Abstract
Purpose
This study evaluates the efficacy of platelet rich plasma (PRP) & porous hydroxyapatite crystals in bone regeneration after surgical removal of mandibular third molar with the help of radiographs and its comparison with control side.
Materials and Methods
A total of 40 patients; both male and female aged between 18 and 35 years, who had impacted mandibular third molars were randomly selected for this study. Twenty patients were taken for control group and 20 patients for study group. The extraction socket of the study group was packed with PRP and hydroxyapatite granules and that of control group was sutured without PRP and hydroxyapatite. The bone density of both extraction sockets were evaluated radiographically using gray level histogram and compared periodically on immediate postoperative day, 1st and 3rd month postoperatively and postoperative sequelae of both the control group and study group in terms of oedema & pain or any other adverse reactions were also assessed.
Results
Data suggested evidence of early bone formation and maturation radiographically in study group as compared to control group. The percentage of facial swelling was numerically greater on the control side as compared to the study side, Pain was also assessed with VAS and it was found that the severity of pain was equal in both study and control groups and the results were not significant.
Conclusion
This study clearly indicated a definitive improvement in the wound healing, increase in bone density, which signifies and highlights the use of PRP and hydroxyapatite granules, certainly as a valid method in inducing and accelerating bone regeneration.
Keywords: Platelet rich plasma, Hydroxyapatite, Bone regeneration, Growth factors, Bone graft, Third molar
Introduction
Healing of bone is a complex process which involves participation of many cell types and growth factors. The healing of fracture or wound is accomplished by the interaction of osteoblasts and extracellular matrix under the influence of various growth factors of which some are secreted by platelets [1]. These factors can activate the proliferation and differentiation of the local osteoprogenitor cells into bone forming cells leading to the formation of new bone matrix and mineralization [2].
Platelet rich plasma (PRP) is an autologous concentrate of platelets suspended in plasma [3, 4]. It is a proven source of growth factors like platelet derived growth factor (PGDF) and transforming growth factor (TGF)-β1 & 2; which is obtained by sequestering and concentrating platelets by gradient density centrifugation. Also used along with bone grafts, PRP gives additional amount of growth factors evidenced by increased radiographic maturation rate of 1.62–2.16 times [2, 3, 5, 6].
By combining with calcium chloride, PRP releases these growth factors [7]. Soft tissue healing is also substantially improved through the application of PRP, by increasing collagen content, promoting angiogenesis and increasing early wound strength [8].
Porous hydroxyapatite (HA) material has been used to fill the bone defects, which has resulted in clinically acceptable responses [9]. It has been shown that porous hydroxyapatite has an excellent bone conductive property which permits the growth of osteogenic cells from existing bone surfaces into adjacent bone graft material [10]. Recently the combination of PRP with porous hydroxyapatite has been of great interest and has shown better healing as compared to porous hydroxyapatite used with saline [11]. Studies have shown that hydroxyapatite is well tolerated by the surrounding tissues with no evidence of inflammation and is appropriate for application in humans [12, 13].
The purpose of this prospective clinical study was to evaluate the osseous regeneration, clinically & radiographically, after surgical removal of mandibular third molars with combination of PRP and porous hydroxyapatite.
Materials and Methods
Selection of Patients
The present study was undertaken at the Department of Oral and Maxillofacial Surgery, Modern Dental College & Research Centre; Indore with due permission of the ethical committee. A total of 40 patients; both male and female aged between 18 and 35 years, who had impacted mandibular third molars were randomly selected for this study. Twenty patients were taken for control group and 20 patients for study group. Patients were selected randomly irrespective of age, sex, caste, religion and socio-economic status and patients with platelet counts within physiological limits (1.5–4.5 lacs/cmm) were included.
The exclusion criteria included systemic diseases, possible compromised immune system, platelet count less than 1.5 lacs/cmm, and documented allergy to any component of the bone substitute or local anaesthetics, drugs.
All patients voluntarily gave consent and signed an informed consent form before participating in the study.
Graft Material
The graft material used in this study was G-Bone, a bioceramic composite material which contains 90 % hydroxyapatite and 10 % beta-tricalcium phosphate. This material is available in three forms i.e. blocks, cylinder and granules. We used granules in this study. These granules are white in colour measuring 1–2 mm in size and with porosity of 500–1200 μmm.
The material is dispensed in a sterile disposable 2 cc syringe in a blister pack.
Preparation of PRP
PRP was prepared from 10 ml of patient’s blood using double centrifugation method. Blood was collected in 5 ml glass tubes, each containing 0.5 ml of 3.2 % sodium citrate [14–16].
The PRP so formed was assessed for platelet concentration and platelet count 3–4 times than the normal was considered acceptable for patient use. Platelet count was calculated using SYSMEX KX21 AUTOMATED HEMATOLOGY ANALYZER and slide preparation was also done and count done in Neuber’s counting chamber (Photographs 1, 2).
Photograph 1.
Peripheral blood smear
Photograph 2.
PRP smear
The values were calculated using the following equations [17]:
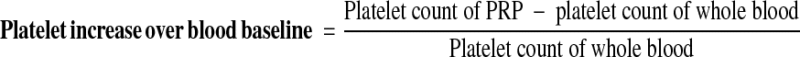 |
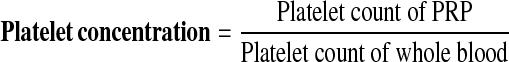 |
.
Activation of PRP
About 10–15 min prior to use of the PRP in the surgical procedure, a coagulated gel was prepared by mixing PRP with 10 % calcium chloride in a ratio of 10:1, i.e. 0.1 ml of calcium chloride for every 1 ml of PRP [11, 17, 18] (Photograph 3).
Photograph 3.
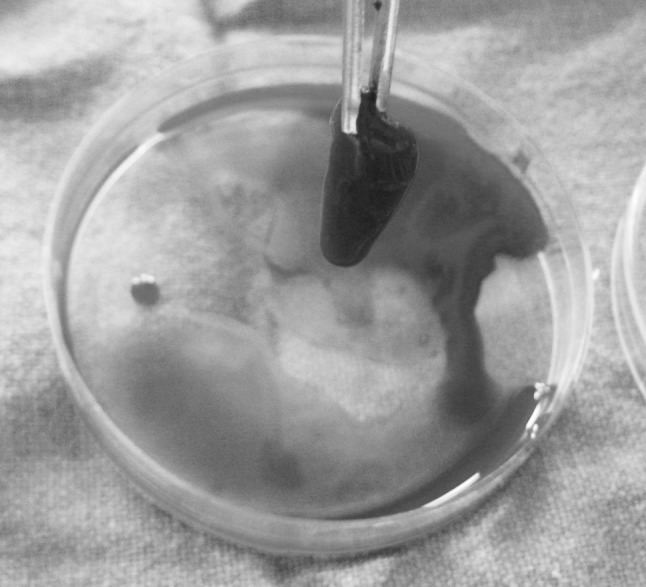
PRP gel formation
Surgical Procedure
All the impacted teeth were extracted under local anesthesia.
Patients were put on an antibiotic course commencing 1 day before surgery to be continued post operatively for 5 days. Under all aseptic precautions and local anaesthesia the impacted mandibular third molars were removed surgically by a single operator.
A mixture of PRP and hydroxyapatite (0.5 gm of porous hydroxyapatite was mixed with every 2 ml of PRP) [12] was placed in study socket (Photograph 4). The wound was closed primarily with 3–0 black braided silk.
Photograph 4.
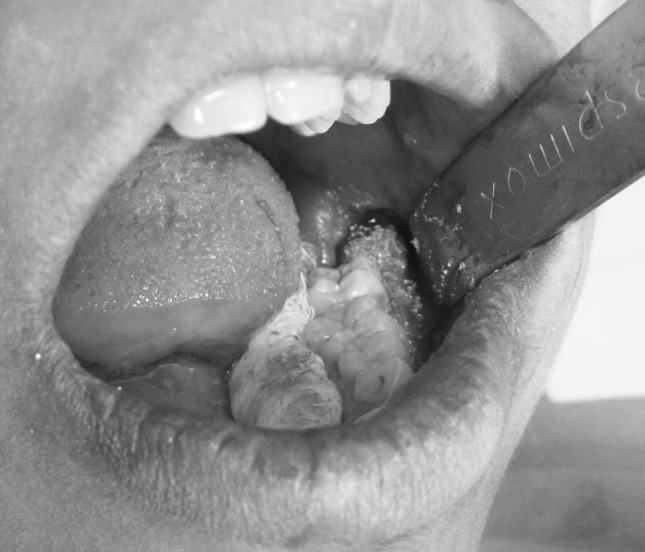
Placement of PRP gel and hydroxyapatite granules in socket
Postoperative Instruction
All the patients were given routine post operative instructions.
All patients were given Cap. Amoxicillin 500 mg, Tab. Diclopep, Tab. Flagyl thrice a day for 5 days.
The patients were asked to rate the pain intensity on five point Visual Analogue scale (VAS).
Swelling was measured preoperatively and on 2nd, 3rd and 7th postoperative day using Suture material (3–0 silk) and metal scale with marking.
Osseous regeneration/bone density was evaluated with the help of standardized intraoral periapical radiographs.
Criteria for radiographic evaluation:
Radiopacity of bone filling the socket.
Presence of trabecular bone formation.
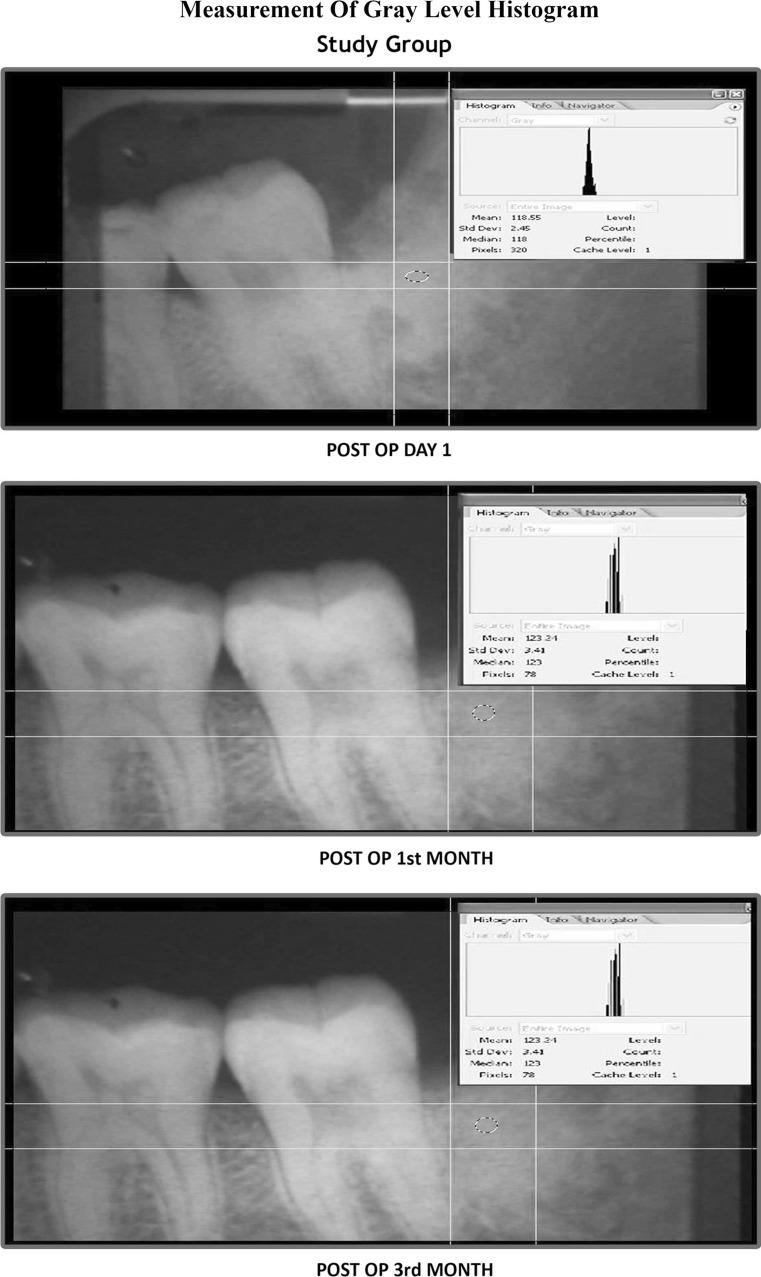
On first post operative day IOPA radiographs were taken and evaluated by the gray level histogram and the procedure was repeated at 1 and 3 months postoperatively. All the IOPA images were digitalized with the help of scanner (“DENTAMERICA\CAMREX\AviCap.exe”). The mean gray level histogram values of the scanned IOPA images of the extraction sockets were obtained in Adobe Photoshop CS2 software [19] (Photograph 5).
Photograph 5.
Measurement of gray level histogram
Results
Results were calculated using the mean value and standard deviation for each of the parameters considered and checked for statistical significance using unpaired ‘t’ test.
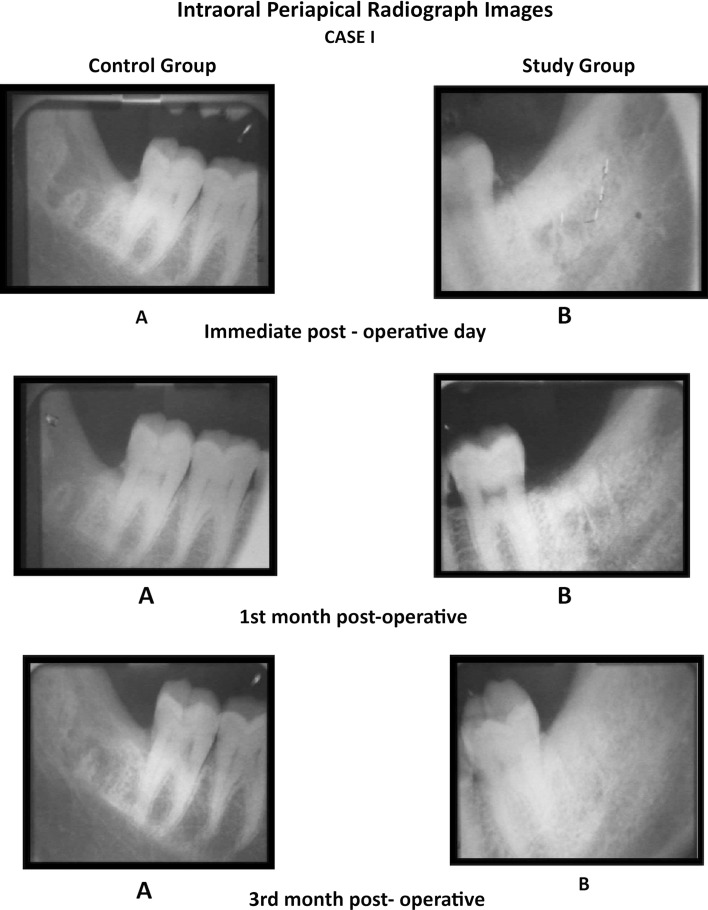
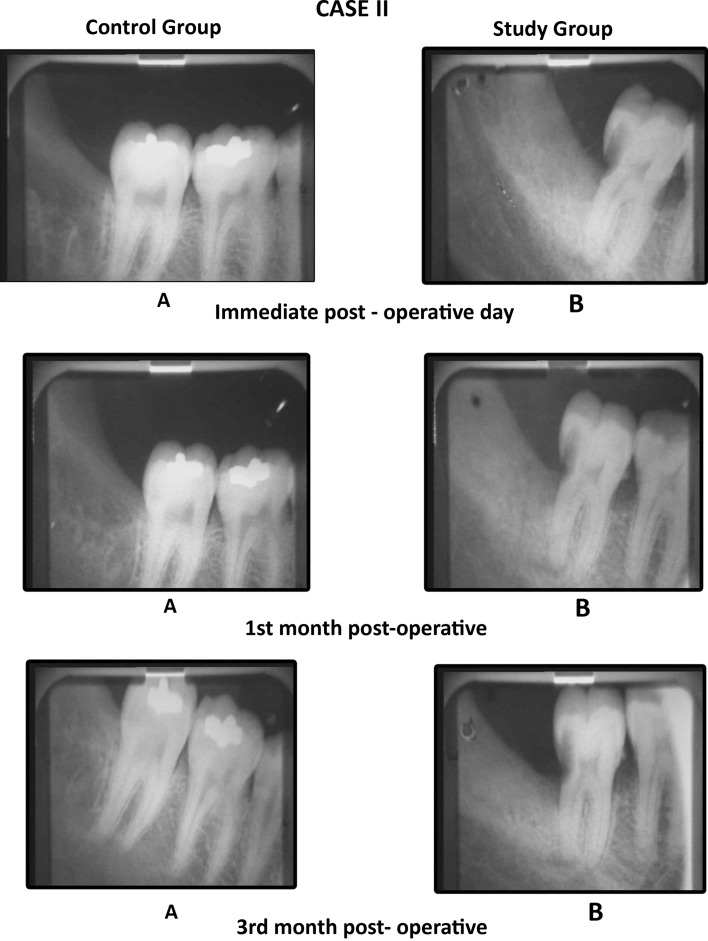
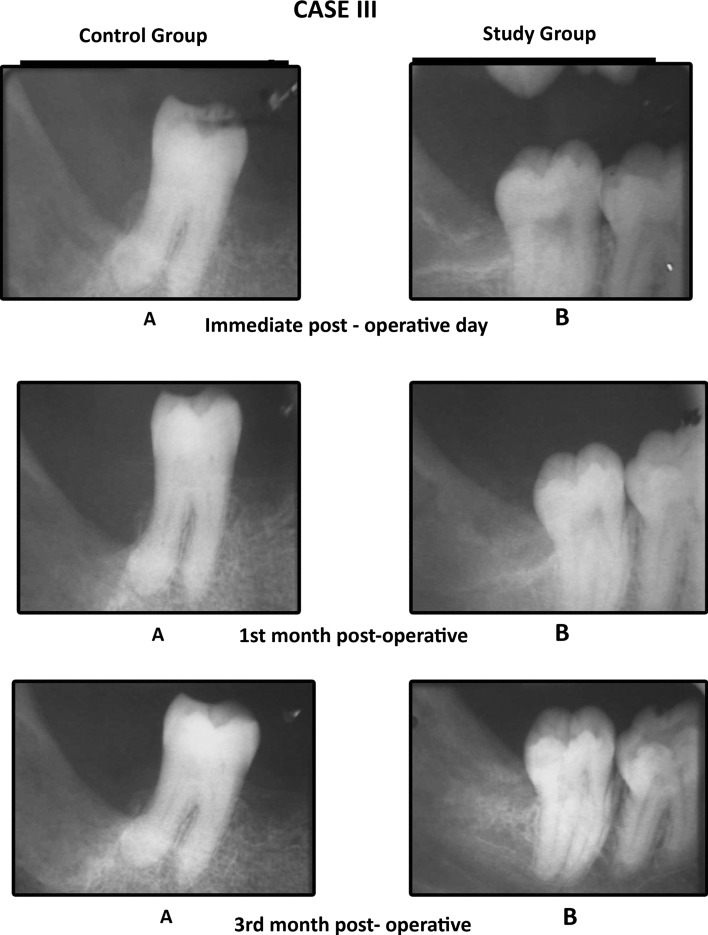
We found that the mean values of gray level histogram were highly significant at 3rd month postoperatively and was significant at immediate post-operative and first month postoperative period. Data suggested evidence of early bone formation and maturation radiographically in study group as compared to control group at 1st and 3rd month postoperatively (Graph 1). We found dense radiographic bone healing on the study side as compared to control side (Cases 1, 2, 3). Evidence of trabecular bone formation was visible as early as 1 week in study group. The results were better in study group than in the control group.
Graph 1.
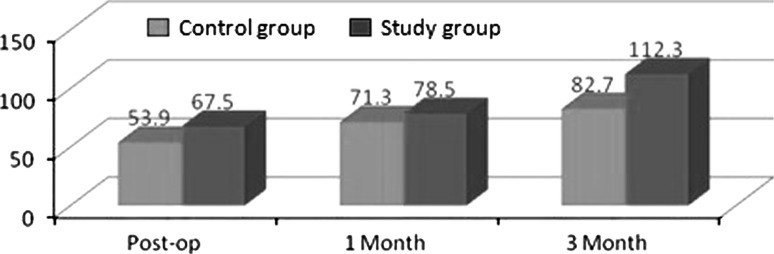
Mean values on different days for gray level histogram values between control and study group
Case 1.
Comparison of the IOPA radiographic images
Case 2.
Comparison of the IOPA radiographic images
Case 3.
Comparison of the IOPA radiographic images
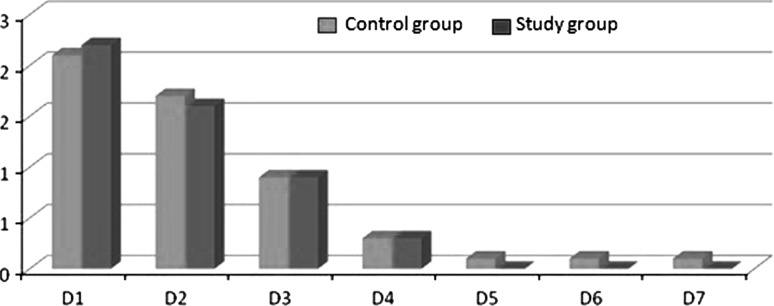
We observed that the percentage of facial swelling was numerically greater on the control side as compared to the study side at 2nd and 3rd post operative day (Graph 2). Pain was also assessed with VAS and it was found that the severity of pain was equal in both study and control groups and the results were not significant. However, the intensity of pain was higher on the day of surgery and gradually reduced (Graph 3).
Graph 2.
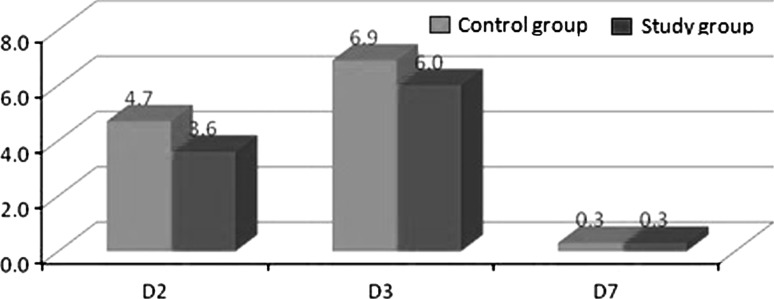
Mean values on different days for % of Facial Swelling between control and study group
Graph 3.
Mean values on different days for VAS for Pain between control and study group
Discussion
Use of PRP is an innovative technique to improve bone healing. A number of studies have been done to check the efficacy of PRP with different bone substitutes. Various studies have verified that PRP when combined with autogenous bone results in considerably faster radiographic maturation and histomorphometrically denser bone regeneration [5, 3]. But they have their own disadvantages like, donor site morbidity, limited availability, post operative discomfort for the patient and increased surgical time. On the other hand, varying results of success have been demonstrated when PRP is added to allografts [20]. But there is a risk of transmission of HIV infection occurring from banked bone. Thus the use of other bone substitutes like alloplastic bone substitute is recommended. Hydroxyapatite (HA) is an osteoconductive material which is biologically inert, readily available, easily adaptable to the site in terms of size and shape. Hydroxyapatite does not exhibit osteoinductive capacity but does demonstrate significant osteoconductive potential [12]. PRP and HA offers an interesting and clinically useful modality to the clinician in treating periodontal osseous defects [12, 13].
The present study was undertaken to study the efficacy of platelet-rich plasma in regeneration of bone when mixed with porous hydroxyapatite and grafted in mandibular third molar socket. This was in accordance with the study by Dr. Kazuhiro Okuda (2005) [13].
The bone formation was measured at different intervals post-operatively. It was observed after a time interval of 3 months that, the sockets filled with the combination of graft and activated platelet rich plasma, showed increase in bone formation, compared to sockets which were left as such and the results were confirmed radiographically. This was in accordance with the study by R.E. Marx (1998) [2].
In our study PRP was prepared from 10 ml of patient’s blood and centrifuged twice in a clinical tabletop centrifugation machine as advocated by, Rick C. Tsay et al. [1], Aron Ganshor [11], Regina Landesberg et al. [21].
To date, there are no clear dose response curves of platelet concentration and bone enhancement; although a 3–5 times increase from the whole blood baseline level is considered a clinical benchmark criteria. The platelet count of PRP in our study was 4–5 times the baseline value as done by Marx et al. [1, 2, 5, 22].
In our study calcium chloride alone was used to form PRP gel [7, 18, 23]. The platelet gel was free of any antigen antibody reaction as it was prepared from patients own blood without the risks of transmitting allogenous material or material of bovine origin [7].
Regina Landesberg [21] and Robert Marx [2] reported that the use of EDTA as anticoagulant was potentially harmful for platelets. So we used 3.2 % sodium citrate as it maintains & preserves platelet membrane integrity [14–16].
The density of the bone formed in the extraction socket was calculated by measuring the gray level histogram values of the digitalized IOPA radiographic images. This method used to assess the bone density was based on the suggestion given by Damante et al. [19].
Radiopacity of the bone filling the socket and presence of trabecular bone formation was evaluated using gray level histogram. The results were better in the study group than in the control group.
We also assessed the percentage of facial swelling in both study and control groups according to the formula given by Mohammad Amin et al. [24]. The percentage of facial swelling was numerically greater on the control side as compared to the study side at 2nd and 3rd post operative day.
Pain was also assessed with VAS and it was found that the severity of pain was equal in both study and control groups and the results were not significant.
Our findings are supported by Deepti Simon et al. [25] who in their study reported evidence of early bone formation radiographically in the third molar extraction group treated with PRP as compared to that of non PRP group.
Jerome Mancuso et al. [26] reported more dense radiographic bone healing, decrease in swelling post operatively, better haemostasis, and lower level of pain on VAS in the third molar extraction group treated with PRP.
Similarly Anitua [27] reported improved epithelization and bone density when PRP was placed in the extraction sockets.
The limitation of the present study was that 3 months post-operative follow up period was short to comment on the efficacy of PRP in complete bone regeneration process but adequate enough to evaluate the effects of PRP in initiating and enhancing both hard and soft tissue healing. Long term follow up along with histological study of the bone is required for assessment of the efficacy of PRP.
Conclusion
This study clearly indicated a definitive improvement in the wound healing, increase in bone density, which signifies and highlights the use of PRP and hydroxyapatite granules, certainly as a valid method in inducing and accelerating bone regeneration. The preparation of PRP is a simple, chair side procedure, safe, cost effective and is made in the preoperative period and demonstrates good results. An added benefit of PRP noted in the present study is its ability to form a biological gel that provides clot stabilization. The use of hydroxyapatite granules (G-bone) is cost effective for the patient as compared to other bone graft materials available in the market.
The present study was done in extraction sockets with a follow up of 3 months, further clinical trials with longer duration and in different sites for e.g. in apicoectomy, & other bone defects should be done to get more affirmative and conclusive results.
References
- 1.Tsay RC, Vo J, Burke A, Eisig SB, Lu HH, Landesberg R. Differential growth factor retention by platelet rich plasma. J Oral Maxillofac Surg. 2005;63:521–528. doi: 10.1016/j.joms.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Geoegeff KR. Platelet rich plasma growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–646. doi: 10.1016/S1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 3.Marx RE. Platelet rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Marx RE. Platelet rich plasma (PRP): What is PRP and what is not PRP? J Implant Dent. 2001;10(4):225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Marx R, Garg A. Dental and craniofacial applications of platelet rich plasma. Chicago: Quintessence; 2005. [Google Scholar]
- 6.Kanno T, Takabashi T, Tsujjsawa T, Ariyoshi W. Platelet rich plasma enhances human osteoblast-like cell proliferation and differentiation. J Oral Maxillofac Surg. 2005;63:362–369. doi: 10.1016/j.joms.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Leao MP, Magini RDS, Machado AMB, Bonfim LKD, Bindo MJF, Pick B, Silva SMLMD (2008) Plasma rich in platelets activation with calcium chloride just. http://www.iadr.confex.com/iadr/2006Orld/techprogram/abstract_76718.htm. Accessed 22 Jan 2009
- 8.Hom DB, Linze BM, Huang TC (2007) The healing effects of autologous platelet gel on acute human skin wounds. Arch Facial Plast Surg 9:174–183 [DOI] [PubMed]
- 9.Kenney EB, Lekovic V, Han T, Carranza FA, Jr, Dimitrijevic B. The use of a porous hydroxylapatite implant in periodontal defects. Clinical results after six months. J Periodontal. 1985;56(2):82–88. doi: 10.1902/jop.1985.56.2.82. [DOI] [PubMed] [Google Scholar]
- 10.Holmes RE, Wardrop RW. Hydroxyapatite as a bone graft substitute in orthognathic surgery: histologic and histometric findings. J Oral Maxillofac Surg. 1998;46(8):661–671. doi: 10.1016/0278-2391(88)90109-7. [DOI] [PubMed] [Google Scholar]
- 11.Gonshor A. Technique for producing platelet rich plasma and platelet concentrate: background and process. Int J Periodontics Restorative Dent. 2002;22:547–557. [PubMed] [Google Scholar]
- 12.Okuda K, Tai H, Tanabe K, Suzuki H, Sato T, Kawase T, Saito Y, Walff L, Yoashie H. Platelet rich plasma combined with porous hydroxyapatite graft for the treatment of intrabony periodontal defects in humans. A comparative controlled clinical study. J Periodontal. 2005;76:890–898. doi: 10.1902/jop.2005.76.6.890. [DOI] [PubMed] [Google Scholar]
- 13.Lykins CL, Friedman CD, Costantino PD. Hydroxyapatite cement in craniofacial skeletal reconstruction and its effects on the developing craniofacial skeleton. Arch Otolaryngol Head Neck Surg. 1998;124:153–159. doi: 10.1001/archotol.124.2.153. [DOI] [PubMed] [Google Scholar]
- 14.Kanno T, Testu T, Tsujjsawa T, Ariyoshi W. Platelet rich plasma enhances human osteoblast like cell proliferation and differentiation. J Oral Maxillofac Surg. 2005;52:161–165. doi: 10.1016/j.joms.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Casati MZ, de Vasconcelos Gurgel BC, Gongalves PF, Pimentel SP, et al. Platelet rich plasma does not improve bone regeneration around peri-implant bone defects—a pilot study in dogs. Int J Oral Maxillofac Surg. 2007;36:132–136. doi: 10.1016/j.ijom.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Wu W, Chen F, Liu Y, et al. Autogenous injectable tissue engineered cartilage by using platelet rich plasma: experimental study in a rabbit model. J Oral Maxillofac Surg. 2007;65:1951–1957. doi: 10.1016/j.joms.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 17.Faleh M, Tamini FM, Montalvo S, Tresguerres I. A comparative study of 2 methods for obtaining platelet rich plasma. J Oral Maxillofac Surg. 2007;65:1084–1093. doi: 10.1016/j.joms.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Sonnleitner D, Huemer P, Sullivan DY. A simplified technique for producing platelet rich plasma and platelet concentrate for intraoral bone grafting techniques: a technical note. Int J Oral Maxillofac Implants. 2000;15:879–882. [PubMed] [Google Scholar]
- 19.Damante JH, Guerra EN da S, Ferreria O. Spontaneous resolution of simple bone cyst. Dentomaxillofac Radiol. 2002;31:182–186. doi: 10.1038/sj.dmfr.4600696. [DOI] [PubMed] [Google Scholar]
- 20.Hanna R, Trejo PM, Weltman RL. Treatment of intrabony defects with bovine-derived xenograft alone and in combination with platelet rich plasma: a randomized clinical trial. J Periodontol. 2004;75:1668–1677. doi: 10.1902/jop.2004.75.12.1668. [DOI] [PubMed] [Google Scholar]
- 21.Landesberg R, Roy M, Glickman RS. Quantification of growth factors levels using a simplified method of platelet rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297–300. doi: 10.1016/S0278-2391(00)90058-2. [DOI] [PubMed] [Google Scholar]
- 22.Marx R, Lynch S, Genco R (1999) Platelet rich plasma: a source of multiple autologous growth factors for bone grafts. In: Tissue engineering-application in maxillofacial surgery and periodontics, chap 4. Quintessence, Chicago, pp 71–82
- 23.Tomonori M, Bonfim LKD, Silva SMLMD, et al. Activation of platelet rich plasma by calcium chloride alone and in combination with thrombin. J Oral Maxillofac Surg. 2002;60:638–642. [Google Scholar]
- 24.Amin M, Laskin DM (1983) Prophylactic use of indomethacin for prevention of postsurgical complications after removal of impacted third molars. J Oral Surg May:440–451 [DOI] [PubMed]
- 25.Simon D, Maneul S, Geetha V, Naik BR. Potential for osseous regeneration of platelet rich plasma—a comparative study in mandibular third molar sockets. Indian J Dent Res. 2004;15(4):133–136. [PubMed] [Google Scholar]
- 26.Manscuso JD. Platelet rich plasma: a preliminary report in routine impacted mandibular third molar surgery and the prevention of alveolar osteitis. J Oral Maxillofac Surg. 2003;60:630–635. [Google Scholar]
- 27.Anitua E. Platelet rich in growth factors: preliminary results of use in the preparation of future site for implants. Int J Oral Maxillofac Implants. 1999;14:529–535. [PubMed] [Google Scholar]