Abstract
Aim
To evaluate the effects of autologous platelet rich fibrin gel (PRF gel) on bone regeneration following extraction.
Materials and Methods
The study design was approved by the Institutional Ethical Committee. Study sample consisting of a total of 22 patients requiring bilateral transalveolar third molar extractions were included after written informed consent. One side was randomly chosen as case and the other side was the control. Autologous PRF gel was prepared from Fresh blood obtained from the patient. The PRF gel was placed in the extraction site and primary closure was obtained.
The patient was called for a follow up on the first post op day, 1st week, one month, three month and six months post op. Regeneration of bone was measured using serial radiographs (RVG) at immediate post op, one, three and six months. This was then compared with the bone regeneration seen in the control group, with the radiographs taken at same intervals, to estimate the difference in bone regeneration if any. RVGs were assessed for amount of radiologic bone filling by the method described by Matteo Chiapasco et al.
Results and Conclusion
Higher mean pixels was recorded in cases compared to controls at all the time intervals viz., immediate post op, 1 month post op, 3 months post op and 6 months post op. However, the difference in the mean pixels recorded between the two groups was not statistically significant (P > 0.05). For complete analysis, further follow up of the present patients and a larger sample size is required to obtain a conclusive result of the Bone Regeneration in extraction sockets with PRF gel.
Keywords: Platelet rich fibrin, Platelet gel, Post extraction socket, Bone regeneration
Introduction
Platelet-rich fibrin gel was first described by Choukroun et al. [1] in France. It has been referred as the second generation platelet-fibrin gel concentrate. It has the following advantages;
Ease of preparation.
Lack of biochemical handling of blood.
Strictly autologous nature, over the traditionally prepared platelet-rich plasma (PRP) gels, as bovine thrombin used in the preparation of PRP gel may lead to development of antibodies to factors 5, 9 and thrombin, resulting in the risk of life threatening coagulopathies [1].
Various uses exist for the gel. Some of which is as follows:
It can be used in conjunction with bone grafts.
It can be used as a scaffold to promote bone growth and maturation.
Wound sealing.
Wound healing and
Hemostasis.
In an experimental study osteoblast cell cultures were used to investigate the influence of PRP and PRF on proliferation and differentiation of osteoblasts. In this study the affinity of osteoblasts to the PRF scaffold appeared to be superior [2]. If such a method of accelerating wound healing in the socket is developed, then, oral rehabilitation by placement of implants without a waiting period can be developed. This method can also be extended to other areas of the body, and also may help in accelerated post-trauma healing. Hence a study was carried out to check for the effect of autologous platelet rich fibrin gel on bone regeneration.
Study Type
Prospective, clinical investigation
Aim
To evaluate the effects of autologous platelet rich fibrin gel (PRF gel) on bone regeneration following transalveolar extractions.
Materials and Methods
Study sample consisting of a total of 22 patients requiring bilateral transalveolar third molar extractions were included after informed consent. One side was randomly chosen as case and the other side was the control.
Criteria for Inclusion of Patients
Patient willing to give informed consent.
Patients above 18 years of age.
Patient indicated for transalveolar extraction of bilateral third molars.
Patient with a blood concentration of thrombocytes within the normal range (1.5–3.5 lakh cells/cubic cm).
In areas where primary closure is possible.
Criteria for Exclusion of Patients
The presence of uncontrolled diabetes, immune disease, or other contraindicating systemic conditions.
Radiation therapy/Chemotherapy in the 12 month period earlier to the proposed therapy.
Uncontrolled periodontal disease.
Presence of any acute local infection.
A smoker.
Pregnant women, children, elderly (>60 years), physically and mentally challenged, terminally and seriously ill.
An unwillingness to commit to a long-term post-therapy maintenance program.
Methodology
Preparation of PRF gel
The required quantity of blood (9 ml) is drawn into 10 ml Syringe (Fig. 1). From this 4.5 ml is used for routine blood investigation and the other 4.5 ml is transferred to tube containing 0.5 ml anticoagulant [acidulated citrate dextrose (ACD)] and centrifuged using a table-top centrifuge for 20 min at 360–400 rpm (Fig. 2). The resultant product consists of the following layers.
The top most layer, consisting of acellular platelet poor plasma (PPP).
The second layer consists of platelet rich plasma (PRP).
RBC at the bottom (Fig. 3).
Fig. 1.
Withdrawing blood by venipucture
Fig. 2.

Centrifugation of blood
Fig. 3.

Separation of blood into three layers
After which the top layer consisting of PPP will be discarded as Platelet count is minimal. The second layer will be transferred to a neatly incubated test tube. Calcium Gluconate is added to this solution (PRP). For 2 ml of PRP, 0.5 ml of Calcium Gluconate is added and allowed to stand for 10 min for the standardization of the gel. The resultant gel obtained is platelet rich fibrin.
This gel is placed into the extracted tooth sockets of the patients and sutured (Figs. 4 and 5). The variation of the platelet counts from the each individual patient has been observed and recorded both the base line platelet count and the platelet count in the PRF gel. Patients with a baseline platelet count greater than 1,00,000 cells/cu mm were only selected. An average increase of platelet count up to four fold (1:4) was seen in all cases after the centrifuge. Further follow up of the Patients was done in the next consultation with RVGs regularly for 1st, 3rd, 6th month.
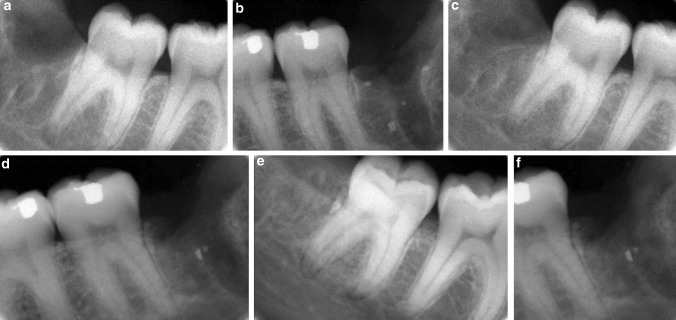
Fig. 6.
a 1st month post op, RVG Image with PRF gel b 1st month post op, RVG Image without PRF gel c 3rd month post op, RVG Image with PRF gel d 3rd month post op, RVG Image without PRF gel e 6th month post op, RVG Image with PRF gel f 6th month post op, RVG Image without PRF gel
Fig. 4.
PRF Gel
Fig. 5.
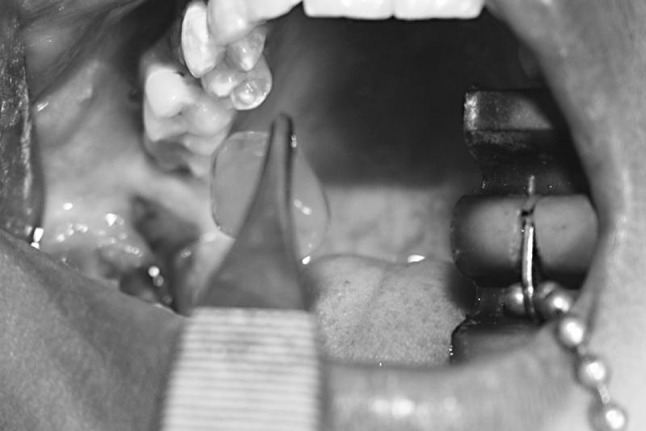
Placement of PRF Gel into the post extraction socket
Radio Visio-Graphic (RVG) Analysis
After Placing the PRP Gel in the Socket region an Immediate RVG was taken. Patient was recalled at regular intervals of 1st, 3rd and 6 months post op to take RVG’S. The Radiolucency of the Socket region was measured using the Corel Draw Software. The size of the residual defect was calculated by the technique described by Matteo Chiapasco et al. [3]. The radiographs were converted to a digital format by a scanner connected to a computer and through the transfer of RVG images to the Corel Draw software. They were then converted to grayscale tonalities of 256 using Corel Photo paint Software.
A person blinded of the PRF primary closure marked the size of the residual cavity using the software. The area marked was converted into a histogram which gave the number of pixels in the residual cavity. The number of pixels in the residual cavity was calculated on all RVGs. This indicated the size of the defect. The number of pixels in the residual defect in the immediate post-operative radiograph was fixed at 100 %. The decreasing number of pixels in the surgical defect overtime gave us the relative bone filling in the area of the lesion. The percentage of bone filling was then calculated. The residual cavity defect and regenerated bone density in both the study group and control group were also calculated using Radio Visio-Graphs to rule out bias. Comparing the bone regeneration results of the participants on test side and Control side with 6 months follow up.
The Statistical analysis is as follows.
Null Hypothesis: There is no significant difference in the mean pixels between two groups i.e. μ1 = μ2.
Alternate Hypothesis: There is a significant difference in the mean pixels between two groups i.e. μ1 ≠ μ2.
Level of Significance: α = 0.05.
Statistical test used:t test.
Decision Criterion: We compare the P value with the level of significance. If P < 0.05, we reject the null hypothesis and accept the alternate hypothesis. If P ≥ 0.05, we accept the null hypothesis.
Computations: The Table 1 gives us the various computations and the P value.
Table 1.
Statistical analysis of the data
| Time interval | Group | N | Mean | Std dev | Mean diff | t | P value |
|---|---|---|---|---|---|---|---|
| Immediate post-op | Case | 22 | 1,272.41 | 383.63 | 9.500 | 0.090 | 0.929 |
| Control | 22 | 1,262.91 | 314.01 | ||||
| 1st month follow-up | Case | 22 | 1,144.55 | 385.32 | −58.545 | −0.569 | 0.573 |
| Control | 22 | 1,203.09 | 291.12 | ||||
| 3rd month follow-up | Case | 22 | 1,036.09 | 330.30 | −120.727 | −1.293 | 0.203 |
| Control | 22 | 1,156.82 | 287.36 | ||||
| 6th month follow-up | Case | 22 | 979.41 | 325.21 | −135.318 | −1.454 | 0.153 |
| Control | 22 | 1,114.73 | 291.09 |
Higher mean pixels was recorded in cases compared to controls at all the time intervals viz., immediate post op, 1 month post op, 3 months post op and 6 months post op (graph 1). However, the difference in the mean pixels recorded between the two groups was not statistically significant (P > 0.05).
Discussion
In the preparation methods of PRP described by Marx et al. [4] and others, the coagulation process to obtain a gel was initiated with 10 % calcium chloride and bovine thrombin. The introduction of an endogenous initiator of coagulation (usually bovine thrombin), in most of the commercially available methods of PRP preparation has the effect of causing rapid degranulation of platelets and almost immediate liberation of growth factors into the surgical area at the time of preparation [5]. Since growth factors have a limited time of effectiveness, immediate release of growth factors can only affect the immediate stages of wound healing and not the extended period of time needed for bone and soft tissue regeneration.
A platelet-rich fibrin material, which does not use bovine thrombin as an activator, has been described as a platelet-rich fibrin matrix (PRFM) [6]. The proprietary process for PRFM preparation separates the blood cells from the platelets and plasma proteins, during an initial low speed centrifugation of a patient’s blood. A second centrifugation converts fibrinogen to fibrin in the presence of CaCl2 and the fibrin cross-links to form a matrix that contains viable platelets. Carroll et al. [7] have demonstrated, in vitro, that the viable platelets in PRFM released six growth factors in about the same concentration for the 7 day duration of their study. Given prolonged growth factor presence it would be expected that PRFM treatment of an extraction socket might result in enhanced wound healing. To test this hypothesis, a study was designed to compare bone regeneration following bilateral extractions of identically placed third molar teeth which required primary closure to contain the PRF gel within the site. Higher mean pixels was recorded in cases compared to controls at all the time intervals viz., immediate post op, 1 month post op, 3 months post op and 6 months post op. However, the difference in the mean pixels recorded between the two groups was not statistically significant (P > 0.05).
We also observed accelerated soft tissue healing at all the test sites with PRF compared with the control sites. The soft tissue parameters assessed qualitatively were: postoperative swelling, trismus, erythema, pus formation and Wound dehiscence in the first week of extraction.
When combined with bone graft it may facilitate better and faster bone regeneration because of the presence of growth factors [4]. It is an economical alternative to expensive recombinant growth factors when used in conjunction with osseous grafts.
No graft material was added to PRF in this study. It is assumed that the combination of bone grafts with PRF might have further improved the result of our study. We intend to use it in conjunction with bone graft material to further accelerate bone regeneration.
A canine study [8] performed to determine if extraction sites treated with PRFM exhibit enhanced healing compared to sites treated with non-viable materials. Demineralized freeze-dried bone allograft (DFDBA) and membrane, PRFM and DFDBA, and untreated control were used. Clinical and histologic evaluation of healing at 10 days, 2, 3, 6 and 12 weeks showed that healing was more rapid in the PRFM and PRFM and membrane sites. By 3 weeks those sockets had osseous fill. Sites containing DFDBA had little new bone at 6 weeks. So they concluded that PRFM alone may be the best graft for ridge preservation procedures. Some authors [9, 10] have reported that PRF can also be used for accelerated repair of articular cartilages. It has also found applications in periodontal pathologies [11].
We also are carrying on research currently with PRF as a means of local delivery of antibiotics (metronidazole) and analgesic (diclofenac) to improve local wound healing and also to cut down on systemic delivery of drugs.
Conclusion
The present study clearly indicates a definite improvement in the regeneration of bone after third molar surgery in cases treated with PRF as compared to the control group post operatively. This increase in the bone density signifies and highlights the use of PRF, certainly as a valid method in accelerating hard tissue regeneration. For complete analysis, further follows up of the present patients and a larger sample size is required to obtain a conclusive result of the bone regeneration in extraction sockets with PRF gel.
Acknowledgments
We would like to acknowledge the financial aid by the Indian Council Of Medical Research (ICMR) to carry out this study in the planned manner. IRIS ID Number:2009-00470.
References
- 1.Choukroun J, Diss A, Simonpiere A, et al. Platelet rich fibrin (PRF): a second generation platelet concentrate Part 1: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:E37–E44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez AR, Sheridan PJ, Kupp LI. Is platelet rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 2003;18:93–103. [PubMed] [Google Scholar]
- 3.Chiapasco M, Rossi A, Motta JJ, Crescentini M. Spontaneous bone regeneration after enucleation of large mandibular cysts: a radiographic computed analysis of 27 consecutive cases. J Oral Maxillofac Surg. 2000;58:942–948. doi: 10.1053/joms.2000.8732. [DOI] [PubMed] [Google Scholar]
- 4.Marx R, Carlson ER, Eichstaedt RM, et al. Platelet Rich Plasma growth factors enhancement for bonegrafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–646. doi: 10.1016/S1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 5.O’Connell S, Carroll R, Beavis A, et al. Flow cytometric characterization of Cascade platelet-rich fibrin matrix (PRFM); the impact of exogenous thrombin on platelet concentrates (PC) Edison: Musculoskeletal Transplant Foundation; 2006. [Google Scholar]
- 6.Lucarelli E, Beretta R, Dozza B, Tazzari TL, O’Connell S, Ricci F, Pierini M, Squarzoni S, Pagliaro PP, Oprita EI, Donati D. A recently developed bifacial platelet-rich fibrin matrix. Eur Cell Mater. 2010;20:13–23. doi: 10.22203/ecm.v020a02. [DOI] [PubMed] [Google Scholar]
- 7.Carroll RJ, Amoczky SP, Graham S, O’Connell SM. Characterization of autologous growth factors in Cascade platelet rich fibrin matrix (PRFM) Edison: Musculoskelatal Transplant Foundation; 2005. [Google Scholar]
- 8.Simon BI, Zatcoff AL, Kong JJW, O’Connell SM. Clinical and histological comparison of extraction socket healing following the use of autologous platelet-rich fibrin matrix (PRFM) to ridge preservation procedures employing demineralized freeze dried bone allograft material and membrane. Open Dent J. 2009;3:92–99. doi: 10.2174/1874210600903010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuo TF, Lin MF, Lin YH, Lin YC, Su RJ, Lin HW, Wing P. Implantation of platelet-rich fibrin and cartilage granules facilitates cartilage repair in the injured rabbit knee: preliminary report. Clinics. 2011;66(10):1835–1838. doi: 10.1590/S1807-59322011001000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singergy AAE, Haleem AM, Sabry D, Atta HM, Rashed LA, Chu CR, Shewy MTE, Azzam A, Aziz MTA. The clinical use of human culture-expanded autologous bone marrow mesenchymal stem cells transplanted on platelet-rich fibrin glue in the treatment of articular cartilage defects: a pilot study and preliminary results. Cartilage. 2010;1(4):253–261. doi: 10.1177/1947603510366027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anilkumar K, Geetha A, Umasudhakar, Ramakrishnan T, Vijayalakshmi R, Pameela E. Platelet-rich-fibrin: a novel root coverage approach. J Indian Soc Periodontol. 2009;13(1):50–54. doi: 10.4103/0972-124X.51897. [DOI] [PMC free article] [PubMed] [Google Scholar]