Abstract
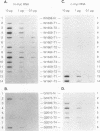
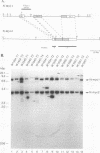
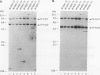
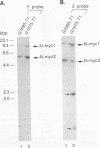
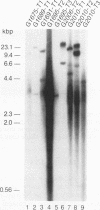
Woodchucks infected with woodchuck hepatitis virus (WHV) and ground squirrels infected with ground squirrel hepatitis virus (GSHV) both develop hepatocellular carcinoma (HCC), but WHV-associated tumors arise more frequently and much earlier in life. These differences are preserved when the oncogenic potentials of the two viruses are examined in the same host (woodchucks). We examined RNA and genomic DNA from tumors arising from WHV- and GSHV-infected woodchucks to determine whether these viruses use the same oncogenic pathway. N-myc RNA was not expressed in normal liver but was expressed in 10 of 13 WHV-associated HCCs examined. Southern blot analysis showed that 7 of 17 WHV-induced tumors (41%) contained rearrangements at N-myc loci due to viral genomic integration. Six of these seven inserts affected N-myc2, and most of these were at the 5' end of the gene. In contrast, only two of seven GSHV-induced woodchuck HCCs expressed N-myc RNA, and only 1 of the 16 tumors (6%) contained a rearranged N-myc allele. The GSHV-associated HCCs all contained numerous viral insertions, so the low frequency of integration into N-myc loci by GSHV was not due to a general block to integration. Four of sixteen GSHV-induced tumors harbored amplified c-myc alleles, and five of seven GSHV tumors tested contained elevated c-myc RNA levels. By contrast, enhanced c-myc RNA levels were observed in only 2 of 13 WHV-induced HCC. We conclude that N-myc overexpression is a regular feature of WHV- but not GSHV-associated hepatocarcinogenesis in a common host. In contrast, c-myc transcriptional deregulation is rarely encountered in WHV-induced HCC but is frequent in GSHV-induced HCC.
Full text
PDF


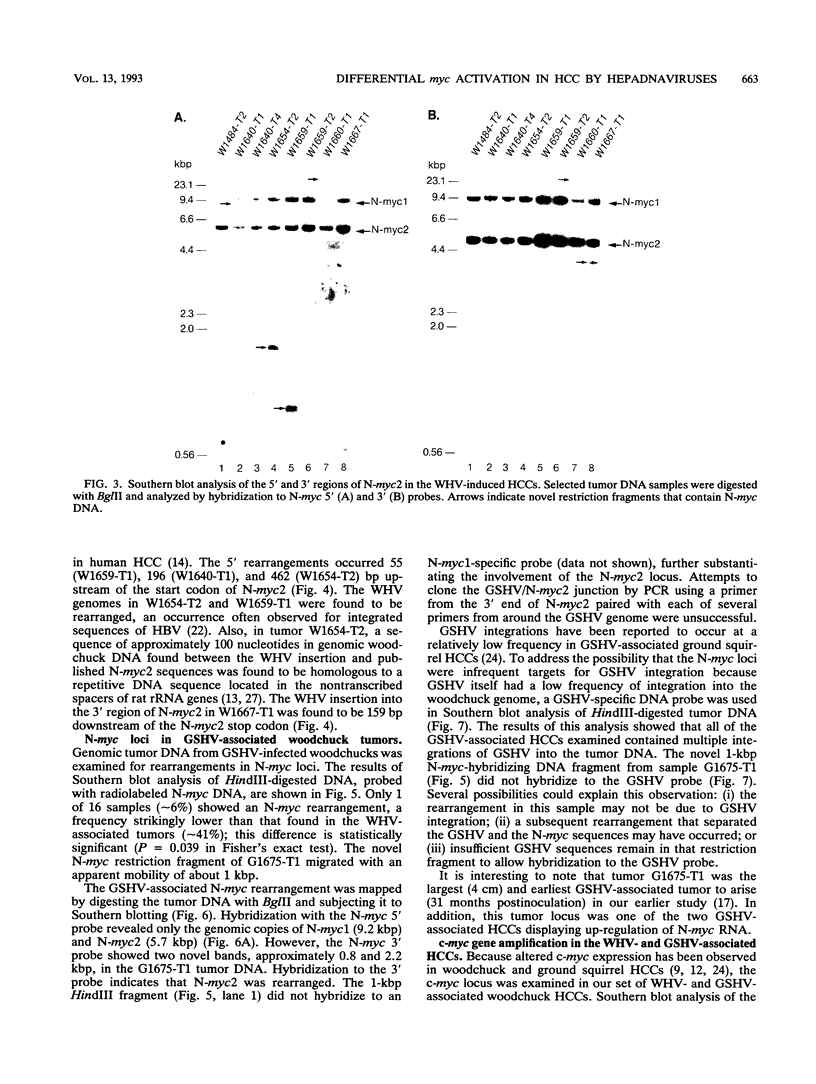
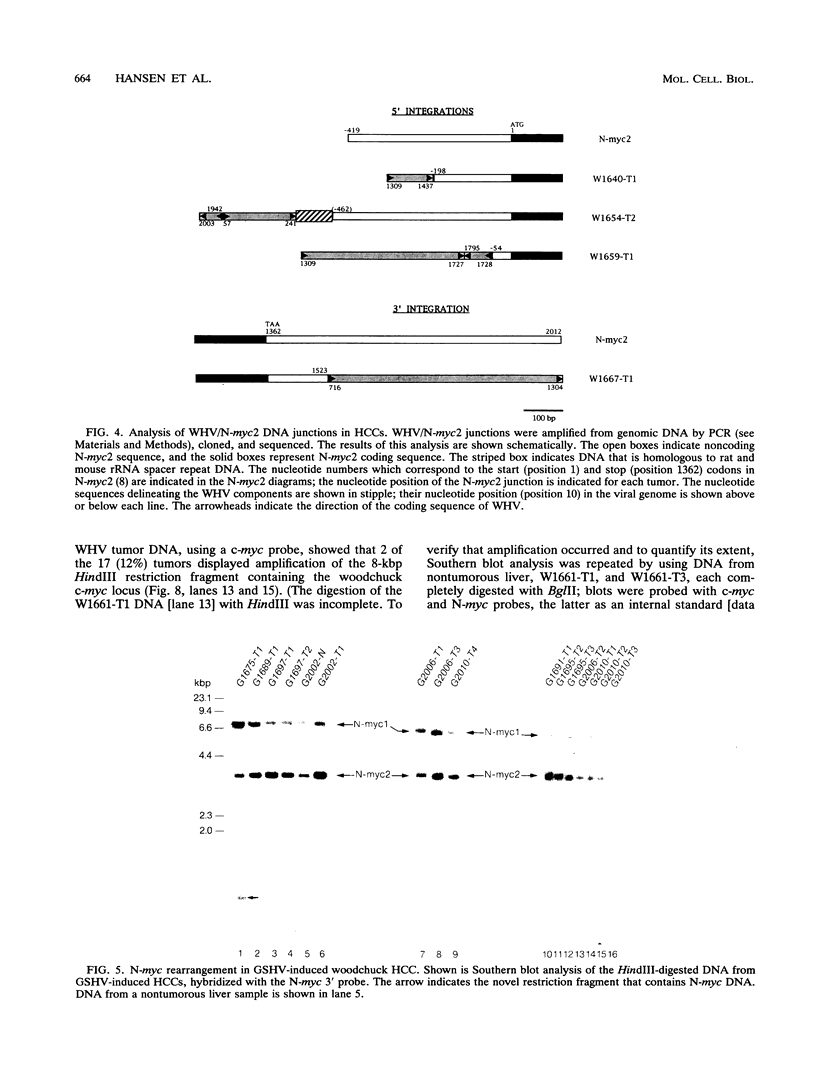
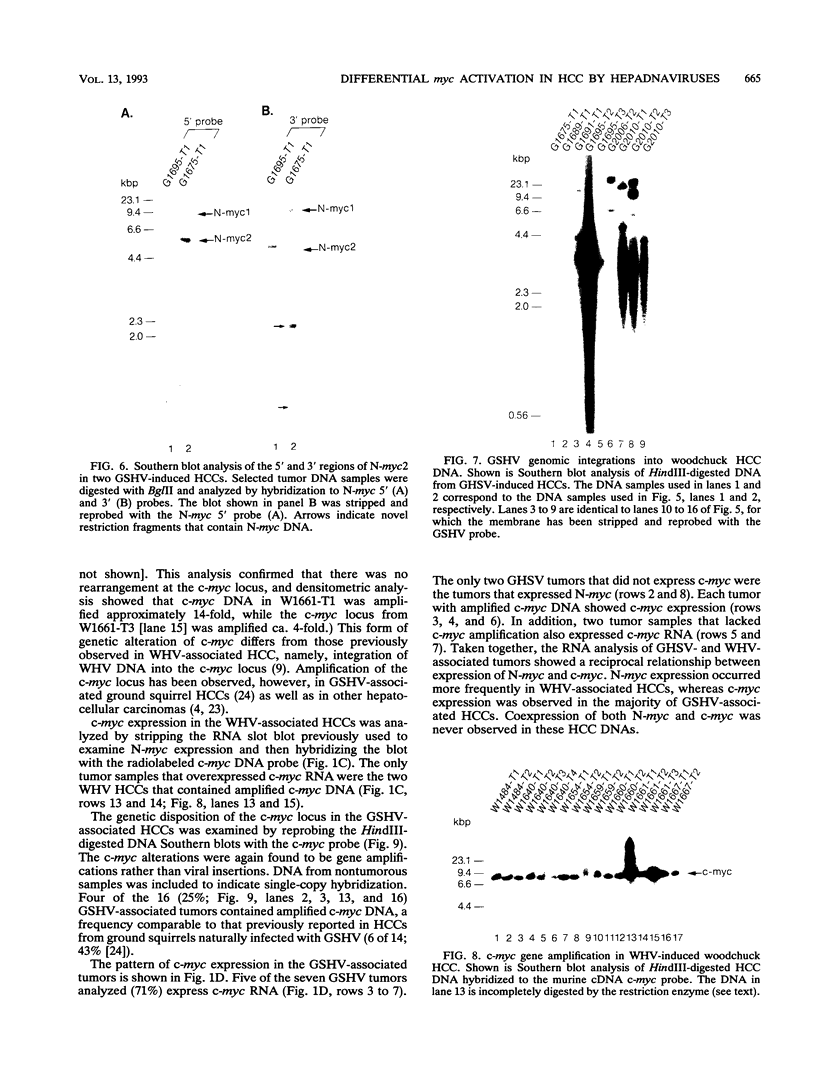


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiss L. E., Friedman J. M. Regulation of N-myc gene expression: use of an adenovirus vector to demonstrate posttranscriptional control. Mol Cell Biol. 1990 Dec;10(12):6700–6708. doi: 10.1128/mcb.10.12.6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley R. P. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988 May 15;61(10):1942–1956. doi: 10.1002/1097-0142(19880515)61:10<1942::aid-cncr2820611003>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Beasley R. P., Hwang L. Y., Lin C. C., Chien C. S. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981 Nov 21;2(8256):1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- Chandar N., Lombardi B., Locker J. c-myc gene amplification during hepatocarcinogenesis by a choline-devoid diet. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2703–2707. doi: 10.1073/pnas.86.8.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fourel G., Trepo C., Bougueleret L., Henglein B., Ponzetto A., Tiollais P., Buendia M. A. Frequent activation of N-myc genes by hepadnavirus insertion in woodchuck liver tumours. Nature. 1990 Sep 20;347(6290):294–298. doi: 10.1038/347294a0. [DOI] [PubMed] [Google Scholar]
- Hsu T., Möröy T., Etiemble J., Louise A., Trépo C., Tiollais P., Buendia M. A. Activation of c-myc by woodchuck hepatitis virus insertion in hepatocellular carcinoma. Cell. 1988 Nov 18;55(4):627–635. doi: 10.1016/0092-8674(88)90221-8. [DOI] [PubMed] [Google Scholar]
- Kodama K., Ogasawara N., Yoshikawa H., Murakami S. Nucleotide sequence of a cloned woodchuck hepatitis virus genome: evolutional relationship between hepadnaviruses. J Virol. 1985 Dec;56(3):978–986. doi: 10.1128/jvi.56.3.978-986.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion P. L., Van Davelaar M. J., Knight S. S., Salazar F. H., Garcia G., Popper H., Robinson W. S. Hepatocellular carcinoma in ground squirrels persistently infected with ground squirrel hepatitis virus. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4543–4546. doi: 10.1073/pnas.83.12.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroczka D. L., Cassidy B., Busch H., Rothblum L. I. Characterization of rat ribosomal DNA. The highly repetitive sequences that flank the ribosomal RNA transcription unit are homologous and contain RNA polymerase III transcription initiation sites. J Mol Biol. 1984 Mar 25;174(1):141–162. doi: 10.1016/0022-2836(84)90369-3. [DOI] [PubMed] [Google Scholar]
- Möröy T., Marchio A., Etiemble J., Trépo C., Tiollais P., Buendia M. A. Rearrangement and enhanced expression of c-myc in hepatocellular carcinoma of hepatitis virus infected woodchucks. Nature. 1986 Nov 20;324(6094):276–279. doi: 10.1038/324276a0. [DOI] [PubMed] [Google Scholar]
- Nagaya T., Nakamura T., Tokino T., Tsurimoto T., Imai M., Mayumi T., Kamino K., Yamamura K., Matsubara K. The mode of hepatitis B virus DNA integration in chromosomes of human hepatocellular carcinoma. Genes Dev. 1987 Oct;1(8):773–782. doi: 10.1101/gad.1.8.773. [DOI] [PubMed] [Google Scholar]
- Popper H., Roth L., Purcell R. H., Tennant B. C., Gerin J. L. Hepatocarcinogenicity of the woodchuck hepatitis virus. Proc Natl Acad Sci U S A. 1987 Feb;84(3):866–870. doi: 10.1073/pnas.84.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger C., Baldwin B., Hornbuckle W. E., Yeager A. E., Tennant B. C., Cote P., Ferrell L., Ganem D., Varmus H. E. Woodchuck hepatitis virus is a more efficient oncogenic agent than ground squirrel hepatitis virus in a common host. J Virol. 1991 Apr;65(4):1673–1679. doi: 10.1128/jvi.65.4.1673-1679.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger C., Ganem D., Varmus H. E. Nucleotide sequence of an infectious molecularly cloned genome of ground squirrel hepatitis virus. J Virol. 1984 Aug;51(2):367–375. doi: 10.1128/jvi.51.2.367-375.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger C., Maragos J. Molecular analysis of the function of direct repeats and a polypurine tract for plus-strand DNA priming in woodchuck hepatitis virus. J Virol. 1989 May;63(5):1907–1915. doi: 10.1128/jvi.63.5.1907-1915.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. K., Zimmerman K., Yancopoulos G. D., Ma A., Alt F. W. Transcriptional down-regulation of N-myc expression during B-cell development. Mol Cell Biol. 1992 Apr;12(4):1578–1584. doi: 10.1128/mcb.12.4.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton L. W., Fahrlander P. D., Tesser P. M., Marcu K. B. Nucleotide sequence comparison of normal and translocated murine c-myc genes. Nature. 1984 Aug 2;310(5976):423–425. doi: 10.1038/310423a0. [DOI] [PubMed] [Google Scholar]
- Takada S., Gotoh Y., Hayashi S., Yoshida M., Koike K. Structural rearrangement of integrated hepatitis B virus DNA as well as cellular flanking DNA is present in chronically infected hepatic tissues. J Virol. 1990 Feb;64(2):822–828. doi: 10.1128/jvi.64.2.822-828.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro F., Morimura S., Hayashi K., Makino R., Kawamura H., Horikoshi N., Nemoto K., Ohtsubo K., Sugimura T., Ueno Y. Expression of the c-Ha-ras and c-myc genes in aflatoxin B1-induced hepatocellular carcinomas. Biochem Biophys Res Commun. 1986 Jul 31;138(2):858–864. doi: 10.1016/s0006-291x(86)80575-7. [DOI] [PubMed] [Google Scholar]
- Transy C., Fourel G., Robinson W. S., Tiollais P., Marion P. L., Buendia M. A. Frequent amplification of c-myc in ground squirrel liver tumors associated with past or ongoing infection with a hepadnavirus. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3874–3878. doi: 10.1073/pnas.89.9.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Fourel G., Ponzetto A., Silvestro M., Tiollais P., Buendia M. A. Hepadnavirus integration: mechanisms of activation of the N-myc2 retrotransposon in woodchuck liver tumors. J Virol. 1992 Sep;66(9):5265–5276. doi: 10.1128/jvi.66.9.5265-5276.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavachev L. P., Georgiev O. I., Braga E. A., Avdonina T. A., Bogomolova A. E., Zhurkin V. B., Nosikov V. V., Hadjiolov A. A. Nucleotide sequence analysis of the spacer regions flanking the rat rRNA transcription unit and identification of repetitive elements. Nucleic Acids Res. 1986 Mar 25;14(6):2799–2810. doi: 10.1093/nar/14.6.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]