Abstract
Objective
To characterize the magnitude and course of alterations in total and free lamotrigine (LTG) clearance (Cl) during pregnancy and the postpartum period, to assess the impact of therapeutic drug monitoring (TDM) on seizure frequency, to determine the ratio to individual target LTG concentration that is associated with increased seizure risk, and to evaluate maternal postpartum toxicity.
Methods
A cohort of women were enrolled before conception or during pregnancy in this prospective, observational study. Visits occurred every 1 to 3 months with review of seizure and medication diaries, examination, and blood sampling. Total and free LTG Cls were calculated. Individualized target concentrations were used for TDM. The ratio to target concentration (RTC) was compared between patients with and without increased seizures. A receiver operating characteristic curve determined the threshold RTC that best predicts increased seizure frequency.
Results
Analysis of 305 samples in 53 pregnancies demonstrated increased total and free LTG Cl in all trimesters above nonpregnant baseline (p < 0.001), with peak increases of 94% and 89% in the third trimester. Free LTG Cl was higher in white compared with black women (p < 0.05). Increased seizure frequency (n = 36 women with epilepsy) in the second trimester was associated with a lower RTC (p < 0.001), and RTC < 0.65 was a significant predictor of seizure worsening. An empiric postpartum taper reduced the likelihood of maternal LTG toxicity (p < 0.05) (n = 27). Newborn outcomes were similar to the general population (n = 52).
Conclusions
These novel data contribute to a rational treatment plan and dosing paradigm for lamotrigine use during pregnancy, parturition, and the postpartum period.
Lamotrigine (LTG) has emerged as a primary maintenance treatment for women with epilepsy and bipolar disorder during their reproductive years.1–3 A major contributor to the use of LTG in this population is the burgeoning pregnancy data that suggest a modest teratogenic risk compared with other antiepileptic drugs (AEDs), with reported rates of major congenital malformations from 1.0% to 5.6%.4–9 However, observational studies have indicated that LTG clearance (Cl) markedly increases during pregnancy,10–13 with seizures worsening in women with epilepsy.10,12 Current treatment guidelines14,15 do not address how to dose AEDs after pregnancy is established or through the postpartum period. The vulnerability of the developing fetus must be considered in the context of the necessary maintenance of AEDs for seizure control.
The magnitude of the reported increases in LTG Cl during pregnancy exceeds that described for AEDs that are primarily eliminated via the P450 system.16 Greater than 90% of LTG is glucuronidated and is controlled primarily by UGT1A420 and to a lesser extent by UGT1A3.17,18 One theory is that the rising sex steroid hormone concentrations enhance expression of UDP-glucuronosyltransferases (UGTs).19,20
An early study (n = 12) reported an increase in LTG oral apparent Cl (>65%) by the second trimester (p < 0.05)13 but was confounded by coadministration with interacting AEDs in several women. An observational study on LTG monotherapy (n = 14) demonstrated a progressive increase in LTG Cl, with a peak increase of >230% above nonpregnant baseline.11 A retrospective study reported an approximately 150% increase in LTG Cl in the second and third trimesters of pregnancy (n = 11).12 These studies noted a rapid decrease in LTG Cl during the early postpartum period.11–13 Previous investigations have focused on total LTG concentrations. LTG is approximately 55% protein bound. The free fraction of other AEDs increases during pregnancy.16
Observations of pregnant women with epilepsy taking a variety of AEDs indicate that approximately 17% to 37% of patients will have an increase in their seizures, 7% to 25% will have a decrease in seizures, and 50% to 83% will experience no significant change.21,24 Most of these investigations have implicated subtherapeutic AED concentrations as a contributor to increased seizures during pregnancy.16,22–24 Generalized tonic–clonic seizures (GTC-Szs) are considered the most dangerous25–29 and may even increase the risk for cognitive dysfunction in the offspring.30,31
The alterations in LTG Cl during pregnancy have important clinical relevance. The Australian Pregnancy Registry reported that the rates of failure of control of convulsive seizures were higher for the LTG group compared with the valproate group and the carbamazepine group, with two fetal losses in the LTG group in association with status epilepticus or prolonged seizures.8 The physicians in this national population-based study did not tend to use therapeutic drug monitoring (TDM) to make LTG dose adjustments during pregnancy. A study of 12 pregnancies on LTG monotherapy reported that 75% of the pregnancies experienced seizure worsening.10 After delivery, toxic side effects occurred in three of seven women who had dose adjustments during pregnancy. A retrospective study of LTG monotherapy noted seizure worsening in 45% of the pregnancies (n = 11), occurring in women who had >60% change in level/dose ratio.12 These retrospective studies did not include a specific paradigm for adjusting LTG dose according to concentration, but did include some adjustments according to their clinical practice.
METHODS
Study population
Women who were pregnant or planning pregnancy who were enrolled in a prospective observational investigation of the pharmacokinetic alterations in AEDs during pregnancy (Emory Women’s Epilepsy and Mental Health Programs) from December 2002 until March 2006 were screened for inclusion in the present study. The Institutional Review Board of Emory University School of Medicine approved the study. Women were informed of all available treatment options, and written informed consent was obtained. Only women who chose to continue LTG during pregnancy were included in this cohort analysis. Subjects were excluded for uncontrolled thyroid disease, severe anemia, ethanol or recreational drug abuse, renal or hepatic dysfunction, poor compliance, age < 17 years, active suicidal ideations, progressive cerebral disease, inability to keep a seizure calendar personally or by a caregiver, and coadministration of medications known to influence the metabolism of LTG. If a patient had more than one pregnancy during the study, only the most recent completed pregnancy was considered in all analyses.
Study design
Women were enrolled at different gestational ages (GAs) including preconception, and follow-up visits occurred every 1 to 3 months during pregnancy and the first postpartum year. Daily calendars were kept for concomitant medication exposures, for any missed doses of LTG, and for recording number and types of seizures for patients with epilepsy. At each visit, a neurologic examination was performed, the daily seizure-medication calendars were reviewed, and intervening illnesses and maternal or newborn complications were recorded. Maternal blood was collected at each study visit and occasionally at obstetric offices, with recording of number of hours since the last dose of LTG. Medical records were obtained for review from the treating obstetrician, the delivery hospital, and the treating pediatrician.
Laboratory measurements
At each collection, maternal blood was centrifuged at 2,750 rpm at 3°C for 10 minutes, and transferred in 600-μL aliquots to polypropylene tubes. The samples were stored at −80°C until assay. Maternal sera samples were assayed for total and free LTG concentrations using the high-resolution high-performance liquid chromatography with ultraviolet detection method supplied by Chromsystems, GmbH (Munich, Germany).32 The system has a limit of detection of 0.25 μg/mL and is linear to 20 μg/mL for LTG. The intra-assay coefficients of variation (CVs) were 3.0%, 2.0%, and 3.2% for samples with means of 1.50, 9.67, and 14.12 μg/mL for LTG during this study. The interassay CVs were 12.3%, 7.5%, and 12.5% for samples with means of 1.50, 9.67, and 14.12 μg/mL for LTG. Free LTG was separated from bound using Centifree YM-30 cartridges (Millipore Corp, Bedford, MA). The ultrafiltrate was assayed in the system described above without modification. The batched assays were performed masked to the patients’ identities and LTG doses.
Data analysis
Statistical analysis was performed by Limin Peng, PhD, Assistant Professor in the Department of Biostatistics, Rollins School of Public Health, Emory University.
LTG clearance
Given that oral bioavailability could not be directly calculated from this population pharmacokinetic approach, we calculated LTG apparent oral Cl. LTG apparent oral Cls were calculated for both total LTG and for free (unbound) LTG: LTG Cl = daily dose (mg/kg)/serum LTG concentration (mg/L).
Total and free LTG Cls were compared between different stages of pregnancy: baseline (preconception), first trimester (<14 weeks GA), second trimester (14 to 28 weeks GA), third trimester (29 to 42 weeks GA), and postpartum. For those patients who did not enroll before conception, baseline (nonpregnant) LTG Cl was calculated from samples obtained at greater than 6 weeks postpartum and if the patient was not breastfeeding.
To investigate the change of LTG Cl among baseline and pregnancy trimesters, we fit a linear mixed model. We examined the change in LTG Cl throughout the course of pregnancy by testing the difference between the effects of the different trimesters. We also investigated the effects of race and age on LTG Cl. In the model, we assumed constant within-patient correlation. We accounted for the influence of hour post dose of maternal blood collection by incorporating linear and quadratic terms of the corresponding variable. Logarithm transformation was taken to meet the assumption of normality.
Analysis of LTG therapeutic drug monitoring and seizure frequency
In a subgroup of women with epilepsy on LTG monotherapy who had adequate baseline information about seizure frequency and preconception target LTG concentrations, we examined the association between LTG concentrations and seizure frequency. Although this observational, prospective study did not dictate a particular treatment paradigm, if a patient was enrolled in the study with epilepsy, primary management of her seizures and LTG dosing was assumed by one of two investigators (P.B.P. or A.K.). Results of the LTG serum concentrations were actively used for TDM. Recommendations to adjust LTG dosages were made according to the patient’s seizure types, epilepsy syndrome, seizure frequency, history of medication-related side effects, and what was considered that individual’s target concentration based on all of these factors. GA of pregnancy and hours post dose that the blood was collected were also considered.
For each month of study participation, we coded the relative frequency of all types of seizures as 1 if the frequency was greater than the baseline monthly frequency, and 0 otherwise. If the patient had a history of GTCSzs, the relative frequency of GTCSzs was analyzed in the same manner. For each month, we calculated the ratio to target concentration (RTC), which denotes the ratio of total LTG concentration/ target LTG concentration from nonpregnant baseline. The t test was used to compare the RTC values of patients who had an increased seizure frequency (of all types) with the RTC values of those who did not, for each trimester.
Determination of RTC threshold for predicting increased seizure frequency
We calculated the threshold of RTC that best predicted increased seizure frequency. Using a receiver operating characteristic curve (ROC), we determined a reasonable threshold value of RTC which would diagnose a high susceptibility to increased seizure frequency.33 We restricted our attention to the second trimester, in which we found a strong association between LTG TDM and seizure frequency. Reduction of the RTC below some threshold was viewed as a positive test for diagnosing increased seizure susceptibility. The ROC curve was obtained by plotting true-positive rate vs false-positive rate.
Association of LTG dose and maternal postpartum toxicity
Patients with epilepsy who had adequate follow-up through the early postpartum stage were assessed for symptoms of LTG toxicity. Toxicity symptoms included dizziness, imbalance, and blurred or double vision. During the latter half of study, the treating physicians (P.B.P., A.K.) began prescribing empiric postpartum taper schedules to decrease the dose by steady increments at postpartum days 3, 7, and 10, with return to preconception dose or preconception dose plus 50 mg to help counteract the effects of sleep deprivation.
Association of LTG exposure and seizures and fetal outcome
Logistic regression models were fitted to binary outcomes (yes/no) for small for gestational age (SGA), low birth weight (LBW), admission to special care nursery or neonatal intensive care unit (NICU), Apgar score at 1 minute ≤ 7, and Apgar score at 5 minutes ≤ 7. The predictors were chosen as the LTG exposure in the first trimester, second trimester, and third trimester, the total LTG exposure during pregnancy, the occurrence of any seizures, and the occurrence of GTCSzs during pregnancy. The LTG exposure was calculated as the area under the curve (AUC) for LTG dose prescribed.
RESULTS
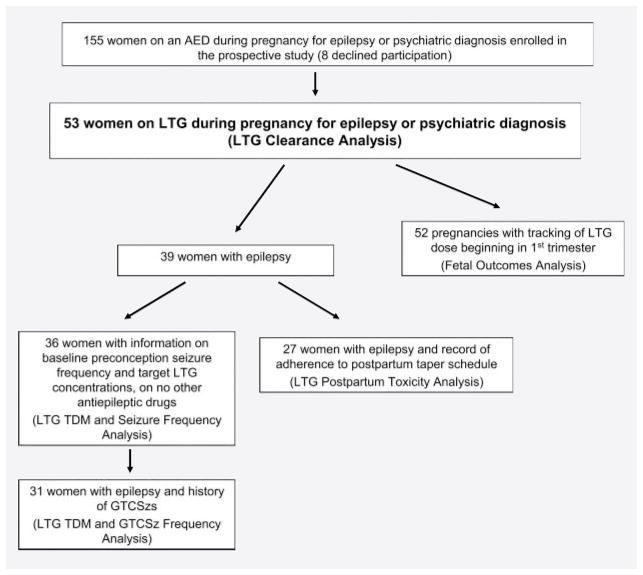
Fifty-three pregnancies in 53 women met criteria for inclusion in this study (figure 1 and table 1). All women, independent of diagnosis, were included in the analysis of alterations in LTG Cl during pregnancy and outcome. Only the patients with epilepsy on LTG monotherapy were included in the analysis of seizure frequency and TDM.
Figure 1.
Flow diagram of women enrolled in the study and information used for data analysis
AED = antiepileptic drug; GTCSz = generalized tonic– clonic seizure; LTG = lamotrigine; TDM = therapeutic drug monitoring.
Table 1.
Study population characteristics
| Total no. of patients | 53 |
| Primary diagnosis | n (% of total) |
| Psychiatric diagnosis | 14 (26.4) |
| Epilepsy | 39 (73.6) |
| Juvenile myoclonic epilepsy | 10 (18.9) |
| Primary generalized epilepsy, unspecified | 2 (3.8) |
| Localization-related epilepsy | 22 (41.5) |
| Epilepsy, unclear whether focal or generalized | 5 (9.4) |
| Racial distribution, n (% of total) | |
| White | 38 (71.7) |
| Black (African-American) | 9 (17.0) |
| Asian | 4 (7.5) |
| White–Hispanic | 2 (3.8) |
| Marital status, n (% of total) | |
| Married | 40 (75.5) |
| Single | 13 (24.5) |
| Tobacco use, n (% of total) | 3 (5.7) |
| Folic acid/vitamin use at time of enrollment, n (% of total) | 53 (100) |
| Mean age (range), y | 31.0 (17–42) |
| Mean education (range), y | 15.4 (10–22) |
| Mean gravida (range) | 2.2 (1–10) |
| Mean gestational age at delivery (range), wk | 38.9 (32.1–41.3) |
LTG clearance across pregnancy and the postpartum period
Total and free LTG Cls were calculated from 305 samples in the 53 women throughout the course of pregnancy and postpartum period (figures 1 and 2, and figures e-1 and e-2 and table e-1 on the Neurology® Web site at www.neurology.org). Six of the 53 women had a preconception baseline sample, and postpartum samples (>6 weeks) were used as nonpregnant baseline in another 19 nonlactating women. Application of the linear mixed model revealed that total and free LTG Cl rates in each of the pregnancy trimesters were all different from baseline Cl rates (p < 0.001; figure 2). Both total and free LTG Cls were different in the second and third trimesters from total and free LTG Cls in the first trimester (p < 0.03). A difference in free LTG Cl was observed between white and black patients (p = 0.031), with the white patients exhibiting a higher Cl. This trend was not significant for total LTG Cl. An increase in hour post dose was associated with an increase in both total LTG Cl and free LTG Cl (p < 0.001). However, the influence of hour post dose was accounted for by incorporating linear and quadratic terms of the corresponding variable in the model. Age had no significant effect on total or free LTG Cl.
Figure 2.
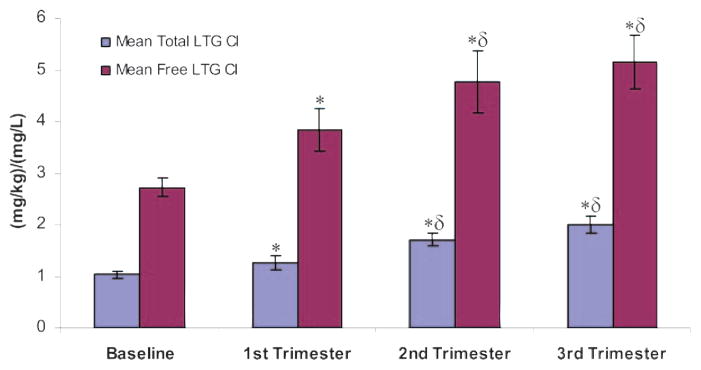
Total and free lamotrigine clearance during pregnancy
Mean (± SE) total lamotrigine clearance (LTG daily dose [mg/kg]/LTG concentration [mg/L]) and mean (± SE) free lamotrigine clearance (LTG daily dose [mg/kg]/unbound LTG concentration [mg/L]) during nonpregnant baseline and each trimester of pregnancy. * p < 0.001 compared with nonpregnant baseline. δ p < 0.03 compared with first trimester. Cl = clearance.
Analysis of LTG therapeutic drug monitoring and seizure frequency
Thirty-six patients of 39 with epilepsy had adequate baseline information about seizure frequency and preconception target LTG concentrations (table 2). Eighty-three RTC calculations were included. The percentage of patients who had an increase in seizures of all types are plotted in figure 3, along with the free LTG Cl by month GA and postpartum. Increased seizure frequency occurred in the highest percentage of patients in the seventh month of gestation (23.5%). For the entire pregnancy, 39% of women had an increase in any type of seizure above preconception baseline, 33% had a decrease in seizures, and 28% had no change.
Table 2.
Percentages of patients who had an increase or a doubling or more in increased seizure frequency compared with preconception baseline, for all seizure types (n = 36) and for generalized tonic– clonic seizures (n = 31) by month gestational age and month postpartum
| Month | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | PP1 | PP2 | PP3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Increased seizures, all types | 8.3 | 16.7 | 8.3 | 19.4 | 16.7 | 17.1 | 23.5 | 14.7 | 11.8 | 5.9 | 16.1 | 14.3 | 10.7 |
| Doubled seizures, all types | 8.3 | 13.9 | 5.6 | 16.7 | 13.9 | 14.3 | 17.6 | 8.8 | 11.8 | 2.9 | 6.5 | 7.1 | 3.6 |
| Increased GTCSzs | 6.7 | 0 | 0 | 3.3 | 6.7 | 10.3 | 3.6 | 0 | 7.1 | 0 | 3.8 | 0 | 0 |
| Doubled GTCSzs | 6.7 | 0 | 0 | 3.3 | 6.7 | 10.3 | 3.6 | 0 | 7.1 | 0 | 3.8 | 0 | 0 |
PP = postpartum month; GTCSz = generalized tonic– clonic seizure.
Figure 3.
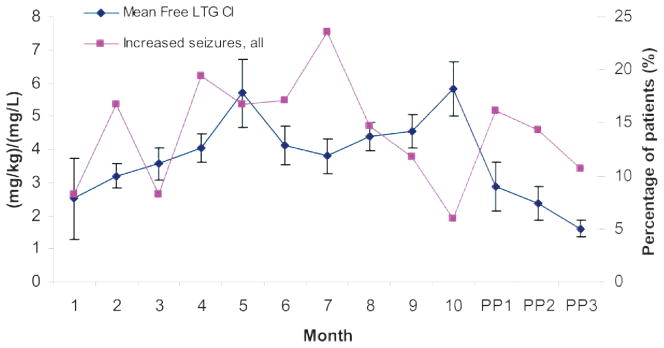
Lamotrigine clearance and seizures during pregnancy and postpartum
Mean (±SE) free lamotrigine clearance (LTG daily dose [mg/kg]/LTG concentration [mg/L]) and percentage of patients who had an increase in seizure frequency compared with preconception baseline, by month gestational age and month postpartum. Cl = clearance.
Comparisons using t tests were performed to compare the RTC values of patients who had an increased seizure frequency (of all types) in the second trimester with the RTC values of those who did not. In the second trimester, increased seizure frequency was associated with a lower RTC in the second trimester (p < 0.001). Using two other thresholds of increased seizure frequency (1.5-fold increase or 2-fold increase in seizures), the same findings occurred (p < 0.001). The associations were not significant in the first trimester or in the third trimester.
Thirty-one patients had a history of GTCSzs. GTCSz frequency doubled or more in 10% of the pregnancies during the sixth month (table 2), but the impact of RTC on GTCSz frequency was not significant. This may be due to the low number of patients with a history of GTCSzs in the past year (n= 9).
Determination of RTC threshold for predicting increased seizure frequency
Using the ROC curve for predicting increased seizure frequency, a high true-positive rate of 83.3% at a low cost of a false-positive rate of 4.2% was achieved at RTC = 0.65. Increasing the threshold to RTC = 0.78 increased the true-positive rate to 100% but increased the false-positive rate from 4.2% to 25.0%.
Association of LTG dose and maternal postpartum toxicity
Among 27 patients with a clear record on adherence to the empiric taper schedule, 4 of 6 patients who did not follow the taper schedule experienced postpartum toxicity, compared with only 3 of 21 patients who adhered to the taper schedule. A χ2 test with continuity correction revealed that nonadherence to the standard taper schedule was associated with a higher risk of experiencing postpartum toxicity (p = 0.040).
Association of LTG exposure and seizures and fetal outcome
The data included 52 patients whose LTG dosage records were tracked since the first trimester of pregnancy. No significant associations were found between the newborn outcomes SGA, LBW, NICU admissions or Apgar scores < 7, and the AUC of LTG prescribed during each trimester or the entire pregnancy, or the occurrence of any seizures or GTCSzs. Neonatal birth weights were comparable to national statistics.34 No stillbirths occurred; one congenital malformation, renal hydronephrosis, was detected, which was nearly resolved by 4 months of age. One newborn was diagnosed with a heart murmur, which resolved by the first well-baby visit. Two deliveries occurred prematurely, at 32 and 36.3 weeks GA.
DISCUSSION
This study demonstrates significant increases in total LTG Cl and free LTG Cl between nonpregnant baseline and all trimesters of pregnancy, and between the first trimester and the latter trimesters of pregnancy. The mean total LTG Cl increased by 94% and the free LTG Cl increased by 89% in the third trimester above the nonpregnant baseline. The magnitude of alteration in total LTG Cl is in the middle of the range of previous reports with smaller patient sample sets.10–13 Substantial interindividual variability in the degree of enhanced Cl has been noted previously11–13 and may contribute to the differences reported. Interestingly, this larger study noted a racial difference, with white patients showing higher LTG Cl rates. The previous studies may have had a different racial mix. Pharmacogenetic variability of UGT1A4 and possibly UGT1A3 polymorphisms35 likely contributes to baseline LTG Cl rates and the degree of enhanced Cl during pregnancy.
Our analysis of the effectiveness of TDM with total LTG concentrations during pregnancy demonstrates improved seizure control compared with previous reports of LTG use during pregnancy.8,10,12 Although 39% of women had an increase in any type of seizure above preconception baseline during pregnancy, 33% actually had a decrease in seizures, and 28% experienced no change. These rates are more consistent with what has been reported for all women with epilepsy on a variety of AED regimens.
The finding of an association between RTC was most robust in the second trimester (14 to 28 weeks), when the largest percentage of patients experienced increased seizures. Our detailed statistical analysis indicates that an RTC < 0.65 in the second trimester is a reliable predictor of seizure worsening. Thus, this data-driven threshold index of a 65% ratio to target LTG concentration should be used for introducing a dosage adjustment for women with epilepsy on LTG during pregnancy. Given that free LTG concentration measurements are not readily available in the clinical setting, measurement of total LTG is adequate for predicting seizure worsening.
Although the RTC does require a simple calculation with consideration of a patient’s target concentration, it is necessary to account for individual differences. The target concentration for each patient is the ideal concentration at which seizures are well controlled without adverse effects. This varies between patients according to epilepsy syndrome, seizure types, and disease severity. Ideally, this target concentration should be established for each woman before pregnancy, but if no preconception baseline is obtained, the practitioner can estimate her target concentration based on these factors.
The return to nonpregnant baseline of total and free LTG Cl rates occurred very quickly and within the first month postpartum (figure 3). More than one quarter of the epilepsy patients did experience postpartum toxicity, which was more likely to occur if the postpartum taper was not performed. The risk of postpartum toxicity must be balanced against the necessary adjustments in LTG dose to maintain seizure control during pregnancy, and an empiric postpartum taper is an effective approach to reduce toxicity.
The lack of an association between the amount of LTG dose administered and newborn outcomes suggests that TDM with adjustment of LTG doses during pregnancy may not adversely affect newborn health. However, this study was not powered to detect an increased risk of congenital malformations and the findings need to be considered in light of a report from the UK Epilepsy and Pregnancy Register of a dose-related risk for LTG in the first trimester and major congenital malformations.7 However, a logistic regression analysis of the International Lamotrigine Pregnancy Registry data showed no difference in the risk for major malformations as a function of dose.4 Additionally, this study did not assess long-term neurocognitive outcomes. Whether the clinical utility of TDM of LTG during pregnancy can be extended to bipolar patients is unclear and under investigation.
This study provides characterization and quantification of the changes in total and free LTG Cl across pregnancy and incorporates this novel data into a TDM approach that can provide the foundation for treatment guidelines to prevent increased seizure frequency and improve maternal and fetal health. Future studies should include formal population pharmacokinetic modeling of the alterations in LTG throughout the course of pregnancy and postpartum stages, individual genotyping of UGT1A4/UGT1A3 polymorphisms, exploration of racial differences, and larger sample sets to more thoroughly assess maternal and fetal outcomes, including long-term neurodevelopment.
Supplementary Material
Acknowledgments
Supported by an NIH Specialized Center of Research (P50 MH 68036), R01 MH-71531, and an NIH grant from the National Center for Research Resources (NCRR M01-RR00039).
GLOSSARY
- AED
antiepileptic drug
- AUC
area under the curve
- Cl
clearance
- CV
coefficient of variation
- GA
gestational age
- GTCSz
generalized tonic– clonic seizure
- LBW
low birth weight
- LTG
lamotrigine
- NICU
neonatal intensive care unit
- PP
postpartum month
- RTC
ratio to target concentration
- SGA
small for gestational age
- TDM
therapeutic drug monitoring
- UGT
UDP-glucuronosyltransferase
Footnotes
Disclosure: Dr. Page B. Pennell has participated in the speaker’s bureau and advisory boards for GlaxoSmithKline and UCB Pharma. She has received research support from GlaxoSmithKline, UCB Pharma, Marinus Pharmaceuticals, and the National Institutes of Health. Dr. D. Jeffrey Newport has received honoraria support from GlaxoSmithKline, Eli Lilly, Pfizer, and AstraZeneca and has received research support from the National Institutes of Health, Eli Lilly, GlaxoSmithKline, and Wyeth. Dr. James C. Ritchie has received research grant support from the National Institutes of Health, the Georgia Cancer Coalition, and the American Foundation for Suicide Prevention. Dr. Archana Koganti has participated in the speaker’s bureaus for Cyberonics and UCB Pharma and has received research support from the National Institutes of Health. Ms. Holley has received salary support for conducting research studies from the National Institutes of Health. Ms. Newman has received salary support for conducting research studies from GlaxoSmithKline, UCB Pharma, Marinus Pharmaceuticals, and the National Institutes of Health. Dr. Zachary N. Stowe has participated in the speaker’s bureaus for GlaxoSmithKline, Pfizer, and Wyeth; serves on the advisory boards for GlaxoSmithKline and Bristol Myers Squibb; and has received research grant support from Pfizer, GlaxoSmithKline, Wyeth, and the National Institutes of Health. Dr. Limin Peng has no conflicts of interest.
References
- 1.Karcescki S, Morrell M, Carpenter D. The expert consensus guideline series: treatment of epilepsy. Epil Behav. 2005;2001:A1–A50. [Google Scholar]
- 2.Sabers A, Dam M, Rogvi-Hansen B, et al. Epilepsy and pregnancy: lamotrigine as main drug used. Acta Neurol Scand. 2004;109:9–13. doi: 10.1034/j.1600-0404.2003.00200.x. [DOI] [PubMed] [Google Scholar]
- 3.Yonkers KA, Wisner KL, Stowe Z, et al. Management of bipolar disorder during pregnancy and the postpartum period. Am J Psychiatry. 2004;161:608–620. doi: 10.1176/appi.ajp.161.4.608. [DOI] [PubMed] [Google Scholar]
- 4.Cunnington M, Ferber S, Quartey G International Lamotrigine Pregnancy Registry Scientific Advisory Committee. Effect of dose on the frequency of major birth defects following fetal exposure to lamotrigine monotherapy in an international observational study. Epilepsia. 2007;48:1207–1210. doi: 10.1111/j.1528-1167.2007.01021.x. [DOI] [PubMed] [Google Scholar]
- 5.Holmes LB, Wyszynski DF, Baldwin EJ, Habecker E, Glassman LH, Smith CR. Increased risk for non-syndromic cleft palate among infants exposed to lamotrigine during pregnancy. Birth Defects Res Part A Clin Mol Teratol. 2006;76:5. Abstract. [Google Scholar]
- 6.Meador KJ, Baker GA, Finnell RH, et al. In utero antiepileptic drug exposure: fetal death and malformations. Neurology. 2006;67:407–412. doi: 10.1212/01.wnl.0000227919.81208.b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrow J, Russell A, Guthrie E, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry. 2006;77:193–198. doi: 10.1136/jnnp.2005.074203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vajda FJ, O’Brien TJ, Graham J, et al. Foetal malformations and seizure control: 52 months data of the Australian Pregnancy Registry. Eur J Neurol. 2006;13:645–654. doi: 10.1111/j.1468-1331.2006.01359.x. [DOI] [PubMed] [Google Scholar]
- 9.Pennell PB. Using current evidence in selecting antiepileptic drugs for use during pregnancy. Epilepsy Currents. 2005;5:45–51. doi: 10.1111/j.1535-7597.2005.05201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Haan GJ, Edelbroek P, Segers J, et al. Gestation-induced changes in lamotrigine pharmacokinetics: a monotherapy study. Neurology. 2004;63:571–573. doi: 10.1212/01.wnl.0000133213.10244.fd. [DOI] [PubMed] [Google Scholar]
- 11.Pennell PB, Newport DJ, Stowe ZN, Helmers SL, Montgomery JQ, Henry TR. The impact of pregnancy and childbirth on the metabolism of lamotrigine. Neurology. 2004;62:292–295. doi: 10.1212/01.wnl.0000103286.47129.f8. [DOI] [PubMed] [Google Scholar]
- 12.Petrenaite V, Sabers A, Hansen-Schwartz J. Individual changes in lamotrigine plasma concentrations during pregnancy. Epilepsy Res. 2005;65:185–188. doi: 10.1016/j.eplepsyres.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Tran TA, Leppik IE, Blesi K, Sathanandan ST, Remmel R. Lamotrigine clearance during pregnancy. Neurology. 2002;59:251–255. doi: 10.1212/wnl.59.2.251. [DOI] [PubMed] [Google Scholar]
- 14.Committee on Educational Bulletins of the American College of Obstetricians and Gynecologists. Seizure disorders in pregnancy. Int J Gynecol Obstet. 1997;56:279–286. [PubMed] [Google Scholar]
- 15.Report of the quality of standards subcommittee of the American Academy of Neurology. Practice parameter: a guideline for discontinuing antiepileptic drugs in seizure-free patients—summary statement. Neurology. 1996;47:600–602. doi: 10.1212/wnl.47.2.600. [DOI] [PubMed] [Google Scholar]
- 16.Pennell PB. Antiepileptic drug pharmacokinetics during pregnancy and lactation. Neurology. 2003;61 (suppl 2):S35–S42. doi: 10.1212/wnl.61.6_suppl_2.s35. [DOI] [PubMed] [Google Scholar]
- 17.Green MD, Tephly TR. Glucuronidation of amines and hydroxylated xenobiotics and endobiotics catalyzed by expressed human UGT1. 4 protein. Drug Metab Dispos. 1996;24:356–363. [PubMed] [Google Scholar]
- 18.Green MD, King CD, Mojarrabi B, Mackenzie PI, Tephly TR. Glucuronidation of amines and other xenobiotics catalyzed by expressed human UDP-glucuronosyltransferase 1A3. Drug Metab Dispos. 1998;26:507–512. [PubMed] [Google Scholar]
- 19.Chen S, Beaton D, Nguyen N, et al. Tissue-specific, inducible, and hormonal control of the human UDP-glucuronosyltransferase-1 (UGT1) locus. J Biol Chem. 2005;280:37547–37557. doi: 10.1074/jbc.M506683200. [DOI] [PubMed] [Google Scholar]
- 20.Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46:1414–1417. doi: 10.1111/j.1528-1167.2005.10105.x. [DOI] [PubMed] [Google Scholar]
- 21.EURAP Study Group. Seizure control and treatment in pregnancy: observations from the EURAP epilepsy pregnancy registry. Neurology. 2006;66:354–360. doi: 10.1212/01.wnl.0000195888.51845.80. [DOI] [PubMed] [Google Scholar]
- 22.Dansky L, Andermann E, Sherwin AL, Anderman F, Kinch RA. Maternal epilepsy and congenital malformations: a prospective study with monitoring of plasma anticonvulsant levels during pregnancy. Neurology. 1980;3:15. [Google Scholar]
- 23.Otani K. Risk factors for the increased seizure frequency during pregnancy and puerperium. Folia Psychiatr Neurol Jpn. 1985;39:33–41. doi: 10.1111/j.1440-1819.1985.tb01942.x. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt D, Canger R, Avanzini G, et al. Change of seizure frequency in pregnant epileptic women. J Neurol Neurosurg Psychiatry. 1983;46:751–755. doi: 10.1136/jnnp.46.8.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pennell PB. Pregnancy in women who have epilepsy. Neurol Clin. 2004;22:799–820. doi: 10.1016/j.ncl.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Yerby MS, Kaplan P, Tran T. Risks and management of pregnancy in women with epilepsy. Cleve Clin J Med. 2004;71 (suppl 2):S25–S37. doi: 10.3949/ccjm.71.suppl_2.s25. [DOI] [PubMed] [Google Scholar]
- 27.Minkoff H, Schaffer R, Delke I, Grunevaum A. Diagnosis of intracranial hemorrhage in utero after a maternal seizure. Obstet Gynecol. 1985;65 (suppl):22S–24S. [PubMed] [Google Scholar]
- 28.Report of the Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: management issues for women with epilepsy (summary statement) Neurology. 1998;51:944–948. doi: 10.1212/wnl.51.4.944. [DOI] [PubMed] [Google Scholar]
- 29.Zahn CA, Morrell MJ, Collins SD, Labiner DM, Yerby MS. Management issues for women with epilepsy: a review of the literature. Neurology. 1998;51:949–956. doi: 10.1212/wnl.51.4.949. [DOI] [PubMed] [Google Scholar]
- 30.Vinten J, Adab N, Kini U, Gorry J, Gregg J, Baker GA. Neuropsychological effects of exposure to anticonvulsant medication in utero. Neurology. 2005;64:949–954. doi: 10.1212/01.WNL.0000154514.82948.69. [DOI] [PubMed] [Google Scholar]
- 31.Gaily E, Kantola-Sorsa E, Hiilesmaa V, et al. Normal intelligence in children with prenatal exposure to carbamazepine. Neurology. 2004;62:28–32. doi: 10.1212/wnl.62.1.28. [DOI] [PubMed] [Google Scholar]
- 32.Riedmann M, Rambeck B, Meijer JW. Quantitative simultaneous determination of eight common antiepileptic drugs and metabolites by liquid chromatography. Ther Drug Monit. 1981;3:397–413. doi: 10.1097/00007691-198104000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Metz CE. Basic principles of ROC analysis. Semin Nuclear Med. 1978;VIII:283–298. doi: 10.1016/s0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- 34.Hamilton BE, Minino AM, Martin JA, Kochanek KD, Strobino DM, Guyer B. Annual summary of vital statistics: 2005. Pediatrics. 2007;119:345–360. doi: 10.1542/peds.2006-3226. [DOI] [PubMed] [Google Scholar]
- 35.Ehmer U, Vogel A, Schutte JK, Krone B, Manns MP, Strassburg CP. Variation of hepatic glucuronidation: Novel functional polymorphisms of the UDP-glucuronosyltransferase UGT1A4. Hepatology. 2004;39:970–977. doi: 10.1002/hep.20131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.