Abstract
Background
Monocyte-derived foam cells are the hallmark of early atherosclerosis, and recent evidence indicates that chemokines play important roles in directing monocyte migration from the blood to the vessel wall. Genetic deletions of monocyte chemoattractant protein-1 (MCP-1, CCL2), fractalkine (CX3CL1), or their cognate receptors, CCR2 and CX3CR1, markedly reduce atherosclerotic lesion size in murine models of atherosclerosis. The aim of this study was to determine whether these 2 chemokines act independently or redundantly in promoting atherogenesis.
Methods and Results
We crossed CX3CL1−/− ApoE−/− and CCR2−/− ApoE−/− mice to create CX3CL1−/− CCR2−/− ApoE−/− triple knockouts and performed a 4-arm atherosclerosis study. Here, we report that deletion of CX3CL1 in CCR2−/− mice dramatically reduced macrophage accumulation in the artery wall and the subsequent development of atherosclerosis. Deletion of CX3CL1 did not reduce the number of circulating monocytes in either “wild-type” ApoE−/− mice or CCR2−/− ApoE−/− mice, which suggests a role for CX3CL1 in the direct recruitment and/or capture of CCR2-deficient monocytes.
Conclusions
These data provide the first in vivo evidence for independent roles for CCR2 and CX3CL1 in macrophage accumulation and atherosclerotic lesion formation and suggest that successful therapeutic strategies may need to target multiple chemokines or chemokine receptors.
Keywords: atherosclerosis, leukocytes, inflammation
Atherosclerosis is a multigenic disease that begins as fatty streaks in early life and progresses to complex plaques that are vulnerable to rupture. Circulating blood monocytes are the precursors of the lipid-laden macrophages that are characteristic of early lesions,1 and it is now widely appreciated that macrophages contribute to the inflammation in both early and advanced lesions.2 Intense investigation of the molecular basis for recruitment of monocytes to atherosclerotic lesions has revealed critical roles for chemokines.
Chemokines are chemotactic cytokines that are synthesized and secreted by vascular wall endothelial and smooth muscle cells in response to cytokines, lipopolysaccharide, or oxidized lipids.3,4 Monocytes undergo chemotaxis in response to several chemokines, including monocyte chemotactic protein-1 (CCL2) and fractalkine (CX3CL1), but the mechanisms by which chemokines recruit and capture circulating monocytes are not well understood. Among the circulating monocytes are “inflammatory” monocytes that are thought to move rapidly from the blood to sites of inflammation, such as atherosclerotic lesions.5 Recent studies have found that these monocytes are uniformly CCR2+6,7 and CX3CR1lo.8 In contrast, CX3CR1 is most highly expressed on CCR2− monocytes that are destined to become resident macrophages.8 These monocytes also traffic to atherosclerotic lesions, albeit somewhat less efficiently than the inflammatory monocytes.9 Nonetheless, deletion of either CCR210 or CX3CR111,12 markedly reduces macrophage recruitment to early athero-sclerotic lesions, which results in significant atheroprotection.
In the present study, we sought to determine whether CCR2 and CX3CR1 act independently or in concert to promote atherosclerosis. The close proximity of CCR2 and CX3CR1 on chromosome 9 makes the creation of mice genetically deficient in both receptors technically difficult. We therefore took advantage of the fact that CX3CL1 is the only known ligand for CX3CR1 and crossed CX3CL1−/− mice with CCR2−/− mice to investigate the relative contributions of CCL2/CCR2 and CX3CL1/CX3CR1 in atherosclerotic lesion development. We performed a 4-arm atherosclerosis study in mice on the apolipoprotein (Apo) E−/− background. Here, we report that the combined genetic deletion of CCR2 and CX3CL1 resulted in dramatic reduction of atherosclerotic lesions and afforded significantly greater atheroprotection than either of the single deletions. These results suggest that CX3CL1 and CCR2 independently promote monocyte recruitment to atherosclerotic lesions.
Methods
Mice
Fractalkine−/−ApoE−/− (CX3CL1−/−ApoE−/−), CCR2−/−ApoE−/−, and CX3CL1−/−CCR2−/−ApoE−/− mice were generated by crossing CX3CL1−/− mice13 with CCR2−/− mice14 and then crossing CX3CL1−/−CCR2−/− mice with ApoE−/− mice. CX3CL1−/−, CCR2−/−, and CX3CL1−/−CCR2−/− mice were maintained on an ApoE+/+ background. CCR2−/− and CCR2−/−ApoE−/− mice were created as described previously.10,14 All mice were backcrossed at least 10 times onto the C57Bl/6 genetic background before mating. Genotyping was performed by polymerase chain reaction and by Transnetyx (Memphis, Tenn). Mice were weaned at 21 to 25 days of age and fed a chow diet containing 4.5% (wt/wt) fat (Ralston Purina, St Louis, Mo) for 21 additional days. Mice were then switched to a high-fat Western diet containing 0.15% cholesterol and 21% fat without added cholate (Harlan Teklad diet No. 88137, Madison, Wis) and maintained for 8 weeks. Mice were matched for gender in each control and experimental group. All mice were housed in a specific pathogen–free facility and were weighed immediately before being euthanized.
Atherosclerosis Analysis
The degree of atherosclerosis was assessed by quantifying lesion sizes on en face, pinned-open aortas and on cross sections of the proximal aortic roots, as described previously.11 After anesthesia (Avertin 0.5 mg/g body weight), mice were perfused through a cannula inserted into the left ventricle with PBS/0.2 mmol/L EDTA followed by a fixative solution (10% phosphate-buffered formalin [Fisher Scientific, Fair Lawn, NJ], 7.5% sucrose, 20 μmol/L butylated hydroxytoluene [Sigma, St Louis, Mo], and 0.2 mmol/L EDTA, pH 7.4]). The heart was removed for sectioning (see below) and the carcass stored in fixative at 4°C for 5 to 7 days before aortic dissection. After removal of any adipose and connective tissue, the aorta was opened longitudinally along the ventral midline from the iliac arteries to the aortic root. After branching vessels were removed, the aorta was pinned out flat, rinsed in 70% ethanol for 5 minutes, stained with Sudan IV for 6 minutes, destained with 80% ethanol for 3 minutes, and then washed and stored in the fixative solution. Aortic images encompassing the entire aorta (arch, thorax, and abdomen) were captured with a mounted Canon G5 digital camera on the macro setting (Canon, Lake Success, NY).
For the aortic root analysis, hearts were dissected by severing the aorta midway between the bifurcation of the innominate artery and the point of entry into the heart. The heart was cut in 2 with a razor blade at mid ventricle such that the plane of the cut was perpendicular to the aorta's exit from the heart. The base of the heart containing the aortic root was perfused with fixative for 30 minutes, then equilibrated in 10% sucrose/PBS for 1 hour, 20% sucrose/PBS for 2 hours, and then 30% sucrose/PBS overnight at 4°C. The following day, hearts were embedded in OCT with the cut side facing down and frozen in isopentane cooled with dry ice. Approximately 80 sections 8 μm thick were cut starting at the appearance of the first aortic sinus valve leaflet. Nine sections 40 μm apart centered on the appearance of the first coronary ostium were chosen for analysis. Sections were stained with oil red O (0.5% in propylene glycol) and then counterstained with Mayer hematoxylin as described previously.11 Images were captured with an Olympus BX60 microscope (Melville, NY) and Zeiss Axiocam HRc camera (Jena, Germany).
Immunohistochemistry
To quantify the macrophage content of the atherosclerotic lesions, we stained the aortic root sections with MOMA-2 (Serotec, Raleigh, NC; MCA519G). Nine sections, 40 μm apart and adjacent to those analyzed for oil red O staining (see above), were selected for analysis. Unless otherwise noted, all procedures were performed at room temperature. Sections were fixed in acetone for 10 minutes, air-dried, washed twice in PBS, and then encircled with a hydrophobic pen. Sections were incubated with 0.1 mol/L glycine/PBS (15 minutes), 3% BSA/PBS (30 minutes), 5% FCS/PBS (30 minutes), and then MOMA-2 (diluted 1:50 in 0.1% BSA/PBS) overnight at 4°C. Sections were rinsed in PBS (5 minutes), and secondary antibody was applied (goat anti-rat Alexa Fluor 488 diluted 0.01 mg/mL in 0.1% BSA/PBS; Molecular Probes A-11006, Carlsbad, Calif) for 90 minutes. After another PBS rinse (5 minutes), slides were mounted with a hard-setting DAPI mounting medium (Vector Laboratories, Burlingame, Calif; H-1500). Sections stained with secondary antibody alone served as staining controls. Images were captured with an Olympus BX60 microscope and Zeiss Axiocam HRc camera.
Image Analysis
All images were analyzed with Adobe Photoshop 6 software (Adobe Systems, Mountain View, Calif) and the Image Processing Tool Kit plug-ins (Reindeer Games, Gainesville, Fla) to demarcate and quantify lesions and MOMA-2–positive areas. The valve leaflets were excluded from the analysis. Data for the aortic pin-outs are reported as the percentage of the aortic surface covered by lesions (total surface area of the atherosclerotic lesions divided by the total surface area of the aorta, in micrometers squared). Data from the root analysis are reported as the total surface area of the atherosclerotic lesions or MOMA-2–positive areas in micrometers squared. Aortic images and aortic root sections were analyzed with a double-blind labeling system, which was decoded after analysis.
Lipid Analysis
Plasma samples were collected by cardiac puncture just before perfusion, after a 4-hour fast. Total cholesterol was determined with a colorimetric assay (cholesterol assay 12217295; Roche Diagnostics, Indianapolis, Ind). Plasma samples from mice with similar total cholesterol levels were pooled and fractionated by fast protein liquid chromatography on a Superose 6 column (Amersham Biosciences, Uppsala, Sweden).
Analysis of Monocyte Subsets
Blood was collected by cardiac puncture of anesthetized mice and depleted of red cells by lysis buffer (0.15 mol/L NH4Cl, 1 mmol/L KHCO3, 0.1 mmol/L EDTA). Leukocytes were washed in staining buffer (PBS, 1% FCS, 0.09% sodium azide) and incubated with rat anti-mouse CD16/CD32 (1:500, or 1 μg/mL; BD Biosciences, San Jose, Calif) for 10 minutes to block Fc receptors. Leukocytes were incubated for 10 minutes at 4°C with combinations of different fluorescent-labeled antibodies, including the following: Ly-6G–FITC (1:200, or 2.5 μg/mL; BD Biosciences), F4/80-APC (1:1,00, or 2 μg/mL; CALTAG Laboratories, South San Francisco, Calif), F4/80-PE (1:100; CALTAG Laboratories), GR-1-APC (1:2000; BD Biosciences), GR-1-biotin (1:200; BD Biosciences), and streptavidin-PacificBlue (1:500, Invitrogen, Carlsbad, Calif). Cells were washed twice between staining steps. Flow cytometry was performed on an LSR II (BD Biosciences) configured with 4 lasers: 50 mW 405 nm, 15 mW 488 nm, 20 mW 630 nm, and 150 mW 532 nm. Data were acquired with BD Diva 5.0 software (BD Biosciences) and analyzed with FlowJo software (version 6.4.7; TreeS-tar, Ashland, Ore).
Statistical Analysis
To analyze differences in the extent of atherosclerosis, MOMA-2 staining, lipid measurements, and body weights, nonparametric Mann–Whitney tests were performed with Prism 4.0 software (GraphPad Software, San Diego, Calif). Probability values <0.05 were considered significant.
The authors had full access to the data and take full responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Atherosclerosis Formation Is Greatly Attenuated in CX3CL1−/−CCR2−/− Mice
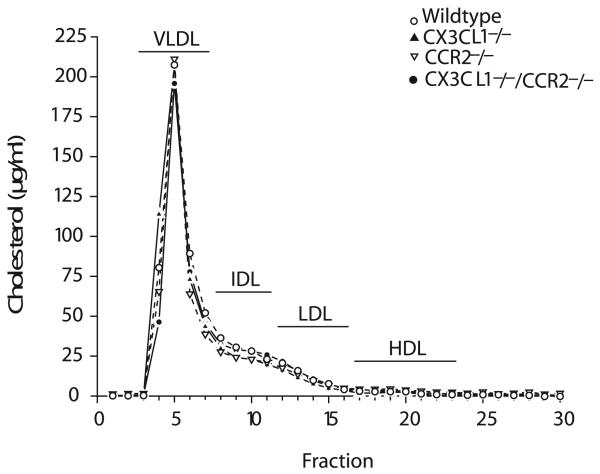
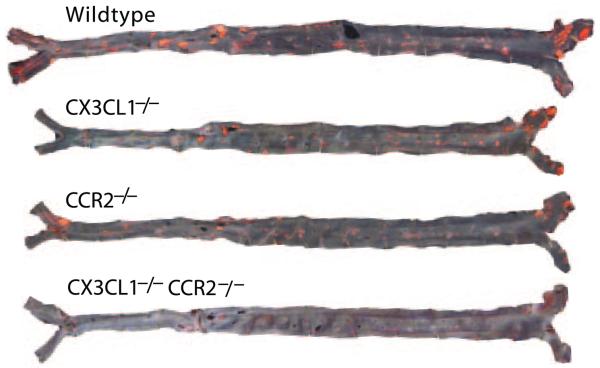
To directly compare the importance of CX3CL1 and CCR2 in the formation of atherosclerotic lesions, we crossed ApoE−/− mice with CX3CL1−/− mice, CCR2−/− mice, or CX3CL1−/−CCR2−/− mice and performed a 4-arm study. All mice were fed the high-fat Western diet for 8 weeks. Total plasma cholesterol levels and weights were determined at the time of euthanasia (Table). Lipoprotein profiles were determined by fast protein liquid chromatography and were similar for all 4 genotypes (Figure 1). Lesion size was quantified in whole aortas pinned out and stained with Sudan IV. Compared with “wild-type” (WT) ApoE−/− mice, statistically significant reductions in total lesion area were not found in the CX3CL1−/− group, although it trended lower (Figure 2). In contrast, lesion size was significantly reduced in CCR2−/− mice compared with either WT or CX3CL1−/− mice. Comparison of the extent of disease in the CCR2−/− mice with the CX3CL1−/−CCR2−/− mice revealed a highly significant 50% reduction in the double knockouts. The results were similar when males and females were analyzed separately (data not shown). Representative aortas of each of the genotypes are shown in Figure 3. To further examine atherosclerotic lesions in these mice, we dissected out the heart and stained serial sections of the aortic root with oil red O. The lesion area in the aortic root in female CX3CL1−/− mice, but not in the males, was significantly less than that in WT ApoE−/− mice (Figure 4A). Lesion area in CCR2−/− mice was significantly less than in CX3CL1−/− mice. Furthermore, aortic root lesion area in CX3CL1−/−CCR2−/− mice was very significantly reduced compared with CCR2−/− mice. Representative stained sections are shown in Figure 4B. With the en face data, these results indicate that compared with either CX3CL1−/− or CCR2−/− mice, the CX3CL1−/−CCR2−/− double-knockout mice had significantly more atheroprotection in both the aortic root and the whole aorta.
Table.
Body Weight and Plasma Cholesterol in ApoE−/− Mice on Different Chemokine-Deficient Backgrounds
| Measurements/Gender | WT | CX3CL1−/− | CCR2−/− | CX3CL1−/−CCR2−/− |
P, CX3CL1−/− vs CX3CL1−/−CCR2−/− |
P, CCR2−/− vs CX3CL1−/−CCR2−/− |
|---|---|---|---|---|---|---|
| Weight, g | ||||||
| Female | 21.7±1.4 (12) | 23.6±1.1 (14) | 21.9±1.4 (13) | 24.4±2.1 (20) | NS | 0.0009 |
| Male | 30.7±2.5 (16) | 31.3±2.4 (18) | 30.9±2.7 (16) | 32.4±4.2 (12) | NS | NS |
| Cholesterol, mg/dL | ||||||
| Female | 931±165 (12) | 1396±184 (14) | 1040±181 (13) | 1081±379 (20) | 0.0005 | NS |
| Male | 1071±245 (16) | 1582±360 (18) | 1368±262 (16) | 1688±400 (12) | NS | 0.0309 |
Mice were 14 weeks of age and had been on the high-fat diet for 8 weeks at the time they were euthanized. Data were analyzed with the 2-tailed Mann–Whitney test. Values in parentheses are number of mice.
Figure 1.
Size fractionation of plasma lipoproteins. Plasma samples of WT, CX3CL1−/−,CCR2−/−, and CX3CL1−/−CCR2−/− mice, fed the Western diet for 8 weeks, with similar total cholesterol levels were pooled and fractionated by fast protein liquid chromatography (n=3 to 4 mice of each geno-type). All mice were on the ApoE−/− genetic background.
Figure 2.
Atherosclerotic lesion areas (en face) in the aorta in WT, CX3CL1−/−, CCR2−/−, and CX3CL1−/−CCR2−/− mice fed the Western diet for 8 weeks. All mice were on the ApoE−/− genetic background. Each symbol depicts the percentage of the total aorta that stained for lipid with Sudan IV in a single mouse. Males (closed circles) and females (open circles) were combined for the analysis. “WT” denotes the ApoE−/− background, without the chemokine receptor deletions. Lesions in CCR2−/− mice were significantly smaller than in CX3CL1−/− mice (2.3% vs 2.9%). Lesion area in CX3CL1−/−CCR2−/− double-knockout mice was significantly less than in CCR2−/− mice (1.2% vs 2.3%). Compared with WT mice, lesion area in CX3CL1−/− CCR2−/− mice was reduced by >65%. Horizontal bars represent mean values for the group (*P≤0.05 and **P≤0.005).
Figure 3.
En face photographs of Sudan IV–stained aortas from WT, CX3CL1−/−, CCR2−/−, and CX3CL1−/−CCR2−/− mice, all on the ApoE−/− background, fed the Western diet for 8 weeks.
Figure 4.
Analysis of atherosclerosis in the aortic root in CX3CL1−/−, CCR2−/−, and CX3CL1−/−CCR2−/− mice fed the Western diet for 8 weeks. All mice were on the ApoE−/− genetic background. The degree of atherosclerosis was determined by staining serial (8 μm) cross sections through the aortic root with oil red O. Lesion size was quantified by digital morphometry, as described in Methods. A, Each symbol represents the mean lesion size in a single mouse, and the horizontal bar represents the mean of the group. Lesion areas in CCR2−/− mice were significantly less than in CX3CL1−/− mice in both males and females, and lesions in CX3CL1−/−CCR2−/− double-knockout mice were significantly smaller than in CCR2−/− mice in both males and females. Lesion areas in female CX3CL1−/−CCR2−/− mice were less than 25% of the areas in WT mice. (*P≤0.05 and **P≤0.005). B, Photomicrographs of oil red O–stained aortas from WT, CX3CL1−/−, CCR2−/−, and CX3CL1−/−CCR2−/− mice, all on the ApoE−/− background, fed the Western diet for 8 weeks.
Macrophage Accumulation Is Markedly Reduced in CX3CL1−/−CCR2−/− Mice
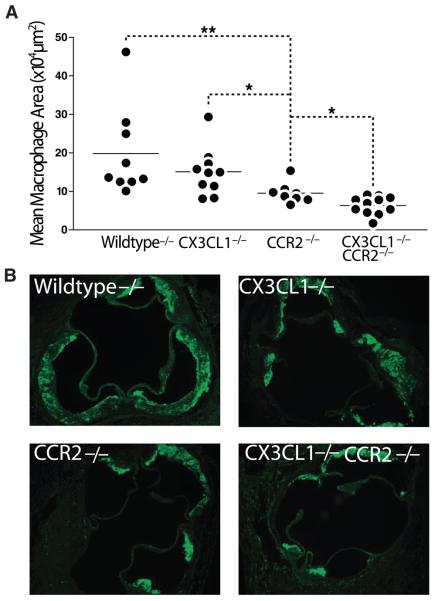
We next asked whether failure to accumulate macrophages in early lesions was the basis for the reduced lesion size in CX3CL1−/−CCR2−/− mice. CCR2−/− mice recruited fewer MOMA-2–positive macrophages into aortic root plaques than either WT ApoE−/− mice or CX3CL1−/− mice (Figure 5). Significantly, this reduction in macrophage presence was even more robust in the CX3CL1−/−CCR2−/− double knockouts.
Figure 5.
Macrophage infiltration of the aortic root in WT, CX3CL1−/−, CCR2−/−, and CX3CL1−/−CCR2−/− female mice, all on the ApoE−/− background, fed the Western diet for 8 weeks. Sections from the aortic root were stained for macrophages with MOMA-2, and the stained area was quantified by digital morphometry, as described in Methods. A, CX3CL1−/−CCR2−/− mice had significantly less MOMA-2 staining than CCR2−/− mice (*P≤0.05 and **P≤0.005). Each symbol represents a single mouse, and the horizontal bar represents the mean value for each group. B, Representative aortic root sections stained with MOMA-2. Sections are adjacent to the ones shown in Figure 3B.
Circulating Monocyte Levels Are Not Affected by CX3CL1 Deletion in CCR2−/− Mice
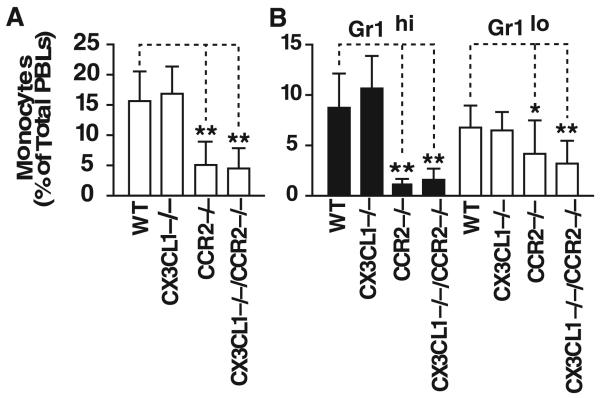
CCR2 facilitates monocyte release from the bone marrow, and CCR2−/− mice have fewer Ly6Cbri monocytes in the blood than WT mice.6,7 We therefore asked whether the reduction in atherosclerotic lesion size in the CX3CL1−/−CCR2−/− mice versus CCR2−/− mice could be accounted for by a further decrease in the number of circulating blood monocytes. After 8 weeks on the diet, CX3CL1−/− and WT mice (on the ApoE−/− background) had similar numbers of total circulating monocytes (Figure 6A). As expected, this number was reduced by >65% in CCR2−/− mice. Notably, deletion of CX3CL1 on the CCR2−/− background did not further decrease the number of circulating monocytes. The Gr1hi (equivalent to Ly6Chi) monocytes are thought to be preferentially recruited into early atherosclerotic lesions. Levels of Gr1hi monocytes were markedly lower in CCR2−/− mice than in WT mice, but they were not further reduced in CX3CL1−/−CCR2−/− mice (Figure 6B). Similarly, the Gr1lo monocyte population was not significantly different in CCR2−/− and CX3CL1−/−CCR2−/− mice.
Figure 6.
Peripheral blood leukocytes (PBLs) in WT, CX3CL1−/−, CCR2−/−, and CX3CL1−/−CCR2−/− mice on the ApoE−/− background fed the Western diet for 8 weeks. A, Total monocytes in each of the 4 genotypes. There was no decrease in the percentage of monocytes in CX3CL1−/− mice vs WT mice. Monocytes were significantly lower in CCR2−/− and CX3CL1−/− CCR2−/− mice than in WT mice, but there was no difference between CCR2−/− and CX3CL1−/−CCR2−/− mice. B, Quantification of Gr1hi and Gr1lo populations of monocytes. There was no difference in the percentage of Gr1hi monocytes in CX3CL1−/− mice vs WT mice. The percentage of Gr1hi monocytes was very significantly reduced in CCR2−/− and CX3CL1−/−CCR2−/− mice compared with WT mice, but there was no difference between CCR2−/− and CX3CL1−/−CCR2−/− mice. Similar results were seen in the Gr1lo population. (*P≤0.05 and **P≤0.005).
Discussion
In this study, we sought to determine whether CX3CL1 and CCR2 act independently or in concert to promote lesion formation through their effects on macrophage accumulation. There are 2 major findings in the present study. First, although deletion of either CX3CL1 or CCR2 reduced macrophage presence and atherosclerosis, atheroprotection in the CCR2−/− mice was more robust. Second, there was an even greater reduction of lesion formation in the CX3CL1−/−CCR2−/− double knockouts, which suggests that CX3CL1 and CCR2 have independent functions in recruiting monocyte/macrophages. These differences, which were not linked to changes in cholesterol levels or lipoprotein profiles, provide mechanistic insights into the role of monocyte chemoattractants in fatty streak formation and early plaque development.
The past 2 decades have witnessed a rapid increase in our understanding of leukocyte migration throughout the body, with the identification of ≈50 chemokines and >20 chemokine receptors. Several chemokines and their cognate receptors have been implicated in atherogenesis, including KC/CXCR2,15 RANTES/CCR5,9,16 CXCR3,17 JE (CCL2)18,19/CCR2,10 and CX3CL120/CX3CR1.11,12 The finding that deletion of individual ligands or their receptors reduces lesion size suggests that these chemokines have specific roles in recruiting and retaining monocytes in the vessel wall; however, the sum of the reductions reported for the individual knockouts easily exceeds 100%, and so, they cannot all be acting independently.
Several lines of evidence support the notion that CCL2/CCR2 and CX3CL1/CX3CR1 are the primary chemokine/receptor pairs that drive atherosclerotic lesion formation. A single-nucleotide polymorphism (SNP) in CCL2 that results in increased plasma levels has been associated with increased risk of myocardial infarction,21,22 as has a CCR2 SNP (V64I).23 Two SNPs (I249V and T280M) have been identified for CX3CR1 and linked to protection from coronary artery disease.24,25 In the mouse, genetic deletion of either CCL218,19 or CCR210,26 reduced diet-induced lesion formation by at least 50%. Similarly, deletion of CX3CR1 robustly reduced lesion size and macrophage recruitment in the aorta.11,12
We sought to determine whether these 2 chemokine/receptor pairs were acting independently or in concert in promoting lesion formation. CCR2 and CX3CR1 are closely linked on the same chromosome, and it is difficult to create double-receptor knockouts. We took advantage of the fact that CX3CL1 is the only known ligand for CX3CR1 and crossed CX3CL1−/− and CCR2−/− mice to investigate the combined effect of the loss of both of these signaling pathways. After 8 weeks on the Western diet, the mice were examined for populations of circulating blood monocytes, recruitment of monocyte/macrophages to the artery wall, and atherosclerotic lesion size in each of the 4 groups.
Recent work identified a population of circulating monocytes that are recruited from the blood to developing atherosclerotic lesions.9,27 By fluorescence-activated cell sorter analysis, these monocytes are Ly6Chi and 7/4bri and express CCR2.6,7 In CCR2−/− mice, this population is greatly reduced in size due to a failure of monocytes to leave the bone marrow, particularly in the setting of infection6 or hypercholesterolemia,7 and their scarcity in the blood may contribute to the impaired macrophage recruitment and atheroprotection seen in CCR2−/− mice. Before the present study, few data were available on monocyte populations in CX3CL1−/− mice, and none were available in the setting of hypercholesterolemia. In agreement with earlier work, we found a marked decrease in the number of Gr1hi monocytes in the CCR2−/− mice fed the high-fat diet.7 In contrast, the Gr1hi monocytes were normal in CX3CL1−/− mice, and significantly, deletion of CX3CL1 did not further decrease this population in CCR2−/− mice. Thus, any differences seen in macrophage accumulation between CCR2−/− and CX3CL1−/−CCR2−/− mice cannot be ascribed to differences in monocyte numbers.
Macrophage content in atherosclerotic lesions was similar in WT and CX3CL1−/− mice, although there was a trend toward a reduction in the CX3CL1−/− mice. As expected, markedly fewer macrophages were found in the lesions in CCR2−/− mice. Tacke et al9 showed that monocytes require CCR2 and CX3CR1 (the receptor for CX3CL1) for direct recruitment to lesions, but it was unclear whether the receptors functioned in sequence or independently. Significantly, we show that deletion of CX3CL1 further reduced macrophage presence in the lesions of CCR2−/− mice. This additive phenotype provides in vivo evidence for independent contributions of CX3CL1 and CCR2 in monocyte/macrophage recruitment to the artery wall. Because this phenotype cannot be attributed to a reduction in circulating monocyte levels, it suggests that CX3CL1 is acting locally at the site of recruitment.
We next asked whether the decreased macrophage recruitment in CX3CL1−/−CCR2−/− double knockouts led to a reduction in atherosclerotic lesion size. As part of this study, we directly compared the extent of atherosclerosis in CX3CL1−/− and CCR2−/− mice, both by en face staining of the entire aorta and by staining serial sections through the aortic root. By both methods, the atheroprotection afforded by deletion of CCR2 was significantly greater than deletion of CX3CL1. Similarly, Teupser et al20 found only modest reductions in the aortic root of CX3CL1−/− mice. Nonetheless, deletion of CX3CL1 in the CCR2−/− mice resulted in a further reduction in lesion size in both the total aorta and in the aortic root, in both males and females, which indicates an additive or synergistic effect. These results are consistent with the macrophage staining results and suggest that CX3CL1 and CCR2 act independently to recruit monocyte/macrophages to the vessel wall at an early step in the development of atherosclerotic lesions. Differences in cholesterol levels or weights of the mice cannot account for these results. In fact, both the weights and total plasma cholesterol levels were greater in the knockout mice than in the WT mice. For example, the CX3CL1−/−CCR2−/− mice were heavier and had slightly higher plasma cholesterol levels than the CCR2−/− mice, yet they had by far the least atherosclerotic lesion formation.
At least 2 distinct models can be envisioned to explain chemokine-dependent recruitment of monocytes to atherosclerotic lesions. In the first, a chemokine such as CCL2 functions as a chemoattractant to bring monocytes to the vessel wall, where they are then localized and captured by other chemokines, such as CX3CL1 and interleukin-8, which are competent cell-adhesion molecules. In this multistep sequential model, different chemokines have distinct but interdependent functions, and genetic deletion of any 1 in the sequence would be expected to produce a similar phenotype (ie, deletion of both chemokines would be no more protective than the individual deletions). Barlic et al28 suggested such a mechanism, based in part on the in vitro findings that oxidized lipids downregulate expression of CCR2 on monocytes but upregulate CX3CR1 expression. However, in vivo data in the present study demonstrate that the simultaneous loss of CCR2 and CX3CL1 results in additive reductions in macrophage recruitment and atheroprotection and thus argue against such a sequential, interdependent recruitment mechanism.
In a second model, CX3CL1 and CCL2 act independently to recruit either the same or different monocytes to the artery wall. Geissmann et al8 described 2 populations of blood monocytes: 1 population is Gr1+(Ly6Chi)CX3CR1loCCR2+, and the other is Gr1− (Ly6Clo)CX3CR1hiCCR2−/−. Tacke et al9 found that both CCR2 and CX3CR1 contributed to migration of the Ly6ChiCCR2+ monocytes to atherosclerotic lesions, and surprisingly, CCR5 (but not CX3CR1) was required for accumulation of the Ly6CloCX3CR1hiCCR2− monocytes.
Because the deletion of both receptors markedly reduces macrophage accumulation in lesions, the present data suggest that CCR2 and CX3CR1 are recruiting different monocytes within the Ly6Chi population. CCL2 and CX3CL1 are expressed in both endothelial cells and smooth muscle, but CX3CL1 is particularly abundant in medial smooth muscle cells.11,28 Different monocytes may thus be recruited to different locations, and the location might depend on where they first encounter chemokines. This mechanism is consistent with a model in which CCR2 and CX3CR1 act independently and nonsequentially.
These results have several clinical implications. First, with regard to atheroprotection, CCR2 appears to be a more promising therapeutic target than CX3CL1. Second, for therapeutic purposes, it might be necessary to block both CX3CL1 and CCR2. In this regard, Martin et al29 recently reported that the viral-encoded protein M3, which binds a broad range of chemokines, provided excellent protection against development of streptozotocin-induced diabetes in mice. Redundancy has been an issue in the chemokine family since the full spectrum of the ligands and receptors became apparent. The present study suggests that it might be necessary to simultaneously block 2 or more chemokine receptors to achieve robust clinical results.
CLINICAL PERSPECTIVE.
The past decade has witnessed a dramatic increase in our understanding of the importance of inflammation in all stages of atherosclerotic heart disease. Subendothelial foam cells, the hallmark of early lesions, are derived from circulating blood monocytes, and recent evidence indicates that chemokines play important roles in directing monocyte migration from the blood to the vessel wall. Genetic deletions of monocyte chemoattractant protein-1 (MCP-1, CCL2), fractalkine (CX3CL1), or their cognate receptors, CCR2 and CX3CR1, have been shown to markedly reduce monocyte recruitment and atherosclerotic lesion size in murine models of atherosclerosis; however, whether these 2 chemokine systems were redundant or made independent contributions was unknown. To address this question, we created double knockouts of fractalkine and CCR2 (on the apolipoprotein E–null background) and performed a 4-arm atherosclerosis study. The results demonstrate that deletion of CCR2 affords significantly more protection than deletion of fractalkine. Significantly, deletion of fractalkine in CCR2-null mice further reduced monocyte recruitment and atherosclerotic lesion size, which indicates that both chemokines make independent and significant contributions to atherogenesis. These data provide the first in vivo evidence for independent roles for CCR2 and CX3CL1 in monocyte recruitment and atherosclerotic lesion formation and suggest that successful therapeutic strategies for atherosclerosis or other classic inflammatory diseases such as rheumatoid arthritis or multiple sclerosis may need to target multiple chemokines or chemokine receptors.
Acknowledgments
We thank Gary Howard and Stephen Ordway for editorial assistance, Mijoung Chang and Daryl Jones for manuscript preparation, and John Carroll for preparation of the figures.
Sources of Funding
This work was funded in part by National Institutes of Health grants 5R01 HL52773 and R01 HL63894 (to Dr Charo) and by NIH/NCRR grant CO6 RR018928 (to J. David Gladstone Institutes).
Footnotes
Disclosures
Dr Lira has received research grants and honoraria from and holds an ownership interest in Schering-Plough.
References
- 1.Ross R. Cell biology of atherosclerosis. Annu Rev Physiol. 1995;57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 3.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 4.Cushing SD, Berliner JA, Valente AJ, Territo MC, Navab M, Parhami F, Gerrity R, Schwartz CJ, Fogelman AM. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990;87:5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 6.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 7.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 9.Tacke F, Alvarez D, Kapla TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 11.Lesnik P, Haskell CA, Charo IF. Decreased atherosclerosis in CX3CR1−/− mice reveals a role for fractalkine in atherogenesis. J Clin Invest. 2003;111:333–340. doi: 10.1172/JCI15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Combadière C, Potteaux S, Gao J-L, Esposito B, Casanova S, Lee EJ, Debré P, Tedgui A, Murphy PM, Mallat Z. Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation. 2003;107:1009–1016. doi: 10.1161/01.cir.0000057548.68243.42. [DOI] [PubMed] [Google Scholar]
- 13.Cook DN, Chen S-C, Sullivan LM, Manfra DJ, Wiekowski MT, Prosser DM, Vassileva G, Lira SA. Generation and analysis of mice lacking the chemokine fractalkine. Mol Cell Biol. 2001;21:3159–3165. doi: 10.1128/MCB.21.9.3159-3165.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr, Broxmeyer HE, Charo IF. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boisvert WA, Santiago R, Curtiss LK, Terkeltaub RA. A leukocyte homologue of the IL-8 receptor CXCR-2 mediates the accumulation of macrophages in atherosclerotic lesions of LDL receptor-deficient mice. J Clin Invest. 1998;101:353–363. doi: 10.1172/JCI1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veillard NR, Kwak B, Pelli G, Mulhaupt F, James RW, Proudfoot AEI, Mach F. Antagonism of RANTES receptors reduces atherosclerotic plaque formation in mice. Circ Res. 2004;94:253–261. doi: 10.1161/01.RES.0000109793.17591.4E. [DOI] [PubMed] [Google Scholar]
- 17.Veillard NR, Steffens S, Pelli G, Lu B, Kwak BR, Gerard C, Charo IF, Mach F. Differential influence of chemokine receptors CCR2 and CXCR3 in development of atherosclerosis in vivo. Circulation. 2005;112:870–878. doi: 10.1161/CIRCULATIONAHA.104.520718. [DOI] [PubMed] [Google Scholar]
- 18.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor–deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 19.Gosling J, Slaymaker S, Gu L, Tseng S, Zlot CH, Young SG, Rollins BJ, Charo IF. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999;103:773–778. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teupser D, Pavlides S, Tan M, Gutierrez-Ramos JC, Kolbeck R, Breslow JL. Major reduction of atherosclerosis in fractalkine (CX3CL1)-deficient mice is at the brachiocephalic artery, not the aortic root. Proc Natl Acad Sci U S A. 2004;101:17795–17800. doi: 10.1073/pnas.0408096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szalai C, Duba J, Prohászka A, Kalina A, Szabó T, Nagy B, Horváth L, Császár A. Involvement of polymorphisms in the chemokine system in the susceptibility for coronary artery disease (CAD): coincidence of elevated Lp(a) and MCP-1-2518 G/G genotype in CAD patients. Atherosclerosis. 2001;158:233–239. doi: 10.1016/s0021-9150(01)00423-3. [DOI] [PubMed] [Google Scholar]
- 22.McDermott DH, Yang Q, Kathiresan S, Cupples LA, Massaro JM, Keaney JF, Jr, Larson MG, Vasan RS, Hirschhorn JN, O'Donnell CJ, Murphy PM, Benjamin EJ. CCL2 polymorphisms are associated with serum monocyte chemoattractant protein-1 levels and myocardial infarction in the Framingham Heart Study. Circulation. 2005;112:1113–1120. doi: 10.1161/CIRCULATIONAHA.105.543579. [DOI] [PubMed] [Google Scholar]
- 23.Ortlepp JR, Vesper K, Mevissen V, Schmitz F, Janssens U, Franke A, Hanrath P, Weber C, Zerres K, Hoffmann R. Chemokine receptor (CCR2) genotype is associated with myocardial infarction and heart failure in patients under 65 years of age. J Mol Med. 2003;81:363–367. doi: 10.1007/s00109-003-0435-x. [DOI] [PubMed] [Google Scholar]
- 24.McDermott DH, Halcox JPJ, Schenke WH, Waclawiw MA, Merrell MN, Epstein N, Quyyumi AA, Murphy PM. Association between polymorphism in the chemokine receptor CX3CR1 and coronary vascular endothelial dysfunction and atherosclerosis. Circ Res. 2001;89:401–407. doi: 10.1161/hh1701.095642. [DOI] [PubMed] [Google Scholar]
- 25.Moatti D, Faure S, Fumeron F, Amara MEW, Seknadji P, McDermott DH, Debré P, Aumont MC, Murphy PM, de Prost D, Combadière C. Polymorphism in the fractalkine receptor CX3CR1 as a genetic risk factor for coronary artery disease. Blood. 2001;97:1925–1928. doi: 10.1182/blood.v97.7.1925. [DOI] [PubMed] [Google Scholar]
- 26.Dawson TC, Kuziel WA, Osahar TA, Maeda N. Absence of CC chemokine receptor-2 reduces atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 1999;143:205–211. doi: 10.1016/s0021-9150(98)00318-9. [DOI] [PubMed] [Google Scholar]
- 27.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barlic J, Zhang Y, Foley JF, Murphy PM. Oxidized lipid-driven chemokine receptor switch, CCR2 to CX3CR1, mediates adhesion of human macrophages to coronary artery smooth muscle cells through a peroxisome proliferator-activated receptor gamma-dependent pathway. Circulation. 2006;114:807–819. doi: 10.1161/CIRCULATIONAHA.105.602359. [DOI] [PubMed] [Google Scholar]
- 29.Martin AP, Alexander-Brett JM, Canasto-Chibuque C, Garin A, Bromberg JS, Fremont DH, Lira SA. The chemokine binding protein M3 prevents diabetes induced by multiple low doses of streptozotocin. J Immunol. 2007;178:4623–4631. doi: 10.4049/jimmunol.178.7.4623. [DOI] [PubMed] [Google Scholar]