]–[16].
It is well known that heterogeneous protein expression level in P. pastoris strongly depends on the biased codon usage [17]–[19]. This problem is minimized by codon optimization technique. Previous studies demonstrated that the codon optimization technique greatly increases (about 1- to 10-fold) the foreign proteins expression in P. pastoris [20]–[21]. In this study, the B. licheniformis keratinase gene was cloned and optimized using different codon optimization strategies. All mutated keratinase genes were expressed in P. pastoris. The aim of the present study was to develop a high-level expression system of keratinase. Furthermore, the biochemical properties of the purified recombinant keratinase including substrate specificity and amino acid sequence were examined. To our knowledge, this is also the first report to improve keratinase production by codon optimization strategies in P. pastoris.
Materials and Methods
Plasmids, Reagents, Media and Strains
B. licheniformis S90 which could efficiently degrade the feathers was stored in our laboratory [22] and grown at 37°C for 2–3 d on feather medium [22]. E. coli DH5α was grown at 37°C for 12–18 h in Luria-Bertani (LB) broth or low sault LB media. P. pastoris X-33 was from our laboratory and cultivated at 30°C for 2–3 d in YPD (1% yeast extract, 2% glucose and 2% peptone) medium or agar. The plasmids pMD20-T was a T-cloning vector and obtained from TaKaRa (China). The pPICZαA was an expression vector for secretion in P. pastoris and purchased from Invitrogen (USA). The pMD20T-GAP plasmids was established and stored in our laboratory (unpublished data).
Total genomic DNA was extracted from B. licheniformis S90 using TIANamp Bacteria DNA kit (Tiangen, China). Plasmid DNA was extracted from E. coli by Plasmid Mini kit I (Omega, USA). The T4 DNA ligase and restriction enzymes were obtained from NEB (USA) and TaKaRa (China) respectively.
B. licheniformis Keratinase Gene (kerAwt) Amplification and Cloning
The DNA fragment encoding the B. licheniformis S90 kerA pro-enzyme was isolated by PCR with the primers kerAwtF (5′-GCTGGTACCGCTCAACCGGCGAAAAATGT-3′, KpnI restriction site underlined) and kerAwtR (5′-CGAGCGGCCGCTTGAGCAGCAGCTTCGACATTGAT-3′, NotI restriction site underlined). Both primers were designed according to the nucleotide sequence of the B. licheniformis S90 keratinase gene, as previous work in our laboratory (GenBank accession no. JN859581). The PCR reaction mixtures (50 µl) contained approx.15 ng of B. licheniformis S90 DNA, 0.2 µM each primer and 25 µl PCR premix (TaKaRa, China). The thermal program included 1 cycle of 8 min at 95°C, 32 cycles of (45 s at 94°C, 45 s at at 59°C, and 1.5 min at 72°C),and 1 cycle of 12 min at 72°C. The purified DNA product was inserted into plasmid pMD20-T and sequenced by TaKaRa (China).
Design of the Mutant kerA using Different Codon Optimization Strategies
The main keratinase gene of B. licheniformis S90 was optimized by partly replacing the P. pastoris preferred codons (synonymous codons). In this study, there were two codon optimization strategies–kerAopti1 and kerAopti2 (Table 1). The corresponding mutations were introduced into kerA by site-directed mutagenesis with the MutanBEST kit (TaKaRa, China) following the manufacturer’s protocol. Both kerAopti1 and kerAopti1 genes were inserted into plasmid pMD20-T.
Table 1. The primers and site-directed mutable points of mutant keratinase genes.
| Mutant gene | Primer | Sequences of primersa | Mutable points |
| kerAopti1 | kerAopti1F | 5′-CCAGCTAAAAATGTTGAAAGGGA-3′ | CCG/CCA (Pro-3); GCG/GCT (Ala-4) |
| kerAopti1R | 5′-GAGCGGTACCAGCAATCC-3′ | ||
| kerAopti2 | kerAopti2F | 5′-GTCAGAAACAGACTCTCCAGCAC-3′ | CCG/CCA (Pro-3); GCG/GCT (Ala-4); CGC/AGA (Arg-322); CGT/AGA (Arg-324) |
| kerAopti1R | 5′-TTGTGAAGCTGAAAGGTTCGGATG-3′ |
The mutable points are underlined.
Construction of the Recombinant Plasmids
A high effective shuttle vector, pPICZαA, was used to express keratinase gene in P. pastoris. The plasmids pMD20T-kerAwt, pMD20T-kerAopti1 and pMD20T-kerAopti2 were digested with NotI and KpnI, and the isolated DNA fragments were recovered and inserted into pPICZαA vector. The recombinant plasmids, pPICZαA–kerAwt, pPICZαA–kerAopti1 and pPICZαA–kerAopti2, were transformed into E. coli DH5α on low salt LB agar plates which contained 25 µg/ml zeocin. The positive clones were identified by colony PCR and sequencing.
About 10–15 µg pure vector pPICZαA containing kerAwt, kerAopti1 and kerAopti2 were linearized using SacI prior to integration into P. pastoris by electroporation (1800 kV, 50 µF, 186 Ω. Bio-Rad, USA). The positive clones were chosen on the YPD-zeocin plates (100 µg/ml zeocin) at 30°C and identified by colony PCR and sequencing.
Expression of Recombinant Keratinase in P. pastoris
A single positive colony was cultivated in 25 mL of BMGY (1% glycerol, 2% peptone, 1% yeast extract, 4×10−5% biotin, 1.34% YNB and pH 6.0, 100 mM potassium phosphate) medium at 29°C under constant agitation at 200–220 rpm until an OD600 = 3–5 was reached (approx. 18–20 h). Then, the cells were collected by centrifugation and resuspended in 50 mL BMMY (1.5% methanol, 2% peptone, 1% yeast extract, 4×10−5% biotin, 1.34% YNB and pH 6.0, 100 mM potassium phosphate) medium. Cultures were induced with absolute methanol to a final concentration of 1.5% every day. The expression culture supernate was harvested every 24 h and stored at −70°C before analysis.
Protein Purification
Recombinant P. pastoris strains were cultivated under the optimized conditions. The expression culture supernatant was clarified by centrifugation and concentrated approx.10-fold via ultrafiltration (MW cut-off, 10000 Da, Millipore, Germany). The supernate containing recombinant keratinase was purified by 2 ml Ni2+-chelating chromatography according to the manuals (Bio-rad, USA). The elution buffer (pH 8.0, 50 mM sodium phosphate, 500 mM imidazole and 300 mM NaCl) containing purified keratinase was stored at −70°C before analysis.
Glycoprotein Staining and Deglycosylation of Keratinase
The SDS-PAGE analysis of purified keratinase with glycoprotein staining experiment was performed using glycoprotein-stain kit (Sangon Biotech, China). The purified enzyme was deglycosylated by Endoglycosidase Hf at 37°C for 3 h following the manufacturer’s instructions (NEB, USA). The deglycosylated proteinase was determined by SDS-PAGE.
SDS–PAGE
SDS–PAGE was carried out on a 12% running gel and stained with Coomassie Blue [23].
Analysis of kerAwt, kerAopti1 and kerAopti2 Gene Copy Numbers
kerAwt, kerAopti1 and kerAopti2 gene copy numbers of generated recombinant P. pastoris strains were detected by the previous reports [24]–[26]. Genomic DNA was extracted from positive P. pastoris transformants with the yeastgen DNA kit (Cwbiotech, China) according to the instruction manuals. The modified real-time quantitative PCR (qPCR) method is used to determine copy numbers in this study. The glyceraldehyde-3-phosphate dehydrogenase (GAP) gene of P. pastoris was used as reference gene. All primers were deseigned by Primer 5.0 software (Table 2). Real-time qPCR was performed in a total volume of 10 µl which contained 1µl of DNA, 0.4 µl of 10 µM each primer, 3.2 µl of ddH2O and 5µl of 2×SYBR® Premix Ex Taq™ II (TaKaRa, China). All real-time PCR reactions were carried out in triplicate on CFX96™ Real-Time PCR Detection System (Bio-rad, USA) using the following program: 95°C for 30 s, 40 cycles of 95°C for 10 s and 60°C for 30 s. Specificity of amplicons was examined by melting curve analysis after 40 cycles and by agarose gel electrophoresis analysis.
Table 2. Primer sequences for quantitative real-time PCR.
| Gene | Primer | Sequence 5′-3′ | Product size (bp) |
| kerA | RT-kerAF | GTAAAAGTAGCCGTCCTG | 174 |
| RT-kerAR | CAACGCCTAATACACCC | ||
| GAP | RT-GAPF | ATGACCGCCACTCAAAAG | 98 |
| RT-GAPR | CACCAGTGGAAGATGGAAT | ||
| ACT1 | RT-ACT1F | ACACACAGTGTTCCCATCGGT | 231 |
| RT-ACT1R | AAGAACTGGGTGCTC TTCTG |
The pMD20T-kerA and pMD20T-GAP plasmids were used to construct the standard curve. Briefly, the double standard curves of kerA and GAP were established using 10-fold serial dilutions of the above plasmids ranging from 103 to 108 copies/µl. The cycle threshold (Ct) values of real-time qPCR in every dilution were determined thrice and plotted against the logarithm of the corresponding template gene copy numbers. Each standard curve was generated by linear regression of the plotted points. The total gene copy numbers of GAP and target gene in the genomic DNA sample were determined by relating the Ct values to the standard curves. Finally, the target gene copy numbers integrated in the genome of recombinant P. pastoris would be calculated by the target gene/GAP copy numbers ratio because P. pastoris contains only one copy GAP [27].
Analysis of kerAwt, kerAopti1 and kerAopti2 mRNA Expression Levels
The real-time qPCR method is used to determine kerAwt, kerAopti1 and kerAopti2 mRNA expression levels in present study. Total RNA was extracted from positive recombinant P. pastoris induced with 1.5% methanol for 96 h using RNAiso plus (TaKaRa, China). The reverse-transcription of total RNA (1 µg) was performed by PrimeScript® RT reagent kit with gDNA Eraser (Perfect Real Time) (TaKaRa, China). All primers were deseigned by Primer 5.0 software (Table 2). The reaction mixtures and program of real-time qPCR were identical to the above method. For normalization, the ACT1 gene (housekeeping gene) was used as the endogenous control gene. The relative gene mRNA expression (GE) levels for target gene of different strains were treated using the methods from Pfaffl [28]. In this case, the transcription levels of target genes were determined.
Keratinase Activity Assay
After removal of cells by centrifugation, keratinase activity was detected by the previous method [9]. Briefly, 0.2 ml protease was mixed with 20 mg of substrate keratin azure (Sigma-Aldrich, USA) and 3.8 ml of 0.05 M Tris-HCl buffer (pH 7.5) in a shaking incubator (220 rpm) at 50°C for 1 h. One unit of keratinase activity was defined as the quantity of protease that caused an increase in 0.01 absorbance units (595 nm, A595) for 1 h.
In addition, the activity of keratinase was also detected using chicken feather meal as substrate [9]. The enzyme (0.2 ml) was mixed with 20 mg of feather meal and 3.7 ml of 0.05 M Tris-HCl buffer (pH 7.5) in a shaking incubator (220 rpm) at 50°C for 1 h. Then, 0.1 ml 100% (w/v) trichloroacetic acid was added to the above mixture to terminate the reaction. After incubation for 1 h at 4°C, the reaction supernate was collected by centrifugation. One unit of keratinase activity was defined as the quantity of protease that caused an increase in 0.01 absorbance units (280 nm, A280) for 1 h.
Determination of Optimal Temperature and pH
The keratinolytic optimum temperature was examined at 30 to 80°C and pH 7.5. The optimal pH of its activity was measured at the detected optimum temperature by using buffers with pH ranging from 5.0 to 10.0. The used buffers were 0.05 M citrate phosphate buffer (pH 5–6) and 0.05 M Tris-HCl buffer (pH 7–10).
The temperature stability of keratinase was detected by heating protease at various temperatures (50, 60 and 70°C) for 30 min, and the residual activity was examined at 50°C and pH 7.5 for 1 h. To determine the keratinolytic thermal stability, the purified protease sample was incubated 0.05 M Tris-HCl buffer (pH 7.5) in the absence of keratin azure at 50°C for 30, 40, 50, 60, 90 and 120 min before detecting its activity. All assays were carried out in quadruplicate.
Results and Discussion
Cloning of kerAwt, kerAopti1 and kerAopti2 Gene
To facilitate the protein purification, the pro-enzyme sequences of kerAwt, kerAopti1 and kerAopti2 were cloned in fusion with the yeast alpha-factor signal sequence, which allows secretion of pro-enzyme in to the culture medium. The transcription of the gene fusion was under the control of alcohol oxidase 1 promoter (AOX1). As expected, three kinds of keratinase genes (1050 bp) were cloned into pMD20-T simple vector. The nucleotide sequences of keratinase genes indicated that the corresponding mutations were successfully introduced into kerA. However, all keratinase genes encoded the same mature protein (350 amino acids) and had the triad of catalytic residues including Asp-32, His-63 and Ser-220 [11].
Real-time qPCR Approach for Copy Number and Gene Expression Determination
Previous studies suggested that the copy numbers and transcription levels of heterologous gene would influence its expression level in P. pastoris [24]–[26]. Therefore, the keratinase gene copy numbers and transcription levels of every positive strain were also determined in this work.
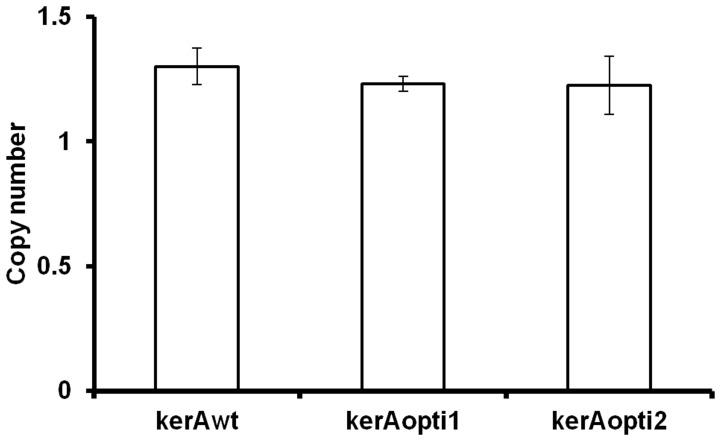
Compared to dot/slot blotting and southern blotting used for heterologous gene copy numbers assay in P. pastoris system, the real-time qPCR was easier, faster and most importantly, absolute quantitative methods [24], [26]. In this work, a modified real-time qPCR method was used to detect the kerAwt, kerAopti1 and kerAopti2 copy numbers in transformants [24]–[26]. The double stand curves for kerA and GAP were constructed by 10-fold serial dilutions of pMD20T-kerA and pMD20T-GAP plasmids. A high correlation between Ct values and the copy numbers was presented for both kerA and GAP determination (R2>0.999). The target gene copy numbers in the genome of selected transformants were then detected with the target gene/GAP copy numbers ratio because P. pastoris contains only one copy GAP within its genomic DNA [27]. Figure 1 showed that there was no significant difference (P>0.05) in the target gene (kerAwt, kerAopti1 and kerAopti2) copy numbers of recombinant P. pastoris strains.
Figure 1. Comparative the target gene copy numbers of P. pastori pPICZα-kerAwt, pPICZα-kerAopti1 and pPICZα-kerAopti2.
The values represent means ± SD (n = 3). Results showed no significant difference in gene copy numbers of every positive transformant (P>0.05).
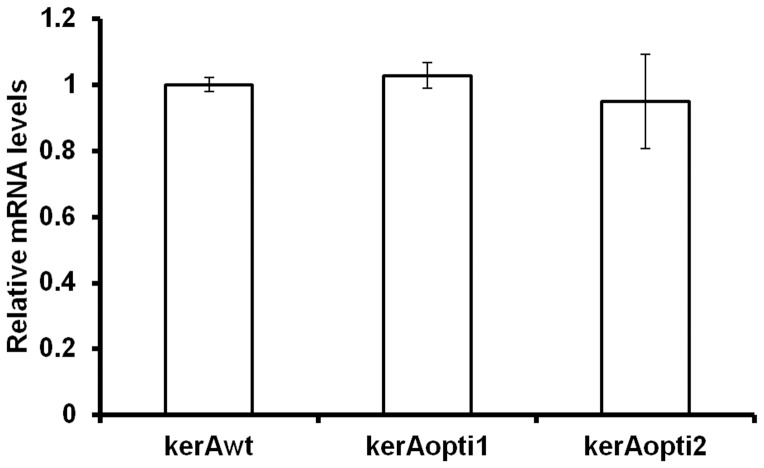
In the present work, the transcription levels of target genes were also determined. The mRNA expression levels of kerAwt, kerAopti1 and kerAopti2 were detected by real-time qPCR according the method from Pfaffl [28]. Results showed that no significant difference (P>0.05) was observed in the target gene (kerAwt, kerAopti1 and kerAopti2) mRNA expression levels of each transformants (Figure 2).
Figure 2. Comparative the target gene relative mRNA levels of P. pastori pPICZα-kerAwt, pPICZα-kerAopti1 and pPICZα-kerAopti2.
The values represent means ± SD (n = 3). Results showed no significant difference in target gene relative mRNA levels of every positive transformant (P>0.05).
SDS-PAGE Analysis of the Keratinase from Recombinant P. pastoris
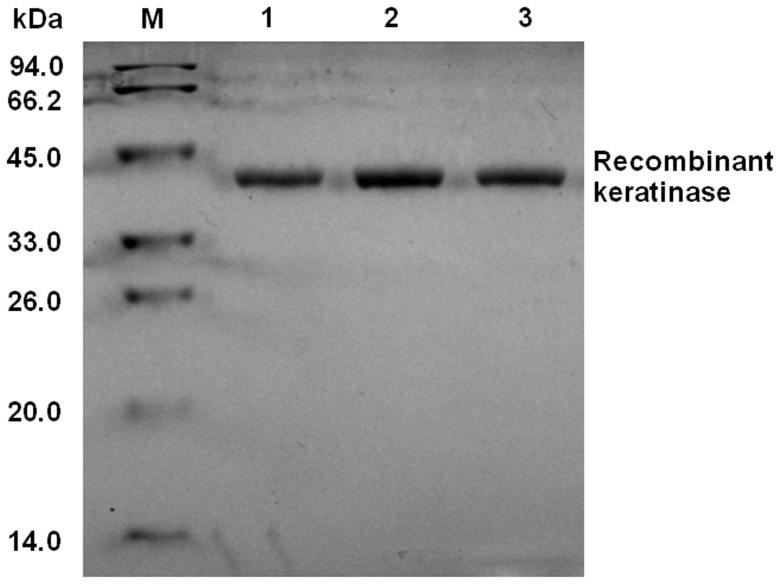
The recombinant P. pastoris pPICZαA-kerAwt, pPICZαA-kerAopti1 and pPICZαA- kerAopti2 were cultivated under the optimized conditions. The expression supernatant containing recombinant keratinase was successfully purified by Ni2+-NTA affinity chromatography, since only one band with MW approx. 39 kDa was presented on SDS-PAGE gel (Figure 3 lanes 1–3). However, the keratinase secreted by B. licheniformis has a MW of 33 kDa [8]. This was probably caused by N-glycosylation [16]. The keratinase amino acid sequence from B. licheniformis S90 has 6 potential glycosylation sites (N-glycosylation sites: 152-N, 293-N and 315-N; O- glycosylation sites: 79-T, 231-S and 327-S) and P. pastoris is capable of adding N- and O- linked oligosaccharide chains to expressed proteins [29]–[30]. Therefore, the molecular mass of recombinant keratinase from P. pastoris was greater than that secreted from B. licheniformis. Similar results have been derived for the difference between the MW of the keratinase of B. licheniformis PWD-1 and B. licheniformis MKU3 expressed in P. pastoris and the calculated weight from the keratinase coded protein [9], [13].
Figure 3. SDS-PAGE analysis.
Lane M: protein MW markers (14–94 kDa); Lane 1: the purified keratinase of P. pastoris pPICZα-kerAwt; Lane 2: the purified keratinase of P. pastoris pPICZα-kerAopti1; Lane 3: the purified keratinase of P. pastoris pPICZα-kerAopti2.
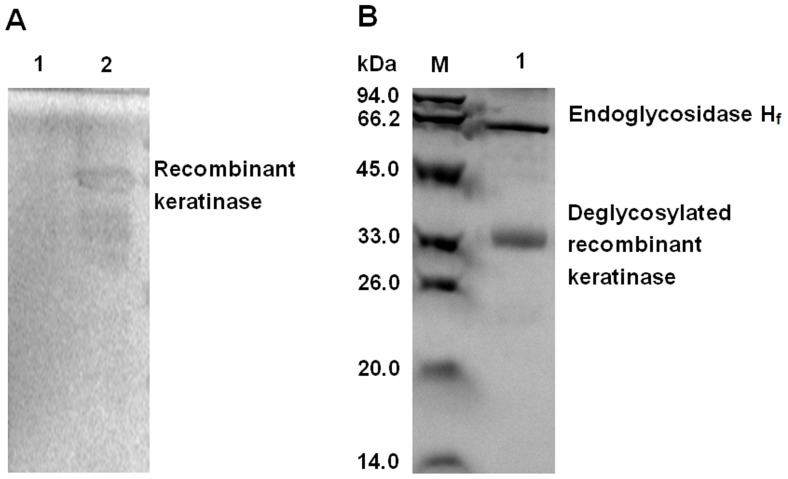
Further, the SDS-PAGE analysis with glycoprotein staining and deglycosylation experiment was used to determine the possible glycosylation of the recombinant keratinase in P. pastoris system attributing to the increased molecular mass. Figure 4A (lanes 2) showed there was a clear band on SDS-PAGE by glycoprotein staining. After deglycosylation of the recombinant keratinase with Endoglycosidase Hf, it presented a single band of approx. 33 kDa in size (Figure 4B lanes 1) which was similar to previous studies [8].
Figure 4. The glycoprotein staining and deglycosylation of purified recombinant keratinase in the SDS-PAGE.
A: The glycoprotein staining experiment. Lane 1: BSA; Lane2: purified glycosylated keratinase. B: The deglycosylation of purified recombinant keratinase. Lane M: protein MW markers (14–94 kDa); Lane 1: deglycosylated keratinase.
Recombinant Keratinases Production by P. pastoris X-33
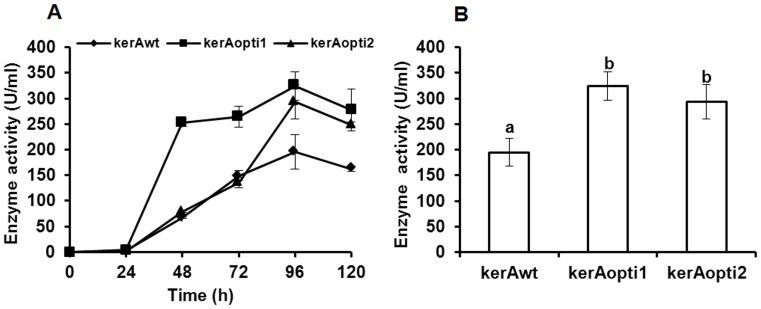
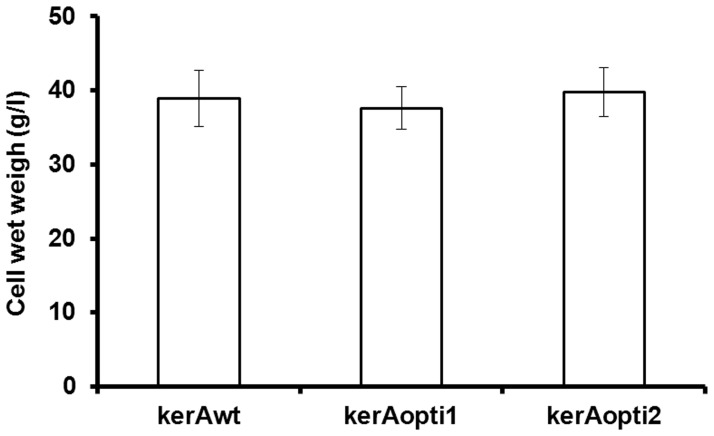
The positive P. pastoris transformants were cultivated in BMMY broth at 29°C and induced by methanol. Keratinolytic activity was detected after 24 h (Figure 5A). The highest keratinase activity produced by P. pastoris pPICZαA-kerAwt, pPICZαA-kerAopti1 and pPICZαA-kerAopti2 was 195 U/ml, 324 U/ml and 293 U/ml respectively at 96 h (Figure 5B). In addition, figure 6 showed there was no significant difference in biomass concentration of all recombinant transformants (P>0.05).
Figure 5. Recombinant keratinases production by P. pastoris X-33.
A: Kinetics of keratinase production by P. pastoris pPICZα-kerAwt, pPICZα-kerAopti1 and pPICZα-kerAopti2. B: Comparative expression of kerAwt, kerAopti1 and kerAopti2 in P. pastoris at 96 h. The values represent means ± standard deviations (SD) of six independent positive transformants. Every transformant was examined in four independent experiments in quadruplicate. a,bMean values were significantly different (P<0.05) when labeled with unlike letters.
Figure 6. Comparative biomass concentration of P. pastori pPICZα-kerAwt, pPICZα-kerAopti1 and pPICZα-kerAopti2 at 96 h.
The values represent means ± SD (n = 3). Results showed there was no significant difference in biomass concentration of every positive transformant (P>0.05).
The keratinase gene of Bacillus licheniformis S90 was optimized for the industrial application value in improving its production and for investigating the effect of different codon optimization strategies on the expression of kerA in P. pastoris. Many studies have suggested that codon biases reduce the metabolic load due to decreasing the diversity of isoacceptor tRNAs and consequently improve heterologous gene expression in host [17], [31]. In addition, the presence of two consecutive rare codons which ought to induce ribosomal frameshift during translation will inhibit the protein expression [17], [32]–[33]. Comparison of P. pastoris codon usage [34]–[36], there were four P. pastoris rare codons CCG (Pro-3), GCG (Ala-4), CGC (Arg-322) and CGT (Arg-324) which should decrease the enzyme expression level in native kerA gene. Schutter et al. (2009) have suggested that the synonymous codon usage bias technique was effective on increasing the expression of foreign proteins [35]. Yao et al. (1998) and Chen et al. (2005) have indicated that the production of phytase gene was great improved in P. pastoris by changing consecutive rare codons [37]–[38]. In this work, the results showed that the keratinase produced by P. pastoris pPICZαA-kerAopti1 (324 U/ml) and pPICZαA-kerAopti2 (293 U/ml) was markedly higher than the keratinase activity from recombinant P. pastoris pPICZαA-kerAwt (195 U/ml) (P<0.05, shown in Figure 5B). However, no significant difference in keratinolytic activity was found between P. pastoris pPICZαA-kerAopti1 and pPICZαA-kerAopti2 (P>0.05, shown in Figure 5B). The reason was probably that the expression level in heterologous expression systems was much lower if the rare codons locate at the N-terminal part of the protein than in other positions [17]. To our knowledge, the activity of keratinase produced from P. pastoris pPICZαA-kerAopti1 is significantly higher than most other reported results (Table 3).
Table 3. Comparison of keratinase activity from different source.
| Enzyme source | Activity(different substrate, U/ml) | Reference | |
| α-keratin (keratin azure) | β-keratin (feather meal or azokeratin) | ||
| B. licheniformis MKU3 | 55.0 | NAa | [12] |
| E. coli pETproK3 | 74.3 | NAa | [12] |
| B. megaterium pWHK3 | 95.0 | NAa | [12] |
| P. pastoris pPICZαA-kerA | NAa | 285.0 | [13] |
| P. pastoris pPZK3 | 135.0 | NAa | [9] |
| P. pastoris pPICZαA-kerAopti1 | 320.0 | 590.0 | This study |
NA, not available.
Biochemical Properties of the Purified Keratinase
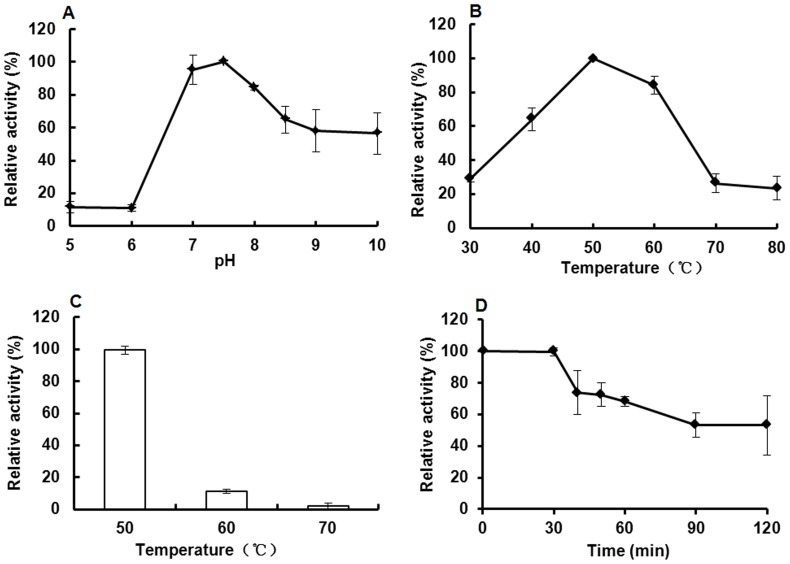
The enzymatic characterization of the purified recombinant keratinase was detected using keratin azure as substrate (Figure 7). The recombinant enzyme was active at pH values from 7–9 and had optimum pH of 7.5 (Figure 8A), which is the same as the keratinase of B. licheniformis or other recombinant keratinases [8]–[9], [12]. In addition, the recombinant keratinase was stable at moderate temperature and had optimum temperature at 50°C (Figure 8B), which was also identical to the previous reports [4], [8]. The recombinant keratinase is much more stable at optimum temperature (50°C), remained approx.50% of the maximum activity at this temperature for 2 h. However, the keratinase from P. pastoris was rapidly inactivated at higher temperatures 60–70°C for 30 min (Figure 8C, D).
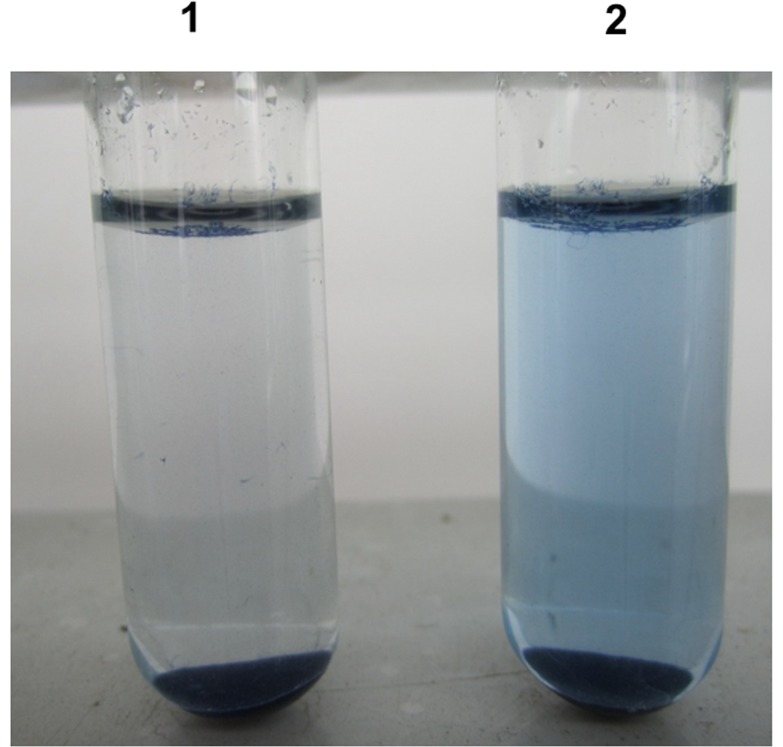
Figure 7. The keratin azure degradation by recombinant keratinase at 50°C after 1 h (1, control without keratinase; 2, keratinase treatment).
Figure 8. Enzymatic properties of the purified keratinase.
A: The optimum pH of keratinase; B: The optimum temperature of keratinase; C: The keratinolytic temperature stability; D: The keratinolytic thermostability. The maximum value was taken as 100%.
Enzymatic Hydrolysis of Different Keratin
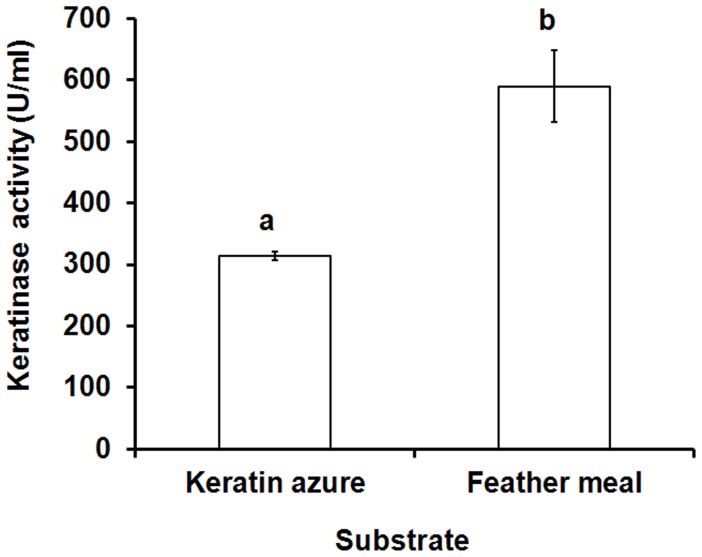
The hydrolytic activity of the recombinant keratinase on α-keratin (keratin azure) and β-keratin (chicken feather meal) was determined (Figure 9). When the substrate chicken feather meal was used, the yield of enzymatic hydrolysis was significantly higher than the substrate keratin azure. These results confirmed that the recombinant keratinase could effectively degrade both α-keratin and β-keratin [8], [10]. The reason was probably that the keratinase preferably cleave aromatic or hydrophobic amino acids at the P1 site [4], [10].
Figure 9. Enzymatic hydrolysis of different keratin.
The values represent means ± standard. a,bMean values were significantly different (P<0.05) when labeled with unlike letters.
Conclusions
The main kerA from B. licheniformis S90 was designed by two codon optimization strategies and successfully expressed in Pichia pastoris. The recombinant P. pastoris pPICZαA-kerAopti1 secreted more keratinase (324 U/ml) than P. pastoris pPICZαA-kerAwt, pPICZαA-kerAopti1 and most other reported results. The broad pH profile, substrate specificity and thermal stability make the recombinant keratinase from P. pastoris pPICZαA-kerAopti1 a suitable applicant for various industrial applications.
Funding Statement
This research was granted by Feed Biotechnology Project of Sichuan Province of China with grant number 2010GZ0193. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Son HJ, Park HC, Kim HS, Lee CY (2008) Nutritional regulation of keratinolytic activity in Bacillus pumilis . Biotechnol Lett 30: 461–465. [DOI] [PubMed] [Google Scholar]
- 2. Kublanov IV, Perevalova AA, Slobodkina GB, Lebedinsky AV, Bidzhieva SK, et al. (2009) Biodiversity of thermophilic prokaryotes with hydrolytic activities in hot springs of Uzon Caldera, Kamchatka (Russia). Appl Environ Microbiol 75(1): 286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Macedo AJ, da Silva WO, Gava R, Driemeier D, Henriques JA, et al. (2005) Novel keratinase from Bacillus subtilis S14 exhibiting remarkable dehairing capabilities. Appl Environ Microbiol 71(1): 594–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta R, Ramnani P (2006) Microbial keratinases and their prospective applications: an overview. Appl Microbiol Biotechnol 70: 21–33. [DOI] [PubMed] [Google Scholar]
- 5. Langeveld JP, Wang JJ, Van de Wiel DF, Shih GC, Garssen GJ, et al. (2003) Enzymatic degradation of prion protein in brain stem from infected cattle and sheep. J Infect Dis 188 (11): 1782–1789. [DOI] [PubMed] [Google Scholar]
- 6. Stark CR, Spencer BE, Shih JCH, Chewning CG, Wang JJ (2009) Evaluation of keratinase stability in pelleted broiler diets. J Appl Poult Res 18: 30–33. [Google Scholar]
- 7. Wang JJ, Garlich JD, Shih JCH (2006) Beneficial effects of versazyme, a keratinase feed additive, on body weight, feed conversion, and breast yield of broiler chickens. J Appl Poult Res 15: 544–550. [Google Scholar]
- 8.Lin X, Lee CG, Casale ES, Shih JC (1992) Purification and characterization of keratinase from a feather-degrading Bacillus licheniformis strain. Appl Environ Microbiol 58(10), 3271–3275. [DOI] [PMC free article] [PubMed]
- 9. Radha S, Gunasekaran P (2009) Purification and characterization of keratinase from recombinant Pichia and Bacillus strains. Protein Expr Purif 64: 24–31. [DOI] [PubMed] [Google Scholar]
- 10. Williams CM, Richter CS, Mackenzie JM, Shih JCH (1990) Isolation, identification, and characterization of a feather-degrading bacterium. Appl Environ Microbiol 56(6): 1509–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin X, Kelemen DW, Miller ES, Shih JCH (1995) Nucleotide sequence and expression of kerA, the gene encoding a keratinolytic protease of Bacillus licheniformis PWD-1. Appl Environ Microbol 61: 1469–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Radha S, Gunasekaran P (2007) Cloning and expression of keratinase gene in Bacillus megaterium and optimization of fermentation conditions for the production of keratinase by recombinant strain. J Appl Microbiol 103: 1301–1310. [DOI] [PubMed] [Google Scholar]
- 13. Porres JM, Benito MJ, Lei XG (2002) Functional expression of keratinase (kerA) gene from Bacillus licheniformis in Pichia pastoris . Biotechnol Lett 24: 631–636. [Google Scholar]
- 14. Romanos MA, Scorer CA, Clare JJ (1992) Foreign gene expression in yeast: A review. Yeast 8: 423–488. [DOI] [PubMed] [Google Scholar]
- 15. Potvin G, Ahmad A, Zhang Z (2012) Bioprocess engineering aspects of heterologous protein production in Pichia pastoris: A review. Biochem Eng J 64: 91–105. [Google Scholar]
- 16. Macauley-Patrick S, Fazenda ML, McNeil B (2005) Heterologous protein production using the Pichia pastoris expression system. Yeast 22: 249–270. [DOI] [PubMed] [Google Scholar]
- 17. Gustafsson C, Govindarajan S, Minshull J (2004) Codon bias and heterologous protein expression. TRENDS Biotechnol 22: 346–353. [DOI] [PubMed] [Google Scholar]
- 18. Boettner M, Steffens C, von Mering C, Bork P, Stahl U, et al. (2007) Sequence-based factors influencing the expression of heterologous genes in the yeast Pichia pastoris–A comparative view on 79 human genes. J Biotechnol 130(1): 1–10. [DOI] [PubMed] [Google Scholar]
- 19. Gvritishvili AG, Leung KW, Tombran-Tink J (2010) Codon preference optimization increases heterologous PEDF expression. PLoS ONE 5(11): e15056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jia H, Fan G, Yan Q, Liu Y, Yan Y, et al. (2012) High-level expression of a hyperthermostable Thermotoga maritima xylanase in Pichia pastoris by codon optimization. J Mol Catal B-Enzym 78: 72–77. [Google Scholar]
- 21. Huang H, Yang P, Luo H, Tang H, Shao N, et al. (2008) High-level expression of a truncated 1,3-1,4-β-D-glucanase from Fibrobacter succinogenes in Pichia pastoris by optimization of codons and fermentation. Appl Microbiol Biotechnol 78(1): 95–103. [DOI] [PubMed] [Google Scholar]
- 22.Hu H, He J, Yu B, Zheng P, Huang Z, et al. (2013) Expression of a keratinase (kerA) gene from Bacillus licheniformis in Escherichia coli and characterization of the recombinant enzymes. Biotechnol Lett 35, 239–244. [DOI] [PubMed]
- 23. Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- 24. Zhu T, Guo M, Tang Z, Zhuang Y, Chu J, et al. (2009) Efficient generation of multi-copy strains for optimizing secretory expression of porcine insulin precursor in yeast Pichia pastoris . J Appl Microbiol 107(3): 954–963. [DOI] [PubMed] [Google Scholar]
- 25. Hartner FS, Ruth C, Langenegger D, Johnson SN, Hyka P, et al. (2008) Promoter library designed for fine-tuned gene expression in Pichia pastoris . Nucleic Acids Res 36(12): e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li K, Gao H, Gao L, Qi X, Gao Y (2012) Recombinant gp90 protein expressed in Pichia pastoris induces a protective immune response against reticuloendotheliosis virus in chickens. Vaccine 30: 2273–2281. [DOI] [PubMed] [Google Scholar]
- 27. Waterham HR, Digan ME, Koutz PJ, Lair SV, Cregg JM (1997) Isolation of the Pichia pastoris glyceraldehyde-3-phosphate dehydrogenase gene and regulation and use of its promoter. Gene 186: 37–44. [DOI] [PubMed] [Google Scholar]
- 28. Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9): e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Choi BK, Bobrowicz P, Davidson RC, Hamilton SR, Kung DH, et al. (2003) Use of combinatorial genetic libraries to humanize N-linked glycosylation in the yeast Pichia pastoris . PNAS 100: 5022–5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li P, Anumanthan A, Gao XG, Ilangovan K, Suzara VV, et al. (2007) Expression of Recombinant Proteins in Pichia Pastoris . Appl Biochem Biotechnol142: 105–124. [DOI] [PubMed] [Google Scholar]
- 31. Andersson GE, Kurland C (1991) An extreme codon preference strategy: codon reassignment. Mol Biol Evol 8(4): 530–544. [DOI] [PubMed] [Google Scholar]
- 32. Hu X, Shi Q, Yang T, Jackowski G (1996) Specific replacement of consecutive AGG codons results in high-level expression of human cardiac troponin T in Escherichia coli . Protein Expr Purif 7(3): 289–293. [DOI] [PubMed] [Google Scholar]
- 33. Spanjaard RA, van Duin J (1988) Translation of the sequence AGG-AGG yields 50% ribosomal frameshift. Proc Natl Acad Sci USA 85(21): 7967–7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang SP, Zubay G, Goldman E (1991) Low-usage codons in Escherichia coli, yeast, fruit fly and primates. Gene 105(1): 61–72. [DOI] [PubMed] [Google Scholar]
- 35. Schutter DK, Lin YC, Tiels P, Van Hecke A, Glinka S, et al. (2009) Genome sequence of the recombinant protein production host Pichia pastoris . Nat Biotechnol 27(6): 561–566. [DOI] [PubMed] [Google Scholar]
- 36. Bai J, Swartz DJ, Protasevich II, Brouillette CG, Harrell PM, et al. (2011) A gene optimization strategy that enhances production of fully functional P-Glycoprotein in Pichia pastoris . PLoS ONE 6(8): e22577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yao B, Zhang CY, Wang J, Fan YL (1998) High-level expression of phytase gene in Pichia pastoris by codon optimization. Science In China (Series C) 28: 237–243. [Google Scholar]
- 38. Chen H, Zhao H, Wang HN, Yang WS, Wu Q, et al. (2005) Increasing expression level of phytase gene(phyA) in Pichia pastoris by Changing Rare Codons. Chin J Biochem Mol Biol 21: 171–175. [Google Scholar]