Abstract
Background
Methylenetetrahydrofolate reductase (MTHFR) C677T, A1298C and methionine synthase reductase (MTRR) A66G polymorphisms are important genetic determinants for homocysteine (Hcy) levels, and are associated with several disorders. These polymorphisms are heterogeneously distributed worldwide. Our objective was to explore the geographical distributions of these polymorphisms in China.
Methodologies
15357 healthy adults were recruited from 10 regions. Buccal samples were collected and genomic DNA was isolated. Genotyping was performed using the fluorogenic 5′-nuclease assay.
Principal Findings
The prevalence of the three polymorphisms among different populations from China varied significantly and showed apparent geographical gradients. For MTHFR C677T, the frequencies of the 677T allele and the 677TT genotype were significantly higher among northern populations and ranged from the lowest values (24.0% and 6.4%, respectively) in Hainan (southern) to the highest values (63.1% and 40.8%, respectively) in Shandong (northern). For MTHFR A1298C, the 1298C allele and the 1298CC genotype frequencies were significantly higher among southern populations and increased from low values (13.1% and 1.4%, respectively) in Shandong to high values (25.7% and 6.7%, respectively) in Hainan. For A66G, the 66G allele and the 66GG genotype frequencies increased from lower values (23.7% and 5.4%, respectively) in Shandong to higher values (29.2% and 8.6%, respectively) in Hainan. The overall frequency of the 677T allele, 677TT genotype, 1298C allele, 1298CC genotype, 66G allele and 66GG genotype in the Chinese Han population was 45.2%, 23.2%, 18.6%, 3.9%, 25.7%, and 6.6%, respectively. No gender differences were found in the prevalence of both the MTHFR C677T and MTRR A66G polymorphisms.
Conclusions
This study indicates that there are marked geographical variations in the prevalence of the three polymorphisms among Chinese Han populations. Our baseline data may be useful for future researches in related fields.
Introduction
Hyperhomocysteinemia (HHcy) is a medical condition characterized by high concentrations of plasma homocysteine (Hcy) and it has been associated with increased risk for many disorders, including vascular and neurodegenerative diseases, pregnancy complications, male infertility, birth defects, diabetes, renal diseases, osteoporosis, neuropsychiatric disorders and cancers [1]–[3]. Plasma levels of Hcy are influenced by both environmental and genetic factors. The main environmental factors include vitamins folate, B12, B6, and B2 [4]–[7]. The major genetic factors are polymorphisms in genes encoding homocysteine metabolizing enzymes, such as methylenetetrahydrofolate reductase (MTHFR) C677T, A1298C and methionine synthase reductase (MTRR) A66G polymorphisms.
MTHFR plays a critical role in catalyzing the irreversible reduction of 5,10-methylenetrahydrofolate to 5-methyltetrahydrofolate, which serves as the methyl donor for the vitamin-B12-depedent remethylation of homocysteine to methionine and for the synthesis of purine, DNA, and RNA. Several polymorphisms in the MTHFR have been identified, however, only the C677T and A1298C polymorphisms have been expressed and confirmed to affect the enzyme activity [8], [9]. The C677T polymorphism is a C to T transition at base pair 677 resulting in an alanine to valine substitution. This polymorphism encodes for a thermolabile variant that decreases enzyme activity by 65% and increases plasma total homocysteine (tHcy) levels especially under the conditions of low dietary folate [10]. The A1298C polymorphism is an A to C transition at base pair 1298 leading to a glutamate to alanine substitution. The polymorphism results in the reduction of the enzyme activity, although to lesser extent than the C677T polymorphism [11]. Neither the homozygous nor the heterozygous state for the A1298C polymorphism is associated with increased plasma homocysteine and/or lower blood folate concentrations. However, combined heterozygosity for the A1298C and C677T polymorphisms is associated with decreased enzyme activity, elevated plasma homocysteine concentrations and reduced plasma folate levels [9].
MTRR is another key enzyme involved in homocysteine metabolism, which is responsible for the remethylation of Hcy to methionine via a vitamin-B12-dependent reaction. The MTRR restores methionine synthase (MTR) activity and is consequently a critical determinant of Hcy levels [12]. The most common polymorphism in the MTRR gene is the substitution of A for G at nucleotide 66 (A66G), which decreases the enzyme activity and the rate of Hcy remethylation [13].
Many epidemiological studies have indicated that the MTHFR C677T, A1298C and MTRR A66G polymorphisms are associated with increased risk for several disorders aforementioned [1], [14]–[20]. The prevalence of the three polymorphisms varies in different geographical regions and ethnic groups. For example, the MTHFR 677T allele frequency is often reported to be high in Europe (24.1–64.3%), and low in Africa (0–35.5%). The MTHFR 1298C allele frequency is approximately 20–70% in Asia, 24–46% in Europe, and 0–15% in America, although it has not been more extensively studied than the C677T [21], [22]. The highest and the lowest MTRR 66G allele frequencies are reported among Hispanics (28.65%) and Caucasians (54.4%), respectively [23]. Additionally, it is of interest that a north-to-south cline of increase in the MTHFR 677T allele frequency has been observed among Europeans and North Americans [24], [25], but a reverse trend has also been reported among Pakistanis, Indians, and Chinese minority groups [26]–[28]. The reasons for these distributional differences are still unclear and can involve both genetic and environmental factors. Nevertheless, accurate information on the prevalence of these polymorphisms may be beneficial to studies of gene-disease associations, population genetics and health impact evaluation.
Heretofore, the studies on the three polymorphisms are performed mainly among European and North American populations, and the data on the distributions of these polymorphisms among Chinese population are limited. The Han nationality is the largest among the 56 nationalities in China, making up 92% of the total population. The Han population are distributed widely from north to south of China. Due to high geographical, social and dietary diversity in China, we hypothesized a varied prevalence of these polymorphisms among the Chinese Han populations residing in different regions. In addition, due to the changing of life styles and dietary habits, like in any other developing countries the rates of Hcy-related diseases, such as stroke, coronary heart disease, hypertension, are markedly increasing in China [29]. It is therefore essential to investigate the heterogeneity in the prevalence of these polymorphisms among the Chinese Han population and compare the genetic markers with other published data of different populations around the world. In this study, we showed the prevalence of the MTHFR C677T, A1298C and MTRR A66G polymorphisms among 15357 Chinese Han adults from 10 geographical regions.
Materials and Methods
Ethics Statement
The study was conducted in accordance with the principles stipulated by the Declaration of Helsinki and all procedures were approved by the ethics review committee of the China Medical University. All specimens and survey data were obtained with written informed consents from all participants prior to study entry and subsequently anonymised.
Study Population
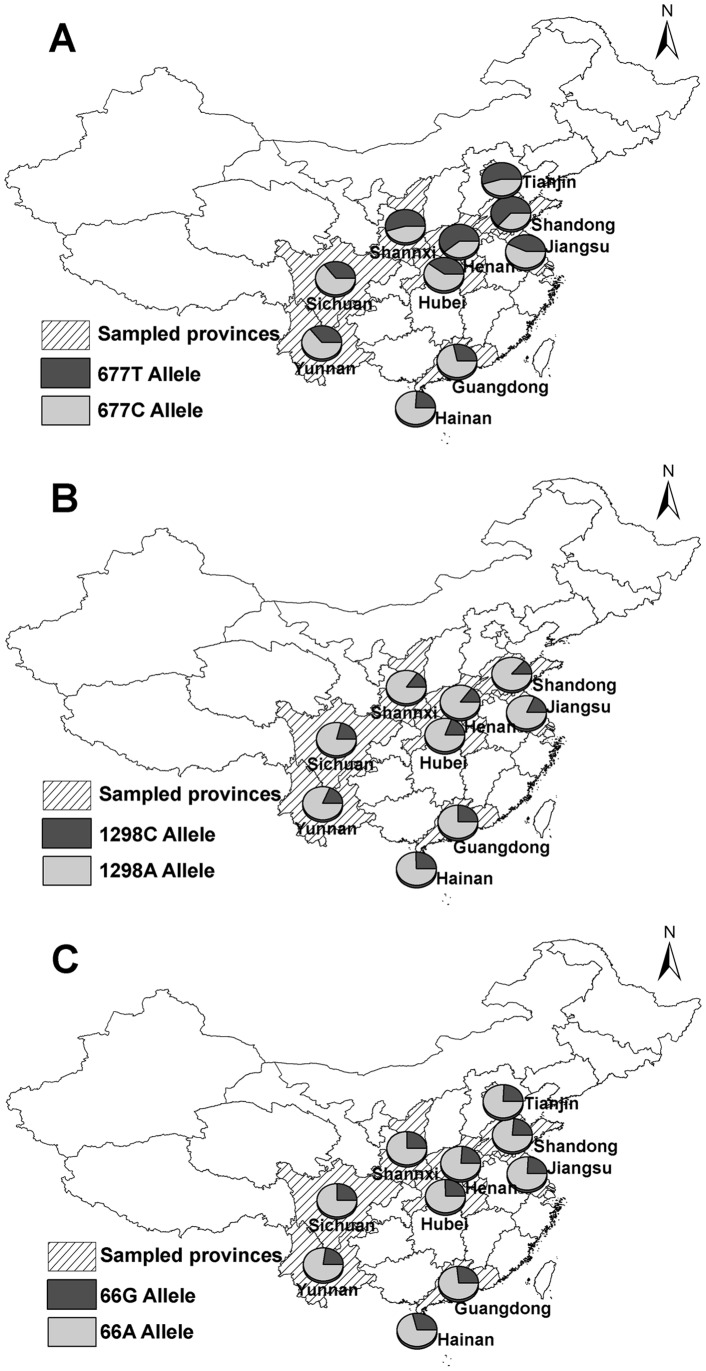
Between October 2008 and February 2011, a total of 13473 unrelated, apparently healthy women aged from 19 to 45 years (mean age, 27±4 years) who came to local maternal and children’s hospital for pre-pregnancy care, were recruited in the study. The hospitals were located in Shandong, Henan, Shannxi, Jiangsu, Hubei, Sichuan, Yunnan, Guangdong, and Hainan provinces, respectively. In addition, 1884 healthy subjects (952 males and 932 females), aged from 18 to 47 years (mean age, 26±5 years), were collected from Dagang Oil Field General Hospital in Tianjin municipality to explore whether the prevalence of the polymorphisms is different between males and females. Figure 1 shows the location of each population in this study.
Figure 1. Map of China showing the distributions of the three polymorphisms in different geographical regions.
Figure 1A, 1B, and 1C show the distributions of the MTHFR C677T, A1298C and MTRR A66G polymorphisms, respectively. Circles indicate the locations of the different populations in this study.
The 10 populations involved in our study were divided into two major groups, the northern and the southern, according to the divide represented by the Yangtze River. The northern group included Shandong (n = 1051), Henan (n = 2661), Tianjin (n = 932), and Shannxi (n = 3090) populations, and the southern group included Jiangsu (n = 477), Hubei (n = 475), Sichuan (n = 2108), Yunnan (n = 106), Guangdong (n = 125), and Hainan (n = 3016) populations.
After obtaining due informed written consent, buccal smears were collected from consenting individuals, dried at room temperature for 1 h and then sent to the laboratory after encoding each sample.
Genotyping
Upon arrival at laboratory, buccal cells were centrifuged and genomic DNA was extracted using the QIAamp DNA Mini Kit (Qiagen, Valencia, CA). The MTHFR C677T, A1298C and MTRR A66G genotypes were determined using the fluorogenic 5′-nuclease assay (Taqman Assay; Applied Biosystems, Foster City, CA). The assays were performed using the Taqman PCR Core Reagent Kit (Applied Biosystems, Foster City, CA) according to manufacture’s instructions. The PCR primers used in the assay and probes (Taqman MGB Probes; Applied Biosystems, Foster City, CA) which were labeled with dyes (FAM or VIC) at the 5′ end, were as follows: for MTHFR C677T, forward primer 5′-GAAAAGCTGCGTGATGATG-3′, reverse primer 5′-TTGAAGGAGAAGGTGTC-3′, probe 1 (VIC-dye labeled) AATCGGCTCCCGC, probe 2 (FAM-dye labeled) AATCGACTCCCGC; for MTHFR A1298C, forward primer 5′-AAGAACGAAGACTTCAAA-3′, reverse primer 5′- TGGGGGGAGGAGCTGAC-3′, probe 1 (VIC-dye labeled) ACACTTGCTTCACT, probe 2 (FAM-dye labeled) ACACTTTCTTCACT; for MTRR A66G, forward primer 5′-AGGCAAAGGCCATCGCA-3′, reverse primer 5′-ATCCATGTACCACAGCTT-3′, probe 1 (VIC-dye labeled) AAGAAATATGTGAG; probe 2 (FAM-dye labeled) AAGAAATGTGTGAG. PCR amplification using approximately 5 ng/sample of genomic DNA was done in a thermal cycler (GeneAmp PCR system 9700; Applied Biosystems, Foster City, CA). Amplifications consisted of an initial step of 95°C for 10 minutes followed by 20 cycles of 92°C for 15 seconds and 60°C for 1 minute. PCR and post-PCR fluorescence analysis were carried out on the Applied Biosystems 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA), and the results were analyzed using the Applied Biosystems Sequence Detection Systems (SDS 2.2.1) software (Applied Biosystems, Foster City, CA).
Statistical Analysis
After the genotype of each individual was obtained, allele and genotype frequencies for the MTHFR C677T, A1298C and MTRR A66G polymorphisms were calculated by direct counting. The confidence intervals of allele and genotype frequencies were computed using the normal approximation and correction for continuity. The χ2 analysis was performed to test Hardy-Weinberg equilibrium among each population under surveyed and compare the differences among the 10 populations with respect to allele and genotype frequencies. The differences in the prevalence of the MTHFR C677T and MTRR A66G polymorphisms between males and females were also examined by χ2 analysis. The comparison of allelic and genotypic frequencies between the northern and southern groups was examined using Kruskal-Wallis test. A P value below 0.05 was taken as statistically significant. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, CN).
Results
Geographical Distribution of MTHFR C677T Allele and Genotype
The allele and genotype frequencies of the MTHFR C677T polymorphism by geographical region are given in Table 1 and Figure 1A. Our data showed a marked heterogeneity in the prevalence of the 677T allele (P<0.0001) and the 677TT genotype (P<0.0001) among the 10 populations. The prevalence of the 677T allele and the 677TT genotype seemed to have an apparent increasing cline from the south to the north. For example, the frequencies of the 677T allele and the 677 TT genotype increased from low values (24.0% and 6.4%, respectively) in Hainan, to intermediate values (28.5–43.5% and 8.3–19.7%, respectively) in Guangdong, Yunnan, Sichuan, Hubei, and Jiangsu, to higher values (54.3–60.2% and 30.4–37.0%, respectively) in Shannxi, Tianjin, and Henan, peaking in Shandong (63.1% and 40.8%, respectively). The frequencies of the 677T allele and the 677TT genotype were significantly higher in the northern than in the southern populations (P = 0.0105 and P = 0.0103, respectively). When taken all populations together, the frequencies of the 677TT genotype and the 677T allele in the Chinese Han population were 23.2% and 45.2%, respectively.
Table 1. Distribution of MTHFR C677T polymorphism among populations from 10 regions in Chinaa.
| Study areaand group | Number of subjects | Genotype (No.) | T allele frequency (%)b | TT genotype frequency (%)c | ||||
| CC | CT | TT | Frequency | 95% CI | Frequency | 95% CI | ||
| Northern * # | ||||||||
| Shandong | 1052 | 154 | 469 | 429 | 63.1 | (60.9–65.1) | 40.8 | (37.8–42.9) |
| Henan | 2661 | 441 | 1236 | 984 | 60.2 | (58.9–61.5) | 37.0 | (35.1–38.8) |
| Tianjin | 932 | 199 | 450 | 283 | 54.5 | (53.1–55.6) | 30.4 | (28.7–32.0) |
| Shannxi | 3090 | 670 | 1482 | 938 | 54.3 | (52.2–56.8) | 30.4 | (27.4–33.4) |
| Southern | ||||||||
| Jiangsu | 477 | 156 | 227 | 94 | 43.5 | (40.3–46.7) | 19.7 | (16.2–23.6) |
| Hubei | 475 | 172 | 223 | 80 | 40.3 | (37.2–43.5) | 16.8 | (13.6–20.5) |
| Sichuan | 2108 | 882 | 936 | 290 | 36.0 | (34.5–37.4) | 13.8 | (12.3–15.3) |
| Yunnan | 124 | 53 | 52 | 19 | 36.3 | (30.3–42.6) | 15.3 | (9.5–22.9) |
| Guangdong | 470 | 241 | 190 | 39 | 28.5 | (25.6–31.5) | 8.3 | (6.0–11.2) |
| Hainan | 3016 | 1763 | 1061 | 192 | 24.0 | (24.6–26.8) | 6.4 | (5.5–7.3) |
| Total | 14405 | 4731 | 6326 | 3348 | 45.2 | (44.6–45.8) | 23.2 | (22.6–23.9) |
Abbreviation: MTHFR, methylenetetrahydrofolate reductase; CC, “wild-type” homozygosity; CT, heterozygosity; TT, mutant homozygosity; CI, confidence interval.
The 10 regions include 9 provinces (Shandong, Henan, Shannxi, Jiangsu, Hubei, Yunnan, Guangdong, and Hainan) and 1 municipality (Tianjin).
The 677T allele frequencies were significantly different among the 10 populations (χ2 = 2166.61, P<0.0001).
The 677TT genotype frequencies were significantly different among the 10 populations (χ2 = 1242.20, P<0.0001).
The 677T allele frequencies were significantly different from the southern populations (χ2 = 6.55, P = 0.0105).
The 677TT genotype frequencies were significantly different from the southern populations (χ2 = 6.59, P = 0.0103).
Geographical Distribution of MTHFR A1298C Allele and Genotype
Table 2 and Figure 1B show the prevalence of the MTHFR A1298C polymorphism by geographical region. The frequencies of the 1298CC genotype varied significantly among different populations (P<0.0001), as well as the frequencies of the 1298C allele (P<0.0001). Geographically, unlike C677T, the distribution of the A1298C polymorphisms showed a reverse trend: increasing from the north to the south. For example, the 1298C allele and the 1298CC genotype frequencies were low in Shandong (13.1% and 1.4%, respectively), intermediate in Jiangsu, Hubei and Sichuan (17.8–20.7% and 3.7–5.0%, respectively), and high in Hainan (25.7% and 6.7%, respectively). The 1298C allele and the 1298CC genotype frequencies were significantly higher in the southern than in the northern populations (P = 0.0201 and P = 0.0389, respectively). In the total sample, the frequencies of the 1298 CC genotype and the 1298C allele were 3.9% and 18.4%, respectively.
Table 2. Distribution of MTHFR A1298C polymorphism among populations from 9 provinces in China.
| Study areaand group | Number of subjects | Genotype (No.) | C allele frequency (%)a | CC genotype frequency (%)b | ||||
| AA | AC | CC | Frequency | 95% CI | Frequency | 95% CI | ||
| Northern * # | ||||||||
| Shandong | 1052 | 791 | 246 | 15 | 13.1 | (11.7–14.6) | 1.4 | (0.8–2.3) |
| Henan | 2661 | 1970 | 625 | 66 | 14.2 | (13.3–15.2) | 2.5 | (1.9–3.1) |
| Shannxi | 3090 | 2243 | 778 | 69 | 14.8 | (13.9–15.7) | 2.2 | (1.7–2.8) |
| Southern | ||||||||
| Jiangsu | 477 | 325 | 134 | 18 | 17.8 | (15.4–20.4) | 3.7 | (2.3–6.0) |
| Hubei | 475 | 318 | 134 | 23 | 18.9 | (16.5–21.6) | 4.8 | (3.1–7.2) |
| Sichuan | 2108 | 1340 | 663 | 105 | 20.7 | (19.5–22.0) | 5.0 | (4.1–5.9) |
| Yunnan | 124 | 82 | 39 | 3 | 18.1 | (13.6–23.5) | 2.4 | (0.5–6.9) |
| Guangdong | 470 | 262 | 181 | 27 | 25.0 | (22.3–27.9) | 5.7 | (3.8–8.2) |
| Hainan | 3016 | 1669 | 1144 | 203 | 25.7 | (24.6–26.8) | 6.7 | (5.9–7.7) |
| Total | 13473 | 9000 | 3944 | 529 | 18.6 | (18.1–19.0) | 3.9 | (3.6–4.3) |
Abbreviation: MTHFR, methylenetetrahydrofolate reductase; AA, “wild-type” homozygosity; AC, heterozygosity; CC, mutant homozygosity; CI, confidence interval.
The 1298C allele frequencies were significantly different among the 9 populations (χ2 = 406.85, P<0.0001).
The 1298CC genotype frequencies were significantly different among the 9 populations (χ2 = 130.73, P<0.0001).
The 1298C allele frequencies were significantly different from the southern populations (χ2 = 5.40, P = 0.0201).
The 1298 CC genotype frequencies were significantly different from the southern populations (χ2 = 4.27, P = 0.0389).
Geographical Distribution of MTRR A66G Allele and Genotype
The prevalence of the MTRR A66G polymorphism is presented in Table 3 and Figure 1C. The frequencies of the 66G allele and the 66GG genotype differed significantly among the 10 populations (P<0.0001 and P = 0.0003, respectively). The prevalence of the MTRR A66G polymorphism increased in a southerly direction. For example, the 66G allele frequency was lowest (23.7%) in Shandong and Henan, intermediate (25.2–26.8%) in Tianjin, Shannxi, Jiangsu, Hubei, Sichuan and Guangdong, and highest (29.2%) in Hainan. The frequency of the 66GG genotype showed similar variability (from 5.4% in Shandong to 8.6% in Hainan). The differences in the 677T allele and the 677TT genotype frequencies between the northern and southern groups did not reach statistical significance (P = 0.2381 and P = 0.2850, respectively). The mean frequencies of the 66G allele and the 66GG genotype in the Chinese Han population were 25.7% and 6.6%, respectively.
Table 3. Distribution of MTRR A66G polymorphism among populations from 10 regions in Chinaa.
| Study areaand group | Number of subjects | Genotype (No.) | G allele frequency (%)b | GG genotype frequency (%)c | ||||
| AA | AG | GG | Frequency | 95% CI | Frequency | 95% CI | ||
| Northern * | ||||||||
| Shandong | 1052 | 611 | 384 | 57 | 23.7 | (21.9–25.5) | 5.4 | (4.1–7.0) |
| Henan | 2661 | 1554 | 951 | 156 | 23.7 | (22.6–24.9) | 5.9 | (4.6–6.4) |
| Tianjin | 932 | 524 | 341 | 67 | 25.5 | (23.5–27.5) | 7.2 | (5.6–9.0) |
| Shannxid | 3090 | 1706 | 1208 | 176 | 25.2 | (24.2–26.3) | 5.7 | (4.9–6.6) |
| Southern | ||||||||
| Jiangsu | 477 | 263 | 185 | 29 | 25.5 | (22.7–28.4) | 6.1 | (4.1–8.6) |
| Hubei | 475 | 261 | 185 | 29 | 25.6 | (22.8–28.5) | 6.1 | (4.1–8.7) |
| Sichuan | 2108 | 1188 | 782 | 138 | 25.1 | (23.8–26.4) | 6.5 | (5.5–7.7) |
| Yunnan | 124 | 73 | 46 | 5 | 22.6 | (17.5–28.3) | 4.0 | (1.3–9.2) |
| Guangdong | 470 | 254 | 180 | 36 | 26.8 | (24.0–29.8) | 7.7 | (5.4–10.5) |
| Hainan | 3016 | 1511 | 1246 | 259 | 29.2 | (28.1–30.4) | 8.6 | (7.6–9.6) |
| Total | 14405 | 7945 | 5508 | 952 | 25.7 | (25.2–26.2) | 6.6 | (6.2–7.0) |
Abbreviations: MTRR, methionine synthase reductase; AA, “wild-type” homozygosity; AG, heterozygosity; GG, mutant homozygosity; CI, confidence interval.
The 10 regions include 9 provinces (Shandong, Henan, Shannxi, Jiangsu, Hubei, Yunnan, Guangdong, and Hainan) and 1 municipality (Tianjin).
The 66G allele frequencies were significantly different among the 10 populations (χ2 = 58.40, P<0.0001).
The 66GG genotype frequencies were significantly different among the 10 populations (χ2 = 31.23, P = 0.0003).
Deviated significantly from Hardy-Weinberg equilibrium (χ2 = 3.97, P = 0.0464).
The 66G allele and the 66GG genotype frequencies were not significantly different from the southern populations (χ2 = 1.39, P = 0.2381 and χ2 = 1.14, P = 0.2850, respectively).
Prevalence of MTHFR C677T and MTRR A66G Polymorphisms by Gender
A total of 1884 samples from Tianjin municipality were collected to explore gender differences in the prevalence of the MTHFR C677T and MTRR A66G polymorphisms. Data obtained from the population are shown in Table 4 and Table 5. For MTHFR C677T, the 677T allele frequency in males and females was 53.7% and 54.5%, respectively. The 677TT genotype frequency in males and females was 29.3% and 30.4%, respectively. The 677T allele and the 677TT genotype frequencies were not significantly different between males and females (P = 0.6321 and P = 0.6157, respectively). For MTRR A66G, the 66G allele frequency in males and females was 23.1% and 25.5%, respectively. The 66GG genotype frequency in males and females was 6.0% and 7.2%, respectively. The 66G allele and the 66 GG genotype frequencies were not significantly different between males and females (P = 0.0893 and P = 0.2930, respectively).
Table 4. Frequencies of MTHFR C677T genotypes and alleles by gendera.
| Gender | Number of subjects | MTHFR C677T genotype, n (%) | Allele frequency | |||||
| CC | CT | TT | 95%CI | C | T | 95%CI | ||
| Malesb | 952 | 208 (21.9) | 465 (48.8) | 279 (29.3) | (26.4–32.3) | 46.3 | 53.7 | (51.5–56.0) |
| Females | 932 | 199 (21.4) | 450 (48.3) | 283 (30.4) | (27.4–33.4) | 45.5 | 54.5 | (52.2–56.8) |
| Total | 1884 | 407 (21.6) | 915 (48.6) | 562 (29.8) | (27.8–32.0) | 45.9 | 54.1 | (52.5–55.7) |
Abbreviations: MTHFR, methylenetetrahydrofolate reductase; CC, “wild-type” homozygosity; CT, heterozygosity; TT, mutant homozygosity; CI, confidence interval.
All participants were from Tianjin municipality.
The 677T allele and the 677TT genotype frequencies were not significantly different from females (χ2 = 0.23, P = 0.6321 and χ2 = 0.26, P = 0.6157, respectively).
Table 5. Frequencies of MTRR A66G genotypes and alleles by gendera.
| MTRR A66G genotype, n (%) | Allele frequency | |||||||
| Gender | No. | AA | AG | GG | 95%CI | A | G | 95%CI |
| Malesb | 952 | 569 (59.8) | 326 (34.2) | 57 (6.0) | (4.6–7.7) | 76.9 | 23.1 | (21.2–25.1) |
| Females | 932 | 524 (56.2) | 341 (36.6) | 67 (7.2) | (5.6–9.0) | 74.5 | 25.5 | (23.5–27.5) |
| Total | 1884 | 1093 (58.0) | 667 (35.4) | 124 (6.6) | (5.5–7.8) | 75.7 | 24.3 | (22.9–25.7) |
Abbreviations: MTRR, methionine synthase reductase; AA, “wild-type” homozygosity; AG, heterozygosity; GG, homozygosity; CI, confidence interval.
All participants were from Tianjin municipality.
The 66G allele and the 66GG genotype frequencies were not significantly different from females (χ2 = 2.89, P = 0.0893 and χ2 = 1.11, P = 0.2930, respectively).
Hardy-Weinberg Equilibrium
The observed genotype frequencies of the MTHFR C677T, A1298C and MTRR A66G polymorphisms were generally in accordance with Hardy-Weinberg equilibrium, with the exception of the MTRR A66G polymorphism among population from Shannxi province.
Discussion
In this study, we examined the prevalence of the C677T and A1298C polymorphisms in the MTHFR gene and the A66G polymorphism in the MTRR gene among a large sample of Chinese adults. Our study subjects were located widely from north to south of China. The frequencies of the MTHFR C677T and A1298C polymorphisms differed significantly between the northern and southern populations.
Our data showed that the prevalence of the MTHFR 677T allele varied between 24.0% and 63.1%. The 677T allele frequencies among subjects from four northern China regions (54.3% in Shanxi, 54.5% in Tianjin, 60.2% in Henan, and 63.1% in Shandong) were much higher than those reported previously for Chinese Han populations (22.2–45.0%) [24], [25], [28], [30]. In addition to these geographical variations, it was previously suggested that 677T allele frequencies varied among ethnic groups. The average 677T allele frequency of our Han subjects was 45.2%, which was lower than that of the Tujia (55.0%) and Lahu (50.0%), and higher than that of the Duar (38.9–45.0%), Man (40.9%), She (40.0%), Xibo (37.6%), Hezhe (35.0%), Uygur (31.3%), Kazakh (30.3), Hui (29.8%), Kyrgyz (28.6%), Dai (10–27.2%), Shui (25.4%), and Jino (21.9%) [21], [28]. Globally, the prevalence of 677T allele ranged from 24.1% to 64.3% among Europeans, 6–64.3% among North Americans, 2–48.7% among South Americans, 0–35.5% among Africans, 8–31.5% among Siberians, and 2.9–28.6% among populations in Oceania [21]. Combined with our data, the frequencies of the 677T allele ranged from 2% to 63.1% in Asia [21].
Another important finding was that the 677T allele and the 677TT genotype frequencies steadily increased from southern to northern China. The geographical gradient presented in our study agrees with that previously reported among Pakistanis [26], Indians [27] and 12 Chinese minority groups [28]. However, our finding seems to contradict observations from Europe and North America, where a reverse trend was reported [24], [25], [31]. Considering the high 677T allele frequencies in Europe, low values in Africa, and the north-to-south cline of increase in the allele frequency across Europe and North American [21], [24], [25], [31], it has been presumed that high T allele frequencies occur in areas where folate intake is adequate and that low frequencies occur in areas where folate intake is inadequate [32], [33]. In China, folate intake is lower in the diet of northern populations than in that of southern populations because of differences in dietary habits [34], [35]. Therefore, we expected lower 677TT genotype frequencies among northern populations and higher frequencies among southern populations. However, our findings were contrary to our expectations. More recently, some investigators hypothesized that ultraviolet (UV) radiation could influence the distribution pattern of MTHFR C677T polymorphism via the photolysis of folate [36]. Lighter skin color and more exposoure to UV radiation may be a disadvantage for 677T allele carriers because of their adverse influence on folate status [37], [38]. However, the UV hypothesis still can not explain why the Chinese northerners, who had lighter skin color [39], less exposure to UV radiation [39] and lower dietary folate intake [34], [35] yet had higher folate deficiency [35] and higher 677T allele frequencies in comparison to the southerners. The reasons for the geographical gradient observed in our study are still unclear, and some other factors, such as evolution and migration in human’s history [40], may also be responsible. Further studies including genetic, nutritional, environmental and demographic factors affecting the polymorphism should be conducted to explain its distributional pattern in China.
The distribution of the MTHFR A1298C polymorphism is not as well studied as that of the C677T. Globally, 1298C allele frequency ranges from 10% to 70% in Asia, 24% to 46% in Europe, 13% to 32.2% in Africa, and 0% to 15% in America [22], [41]. The 1298C allele frequencies in our study ranged from 13.1% in Shandong to 25.7% in Hainan. Taken together, the mean frequency in our study was 18.4%, which is much lower than a previous report of three Han populations (41.7% in Xinjiang, 68.6% in Fujian, and 70.8% in Sichuan) and nine minority groups (25.0% among Kazak, 25.3% among Xibo, 28.3% among Man, 23.8% among Kyrgyz, 42.4% among Shui, 43.3% among Uygur, 44.6% among Jino, 47.8% among Dai, and 48.4% among Hui) [28]. The mean frequency of the1298CC genotype in this study was 3.9%, which is similar to that of Caucasians (4%), Hispanics (4%), and Italians (4.6%), but higher than that of Japanese (1.3%), Mexicans (2.6%), and African Americans (2.1%), and lower than that of Tamilians (10%), Swedish (10.4%), and Lebanese (23.9%) [42]. Interestingly, in contrast to the distribution of C677T, the prevalence of the 1298C allele and the 1298CC genotype showed a decreasing trend from southern to northern China.
The distribution of the MTRR A66G polymorphism is less understood. In this study, we examined the geographical variation in the distribution of the MTRR A66G polymorphism among Chinese adults. Significant deviation from Hardy-Weinberg equilibrium in Shannxi population indicates that selective pressure is acting upon this gene locus. Though the differences between various 66G allele and 66GG genotype frequencies were relatively small, the frequencies increased in a roughly southerly direction. To date, the data on the prevalence of the A66G polymorphism in China are limited. Previous studies of Chinese populations showed that 66G allele and 66GG genotype frequencies had ranges of 20–31% and 2–8%, respectively [15], [43]–[45], which affirmed our observations (25.7% and 6.6%, respectively). Worldwide distributions of the A66G polymorphism showed geographical and ethnic variations. Summaries of previous studies showed that average frequency of the 66G allele was 56.0% in Europe, 26.1% in sub-Saharan Africa, and 28.0% in Asian [46], [47]. Rady et al. [23] reported the allele frequencies among four ethnic populations in Texas: 28.7% among Hispanics, 34% among African Americans, 43.1% among Ashkenazi Jews, and 54.4% among Caucasians. In contrast, the frequency of the 66G allele in our study was relatively low.
The reasons for the distributional differences of these polymorphisms between our study and published reports are still unclear. These differences may derive from differences in study design or the age of the subjects studied, or from the partial ethnic mixing [33]. Additionally, it is possible that genetic drift, living environment, gene-gene and gene-environment interactions could effect the populational distribution of these polymorphisms [31], [32], [39], [48].
The relationship between such geographical and ethnic variation and the prevalence of related diseases is complicated. For example, in southern Italy and Spain, the MTHFR 677TT genotype frequencies are common, but the rates of neural tube defects and cardiovascular diseases are not very high [49], [50]. However, in Mexico, high 677TT genotype frequency is consistent with high rates of neural tube defects [51]. In North America, rates of neural tube defects are high among Hispanics, intermediate among non-Hispanics, and low among Africa Americans, a trend that follows the 677TT genotype frequency [24]. In China, we observed that 677TT genotype frequency decreased from north to south, as did rates of neural tube defects, coronary heart disease, subclinical atherosclerosis, folate deficiency and hypertension [52]–[55]. One possible explanation for these correlational variations is that genetic risks for diseases are influenced by many genetic or environmental factors [24]. Nevertheless, because of higher consistency between 677TT frequency and incidence of Hcy-related disorders in Mexico, North America, and China, the mutation could be considered a genetic risk factor for some disorders. Meanwhile, some specific preventive measures (e.g. folic acid fortification) should be taken in regions where the 677TT genotype is more frequent, such as northern China.
With respect to the MTHFR A1298C polymorphism, because of its weaker effect on enzyme activity and lack of association with elevated homocysteine levels [11], the majority of studies failed to find the association of the polymorphism alone with disorders [31], [56], [57]. However, compound heterozygosity for the C677T and A1298C polymorphisms was associated with elevated Hcy concentrations [9]. This interaction may partly explain previously observed contributions of the A1298C mutation to the development of some disorders [16], [17], [58]. Several epidemiological studies suggested that the MTRR A66G polymorphism was associated with many disorders, including birth defects [15], [18], [59]–[61], cardiovascular diseases [19], [62], [63], and cancers [20]. In addition, it was reported that interaction between the MTRR A66G and MTHFR C677T polymorphisms could increase the risk of neural tube defects [59]–[61] and schizophrenia [2]. The significant gene-gene interactions between the MTHFR C677T, A1298C, and MTRR A66G polymorphisms highlight the importance of multi-locus analyses in the risk prediction of multi-factorial disorders. This phenomenon also reminds us that not only the MTHFR C677T polymorphism, but also the MTHFR A1298C and MTRR A66G polymorphisms should warrant more attention, especially in regions where the MTHFR C677T polymorphism is less frequent and the MTHFR A1298C or MTRR A66G polymorphisms is more frequent, such as southern China.
In interpreting the findings of our study, several aspects can be criticized. Firstly, the data we used for evaluating geographical distributions are all from women. A gender effect may not be avoided because Rozen et al. [64] reported a decreased proportion of 677TT genotypes in female infants. However, when we purposely selected 1884 healthy subjects from Tianjin to explore gender differences, our study failed to find any significant differences in the prevalence of the MTHFR C677T and MTRR A66G polymorphisms between males and females, which is consistent with previous findings [31]. Secondly, our study included only 10 regions. Many other regions in China were not covered. Thirdly, compared with the other regions included in the study, the sample sizes from Yunnan province are small, which could have compromised our estimates. Despite these limitations, our study still has several strengths: (1) heretofore, having the largest sample sizes of all the studies that explore the prevalence of these polymorphisms in China, thus offering more representative and authoritative reference data; (2) reporting the distributions of three polymorphisms in 10 areas in China and specifically investigating the MTRR A66G polymorphism, which is scarcely reported; (3) ruling out possible differences between the polymorphic frequencies of males and females; (4) studying young subjects, thus eliminating possible impact of relationship of gene polymorphisms with a risk of shortened longevity.
In conclusion, our large-scale study indicates that the prevalence of the MTHFR C677T, A1298C and MTRR A66G polymorphisms varies significantly between Han populations residing in different regions of China. Our findings supplement worldwide reports on the three polymorphisms and are helpful for exploring the prevalence of genetic mutations in different populations. These baseline data can also facilitate interpreting the prevalence gradient of diseases associated with those polymorphisms among Chinese Han population. From a public health perspective, the data can help government and experts design preventive measures for specific populations, and evaluate the health care costs-benefits ratio in China, where dietary folic acid fortification has not been initiated.
Acknowledgments
We gratefully acknowledge the assistance and cooperation of the faculty and staff of the local maternal and children’s hospitals and thank all of the participants in our study. We also thank Yongyong Hou from Indiana University, USA and Qiang Zhang from Tianjin Medical University, China for their careful reading of the drafts of this manuscript and the helpful suggestions.
Funding Statement
This study was supported by a grant (No. 81072243) from the National Natural Science Foundation of China (NSFC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Brustolin S, Giugliani R, Felix TM (2010) Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res 43: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lajin B, Alhaj SA, Michati R, Alachkar A (2012) Association between MTHFR C677T and A1298C, and MTRR A66G polymorphisms and susceptibility to schizophrenia in a Syrian study cohort. Asian J Psychiatr 5: 144–149. [DOI] [PubMed] [Google Scholar]
- 3. Eloualid A, Abidi O, Charif M, El HB, Benrahma H, et al. (2012) Association of the MTHFR A1298C variant with unexplained severe male infertility. PLoS One 7: e34111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Homocysteine Lowering Trialists’ Collaboration (2005) Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr 82: 806–812. [DOI] [PubMed] [Google Scholar]
- 5. McKinley MC, McNulty H, McPartlin J, Strain JJ, Pentieva K, et al. (2001) Low-dose vitamin B-6 effectively lowers fasting plasma homocysteine in healthy elderly persons who are folate and riboflavin replete. Am J Clin Nutr 73: 759–764. [DOI] [PubMed] [Google Scholar]
- 6. McNulty H, Dowey LR, Strain JJ, Dunne A, Ward M, et al. (2006) Riboflavin lowers homocysteine in individuals homozygous for the MTHFR 677C→T polymorphism. Circulation 113: 74–80. [DOI] [PubMed] [Google Scholar]
- 7. Yakub M, Moti N, Parveen S, Chaudhry B, Azam I, et al. (2012) Polymorphisms in MTHFR, MS and CBS genes and homocysteine levels in a Pakistani population. PLoS One 7: e33222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, et al. (1995) A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10: 111–113. [DOI] [PubMed] [Google Scholar]
- 9. van der Put NM, Gabreels F, Stevens EM, Smeitink JA, Trijbels FJ, et al. (1998) A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am J Hum Genet 62: 1044–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rozen R (1997) Genetic predisposition to hyperhomocysteinemia: deficiency of methylenetetrahydrofolate reductase (MTHFR). Thromb Haemost 78: 523–526. [PubMed] [Google Scholar]
- 11. Weisberg I, Tran P, Christensen B, Sibani S, Rozen R (1998) A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab 64: 169–172. [DOI] [PubMed] [Google Scholar]
- 12. Gaughan DJ, Kluijtmans LA, Barbaux S, McMaster D, Young IS, et al. (2001) The methionine synthase reductase (MTRR) A66G polymorphism is a novel genetic determinant of plasma homocysteine concentrations. Atherosclerosis 157: 451–456. [DOI] [PubMed] [Google Scholar]
- 13. Olteanu H, Munson T, Banerjee R (2002) Differences in the efficiency of reductive activation of methionine synthase and exogenous electron acceptors between the common polymorphic variants of human methionine synthase reductase. Biochemistry 41: 13378–13385. [DOI] [PubMed] [Google Scholar]
- 14. Wald DS, Morris JK, Wald NJ (2011) Reconciling the evidence on serum homocysteine and ischaemic heart disease: a meta-analysis. PLoS One 6: e16473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeng W, Liu L, Tong Y, Liu HM, Dai L, et al. (2011) A66G and C524T polymorphisms of the methionine synthase reductase gene are associated with congenital heart defects in the Chinese Han population. Genet Mol Res 10: 2597–2605. [DOI] [PubMed] [Google Scholar]
- 16. De Marco P, Calevo MG, Moroni A, Arata L, Merello E, et al. (2002) Study of MTHFR and MS polymorphisms as risk factors for NTD in the Italian population. J Hum Genet 47: 319–324. [DOI] [PubMed] [Google Scholar]
- 17. Palomino-Morales R, Gonzalez-Juanatey C, Vazquez-Rodriguez TR, Rodriguez L, Miranda-Filloy JA, et al. (2010) A1298C polymorphism in the MTHFR gene predisposes to cardiovascular risk in rheumatoid arthritis. Arthritis Res Ther 12: R71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van der Linden IJ, den Heijer M, Afman LA, Gellekink H, Vermeulen SH, et al. (2006) The methionine synthase reductase 66A>G polymorphism is a maternal risk factor for spina bifida. J Mol Med (Berl) 84: 1047–1054. [DOI] [PubMed] [Google Scholar]
- 19. Vijaya LS, Naushad SM, Rupasree Y, Seshagiri RD, Kutala VK (2011) Interactions of 5′-UTR thymidylate synthase polymorphism with 677C→T methylene tetrahydrofolate reductase and 66A→G methyltetrahydrofolate homocysteine methyl-transferase reductase polymorphisms determine susceptibility to coronary artery disease. J Atheroscler Thromb 18: 56–64. [DOI] [PubMed] [Google Scholar]
- 20. Han D, Shen C, Meng X, Bai J, Chen F, et al. (2012) Methionine synthase reductase A66G polymorphism contributes to tumor susceptibility: evidence from 35 case-control studies. Mol Biol Rep 39: 805–816. [DOI] [PubMed] [Google Scholar]
- 21.ALFRED: the ALlele FREquency Databese. Available online: http://alfred.med.yale.edu/alfred/SiteTable1A_working.asp?siteuid=SI001032G. Accessed 26 Apr 2012.
- 22.ALFRED: the ALlele FREquency Databese. Available online: http://alfred.med.yale.edu/alfred/SiteTable1A_working.asp?siteuid=SI003687Y. Accessed 26 Apr 2012.
- 23. Rady PL, Szucs S, Grady J, Hudnall SD, Kellner LH, et al. (2002) Genetic polymorphisms of methylenetetrahydrofolate reductase (MTHFR) and methionine synthase reductase (MTRR) in ethnic populations in Texas; a report of a novel MTHFR polymorphic site, G1793A. Am J Med Genet 107: 162–168. [DOI] [PubMed] [Google Scholar]
- 24. Wilcken B, Bamforth F, Li Z, Zhu H, Ritvanen A, et al. (2003) Geographical and ethnic variation of the 677C>T allele of 5,10 methylenetetrahydrofolate reductase (MTHFR): findings from over 7000 newborns from 16 areas world wide. J Med Genet 40: 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pepe G, Camacho VO, Giusti B, Brunelli T, Marcucci R, et al. (1998) Heterogeneity in world distribution of the thermolabile C677T mutation in 5,10-methylenetetrahydrofolate reductase. Am J Hum Genet 63: 917–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mansoor A, Mazhar K, Ali L, Muazzam AG, Siddiqi S, et al. (2009) Prevalence of the C677T single-nucleotide polymorphism in the methylenetetrahydrofolate reductase gene among Pakistani ethnic groups. Genet Test Mol Biomarkers 13: 521–526. [DOI] [PubMed] [Google Scholar]
- 27. Saraswathy KN, Asghar M, Samtani R, Murry B, Mondal PR, et al. (2012) Spectrum of MTHFR gene SNPs C677T and A1298C: a study among 23 population groups of India. Mol Biol Rep 39: 5025–5031. [DOI] [PubMed] [Google Scholar]
- 28. Mao R, Fan Y, Chen F, Sun D, Bai J, et al. (2008) Methylenetetrahydrofolate reductase gene polymorphisms in 13 Chinese ethnic populations. Cell Biochem Funct 26: 352–358. [DOI] [PubMed] [Google Scholar]
- 29.The World Bank (2011) Toward a healthy and harmonious life in China: stemming the rising tide of non-communicable disease. Available online: http://www.worldbank.org/en/news/2011/07/26/toward-health-harmonious-life-china-stemming-rising-tide-of-non-communicable-diseases. Accessed 9 Feb 2013.
- 30. Ng MC, Wang Y, So WY, Cheng S, Visvikis S, et al. (2004) Ethnic differences in the linkage disequilibrium and distribution of single-nucleotide polymorphisms in 35 candidate genes for cardiovascular diseases. Genomics 83: 559–565. [DOI] [PubMed] [Google Scholar]
- 31. Botto LD, Yang Q (2000) 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epidemiol 151: 862–877. [DOI] [PubMed] [Google Scholar]
- 32. Rosenberg N, Murata M, Ikeda Y, Opare-Sem O, Zivelin A, et al. (2002) The frequent 5,10-methylenetetrahydrofolate reductase C677T polymorphism is associated with a common haplotype in whites, Japanese, and Africans. Am J Hum Genet 70: 758–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gueant-Rodriguez RM, Gueant JL, Debard R, Thirion S, Hong LX, et al. (2006) Prevalence of methylenetetrahydrofolate reductase 677T and 1298C alleles and folate status: a comparative study in Mexican, West African, and European populations. Am J Clin Nutr 83: 701–707. [DOI] [PubMed] [Google Scholar]
- 34. Wang D, He Y, Li Y, Luan D, Yang X, et al. (2011) Dietary patterns and hypertension among Chinese adults: a nationally representative cross-sectional study. BMC Public Health 11: 925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hao L, Ma J, Stampfer MJ, Ren A, Tian Y, et al. (2003) Geographical, seasonal and gender differences in folate status among Chinese adults. J Nutr 133: 3630–3635. [DOI] [PubMed] [Google Scholar]
- 36.Cordain L, Hickey MS (2006) Ultraviolet radiation represents an evolutionary selective pressure for the south-to-north gradient of the MTHFR 677TT genotype. Am J Clin Nutr 84: 1243, 1244–1245. [DOI] [PubMed]
- 37. Jablonski NG, Chaplin G (2000) The evolution of human skin coloration. J Hum Evol 39: 57–106. [DOI] [PubMed] [Google Scholar]
- 38. Branda RF, Eaton JW (1978) Skin color and nutrient photolysis: an evolutionary hypothesis. Science 201: 625–626. [DOI] [PubMed] [Google Scholar]
- 39. Diamond J (2005) Evolutionary biology: geography and skin colour. Nature 435: 283–284. [DOI] [PubMed] [Google Scholar]
- 40. Underhill PA, Shen P, Lin AA, Jin L, Passarino G, et al. (2000) Y chromosome sequence variation and the history of human populations. Nat Genet 26: 358–361. [DOI] [PubMed] [Google Scholar]
- 41. Sazci A, Ergul E, Kaya G, Kara I (2005) Genotype and allele frequencies of the polymorphic methylenetetrahydrofolate reductase gene in Turkey. Cell Biochem Funct 23: 51–54. [DOI] [PubMed] [Google Scholar]
- 42. Sabbagh AS, Mahfoud Z, Taher A, Zaatari G, Daher R, et al. (2008) High prevalence of MTHFR gene A1298C polymorphism in Lebanon. Genet Test 12: 75–80. [DOI] [PubMed] [Google Scholar]
- 43. Shrubsole MJ, Gao YT, Cai Q, Shu XO, Dai Q, et al. (2006) MTR and MTRR polymorphisms, dietary intake, and breast cancer risk. Cancer Epidemiol Biomarkers Prev 15: 586–588. [DOI] [PubMed] [Google Scholar]
- 44. Jiang Y, Xia X, Wang W, Lin L, Xu C, et al. (2012) Hyperhomocysteinemia and related genetic polymorphisms correlate with ulcerative colitis in Chinese Han population in Central China [corrected]. Cell Biochem Biophys 62: 203–210. [DOI] [PubMed] [Google Scholar]
- 45. Stolzenberg-Solomon RZ, Qiao YL, Abnet CC, Ratnasinghe DL, Dawsey SM, et al. (2003) Esophageal and gastric cardia cancer risk and folate-and vitamin B(12)-related polymorphisms in Linxian, China. Cancer Epidemiol Biomarkers Prev 12: 1222–1226. [PubMed] [Google Scholar]
- 46. Lajin B, Alachkar A, Sakur AA (2012) Triplex tetra-primer ARMS-PCR method for the simultaneous detection of MTHFR c.677C>T and c.1298A>C, and MTRR c.66A>G polymorphisms of the folate-homocysteine metabolic pathway. Mol Cell Probes 26: 16–20. [DOI] [PubMed] [Google Scholar]
- 47. Weiner AS, Boyarskikh UA, Voronina EN, Selezneva IA, Sinkina TV, et al. (2012) Polymorphisms in the folate-metabolizing genes MTR, MTRR, and CBS and breast cancer risk. Cancer Epidemiol 36: e95–e100. [DOI] [PubMed] [Google Scholar]
- 48. Ke Y, Su B, Song X, Lu D, Chen L, et al. (2001) African origin of modern humans in East Asia: a tale of 12,000 Y chromosomes. Science 292: 1151–1153. [DOI] [PubMed] [Google Scholar]
- 49. Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, et al. (2012) Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation 125: e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rome: International Center for Birth Defects (2010) International Clearinghouse for Birth defects Monitoring Systems: Annual report 2010 with data for 2008. Available online: http://www.icbdsr.org/page.asp?p=10065&l=1. Accessed 9 Feb 2013.
- 51. Mutchinick OM, Lopez MA, Luna L, Waxman J, Babinsky VE (1999) High prevalence of the thermolabile methylenetetrahydrofolate reductase variant in Mexico: a country with a very high prevalence of neural tube defects. Mol Genet Metab 68: 461–467. [DOI] [PubMed] [Google Scholar]
- 52. Huang J, Wu YF, Liu XQ, Ding D, Zhao LC, et al. (2011) Subclinical atherosclerosis in northern and southern China: the Chinese paradox. J Geriatr Cardiol 8: 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li Z, Ren A, Zhang L, Ye R, Li S, et al. (2006) Extremely high prevalence of neural tube defects in a 4-county area in Shanxi Province, China. Birth Defects Res A Clin Mol Teratol 76: 237–240. [DOI] [PubMed] [Google Scholar]
- 54. Ren A, Zhang L, Hao L, Li Z, Tian Y, et al. (2007) Comparison of blood folate levels among pregnant Chinese women in areas with high and low prevalence of neural tube defects. Public Health Nutr 10: 762–768. [DOI] [PubMed] [Google Scholar]
- 55. Zhao L, Stamler J, Yan LL, Zhou B, Wu Y, et al. (2004) Blood pressure differences between northern and southern Chinese: role of dietary factors: the International Study on Macronutrients and Blood Pressure. Hypertension 43: 1332–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang XW, Luo YL, Wang W, Zhang Y, Chen Q, et al. (2012) Association between MTHFR A1298C polymorphism and neural tube defect susceptibility: a meta-analysis. Am J Obstet Gynecol 206: 251. [DOI] [PubMed] [Google Scholar]
- 57. Chen L, Liu L, Hong K, Hu J, Cheng X (2012) Three genetic polymorphisms of homocysteine-metabolizing enzymes and risk of coronary heart disease: a meta-analysis based on 23 case-control studies. DNA Cell Biol 31: 238–249. [DOI] [PubMed] [Google Scholar]
- 58. Richter B, Stegmann K, Roper B, Boddeker I, Ngo ET, et al. (2001) Interaction of folate and homocysteine pathway genotypes evaluated in susceptibility to neural tube defects (NTD) in a German population. J Hum Genet 46: 105–109. [DOI] [PubMed] [Google Scholar]
- 59. Wilson A, Platt R, Wu Q, Leclerc D, Christensen B, et al. (1999) A common variant in methionine synthase reductase combined with low cobalamin (vitamin B12) increases risk for spina bifida. Mol Genet Metab 67: 317–323. [DOI] [PubMed] [Google Scholar]
- 60. Gueant-Rodriguez RM, Rendeli C, Namour B, Venuti L, Romano A, et al. (2003) Transcobalamin and methionine synthase reductase mutated polymorphisms aggravate the risk of neural tube defects in humans. Neurosci Lett 344: 189–192. [DOI] [PubMed] [Google Scholar]
- 61. Zhu H, Wicker NJ, Shaw GM, Lammer EJ, Hendricks K, et al. (2003) Homocysteine remethylation enzyme polymorphisms and increased risks for neural tube defects. Mol Genet Metab 78: 216–221. [DOI] [PubMed] [Google Scholar]
- 62. Brown CA, McKinney KQ, Kaufman JS, Gravel RA, Rozen R (2000) A common polymorphism in methionine synthase reductase increases risk of premature coronary artery disease. J Cardiovasc Risk 7: 197–200. [DOI] [PubMed] [Google Scholar]
- 63. Li YJ, Li YW, Ding X, Zhao HT, Li Y (2010) Associations of polymorphisms of methionine synthase A2756G and methionine synthase reductase G66A with the risks of coronary artery disease: a meta-analysis. Zhonghua Yu Fang Yi Xue Za Zhi 44: 820–824 (in Chinese) [PubMed] [Google Scholar]
- 64. Rozen R, Fraser FC, Shaw G (1999) Decreased proportion of female newborn infants homozygous for the 677C→T mutation in methylenetetrahydrofolate reductase. Am J Med Genet 83: 142–143. [DOI] [PubMed] [Google Scholar]